Abstract
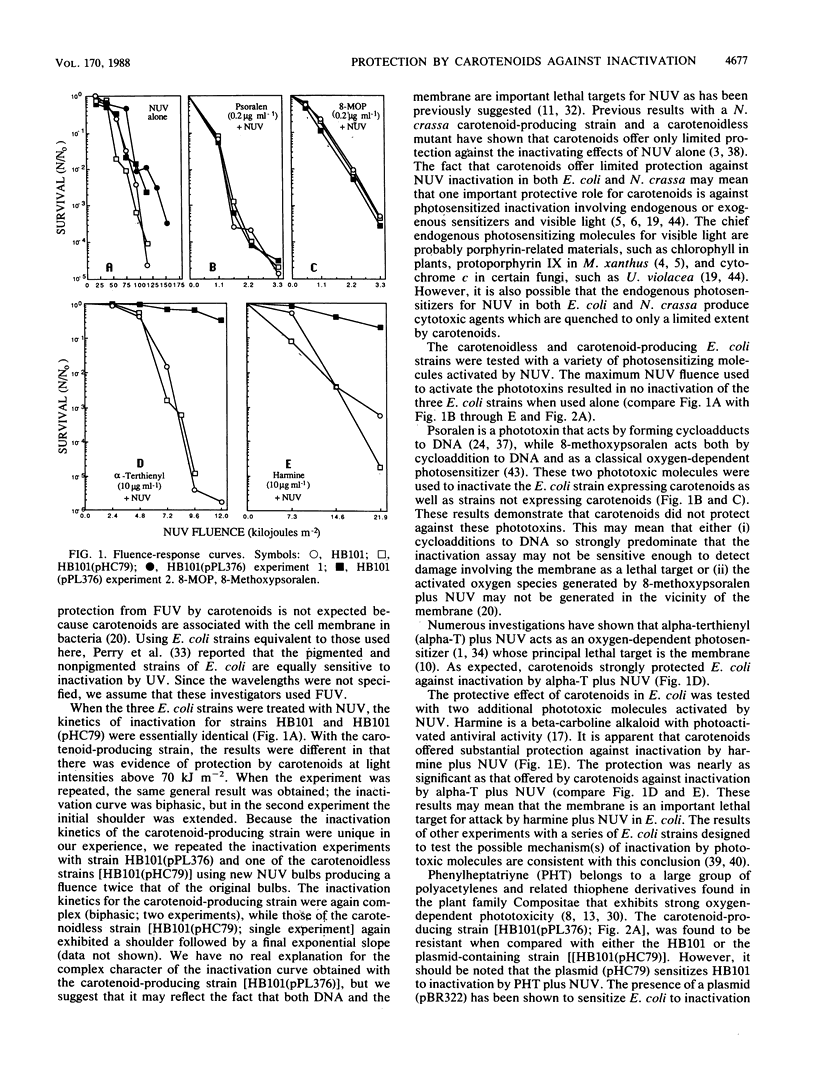
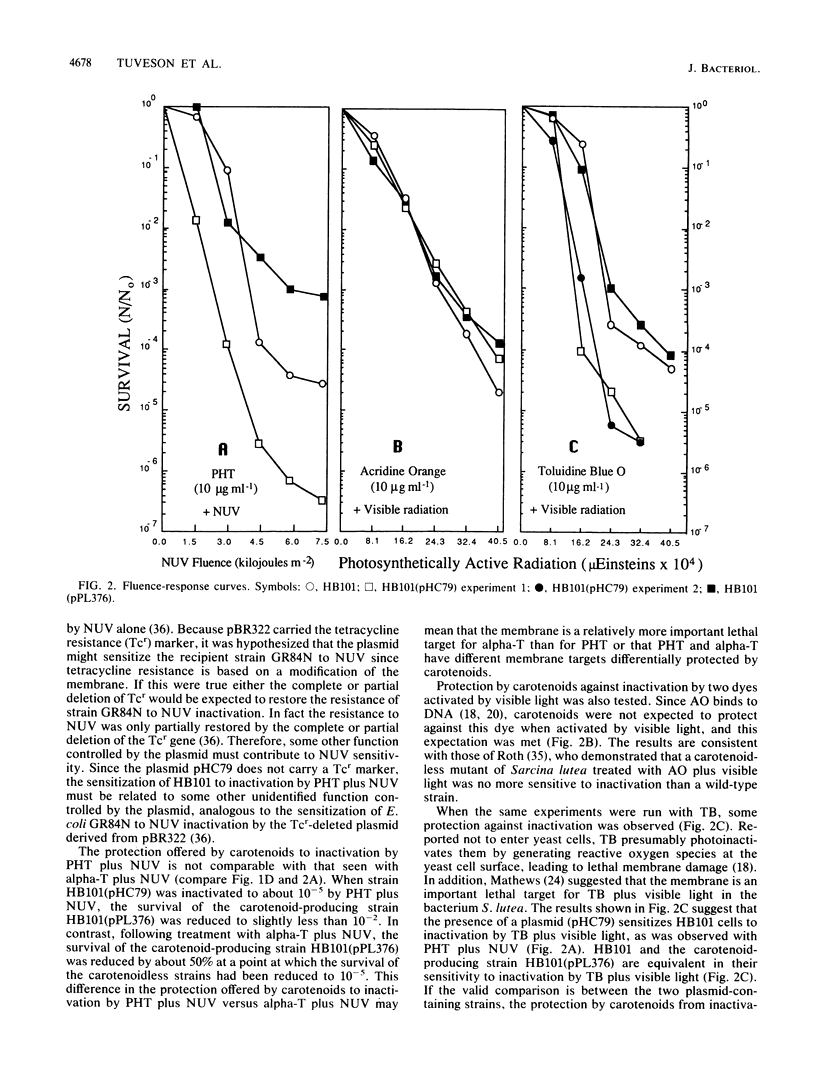
Genes controlling carotenoid synthesis were cloned from Erwinia herbicola and expressed in an Escherichia coli strain. Carotenoids protect against high fluences of near-UV (NUV; 320 to 400 nm) but not against far-UV (200-300 nm). Protection of E. coli cells was not observed following treatment with either psoralen or 8-methoxypsoralen plus NUV. However, significant protection of cells producing carotenoids was observed with three photosensitizing molecules activated by NUV (alpha-terthienyl, harmine, and phenylheptatriyne) which are thought to have the membrane as an important lethal target. Protection of carotenoid-producing cells against inactivation was not observed with acridine orange plus visible light but was seen with toluidine blue O plus visible light.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwood-Smith M. J., Poulton G. A., Ceska O., Liu M., Furniss E. An ultrasensitive bioassay for the detection of furocoumarins and other photosensitizing molecules. Photochem Photobiol. 1983 Jul;38(1):113–118. doi: 10.1111/j.1751-1097.1983.tb08375.x. [DOI] [PubMed] [Google Scholar]
- Blanc P. L., Tuveson R. W., Sargent M. L. Inactivation of carotenoid-producing and albino strains of Neurospora crassa by visible light, blacklight, and ultraviolet radiation. J Bacteriol. 1976 Feb;125(2):616–625. doi: 10.1128/jb.125.2.616-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966 Feb;91(2):535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard R. P., Gordon S. A., Dworkin M. Action spectrum for the photolysis of Myxococcus xanthus. J Bacteriol. 1966 Feb;91(2):896–897. doi: 10.1128/jb.91.2.896-897.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Eisenstark A. Mutagenic and lethal effects of near-ultraviolet radiation (290-400 nm) on bacteria and phage. Environ Mol Mutagen. 1987;10(3):317–337. doi: 10.1002/em.2850100311. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M., SISTROM W. R., COHENBAZIRE G., STANIER R. Y., CALVIN M. Function of carotenoids in photosynthesis. Nature. 1955 Dec 24;176(4495):1211–1215. doi: 10.1038/1761211a0. [DOI] [PubMed] [Google Scholar]
