Abstract
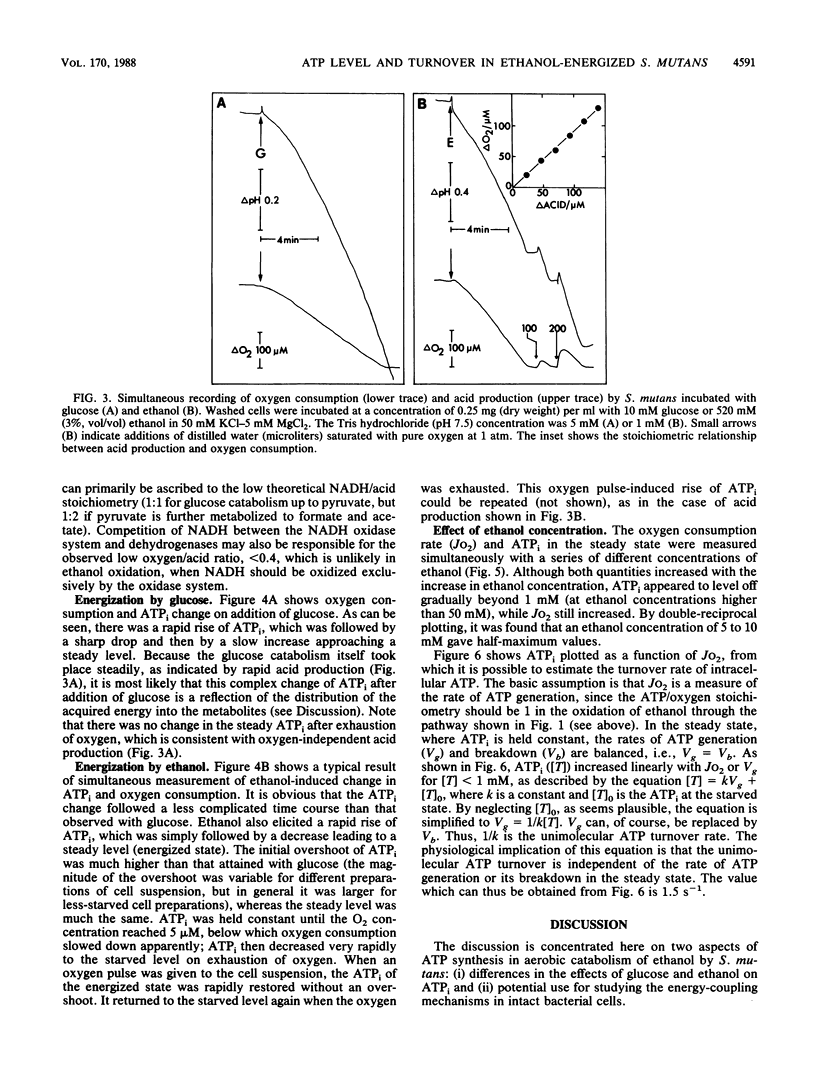
Streptococcus mutans, a group of lactic acid bacteria and a normal inhabitant of the human oral cavity, generates ATP by substrate-level phosphorylation coupled to oxidation of ethanol (an end product of fermentation of sugars) into acetate in the presence of oxygen (K. Fukui, K. Kato, Kodama, H. Ohta, T. Shima moto, and T. Shimono, Proc. Jpn. Acad. 64B:13-16, 1988). Kinetic measurements were made of the cellular responses of S. mutans FA-1 to ethanol in comparison with those to glucose. In contrast to oxygen-independent acid production from glucose, oxygen was absolutely required for acid production from ethanol. Ethanol elicited a marked increase in the intracellular ATP concentration (ATPi) from a starved level to a steady level which was held constant as long as oxygen was present in the medium. Once oxygen was exhausted, ATPi returned to the starved level without delay. On the contrary, ATPi changes induced by glucose, which were independent of oxygen, followed a rather complicated time course before a steady level was established. Both the steady ATPi and the rate of accompanying oxygen consumption were functions of the ethanol concentration. These two parameters were linearly correlated, indicating that the unimolecular ATP turnover rate, which is independent of the rate of ATP generation in the steady state, can be calculated for cells energized by ethanol. The estimated turnover rate was 1.5 s-1 at 37 degrees C, which is comparable to that for other bacteria energized by glucose under nongrowing conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. R., Ugurbil K., Shulman R. G. 31P nuclear magnetic resonance measurements of ATPase kinetics in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5551–5553. doi: 10.1073/pnas.74.12.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadkari D., Stolp H. Energy metabolism of Bdellovibrio bacteriovorus. II. P/O ratio and ATP pool turnover rate. Arch Microbiol. 1976 May 3;108(1):125–132. doi: 10.1007/BF00425102. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E., Maitra P. K. Control of respiration and metabolism in growing Klebsiella aerogenes. The role of adenine nucleotides. Biochem J. 1969 May;112(5):647–656. doi: 10.1042/bj1120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Fukui K., Shimamoto T., Ohta H., Kokeguchi S., Kato K. Effects of oxygen on glucose-limited growth of Streptococcus mutans. Infect Immun. 1987 Jan;55(1):169–173. doi: 10.1128/iai.55.1.169-173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart J. B., Thomas T. D. Effect of oxygen on lactose metabolism in lactic streptococci. Appl Environ Microbiol. 1987 Mar;53(3):533–541. doi: 10.1128/aem.53.3.533-541.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Pera K. A. Oxygen metabolism of Streptococcus mutans: uptake of oxygen and release of superoxide and hydrogen peroxide. J Bacteriol. 1983 Jun;154(3):1236–1244. doi: 10.1128/jb.154.3.1236-1244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