- Gong H. H., Kagan J., Seitz R., Stokes A. B., Meyer F. A., Tuveson R. W. The phototoxicity of phenylheptatriyne: oxygen-dependent hemolysis of human erythrocytes and inactivation of Escherichia coli. Photochem Photobiol. 1988 Jan;47(1):55–63. doi: 10.1111/j.1751-1097.1988.tb02695.x. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Graham E. A., Fong R., Hudson L. L., Towers G. H. Further studies on the antiviral activity of harmine, a photoactive beta-carboline alkaloid. Photochem Photobiol. 1986 Oct;44(4):483–487. doi: 10.1111/j.1751-1097.1986.tb04696.x. [DOI] [PubMed] [Google Scholar]
- Ito T. Cellular and subcellular mechanisms of photodynamic action: the 1O2 hypothesis as a driving force in recent research. Photochem Photobiol. 1978 Oct-Nov;28(4-5):493–508. doi: 10.1111/j.1751-1097.1978.tb06957.x. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. M. Comparative study of lethal photosensitization of Sarcina lutea by 8-methoxypsoralen and by toluidine blue. J Bacteriol. 1963 Feb;85:322–328. doi: 10.1128/jb.85.2.322-328.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. Function of carotenoid pigments in non-photosynthetic bacteria. Nature. 1959 Dec 12;184(Suppl 24):1892–1893. doi: 10.1038/1841892a0. [DOI] [PubMed] [Google Scholar]
- MATHEWS M. M., SISTROM W. R. The function of the carotenoid pigments of Sarcina lutea. Arch Mikrobiol. 1960;35:139–146. doi: 10.1007/BF00425002. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M. Carotenoids quench evolution of excited species in epidermis exposed to UV-B (290-320 nm) light. Photochem Photobiol. 1986 Jan;43(1):91–93. doi: 10.1111/j.1751-1097.1986.tb05596.x. [DOI] [PubMed] [Google Scholar]
- Mathews-Roth M. M. Porphyrin photosensitization and carotenoid protection in mice; in vitro and in vivo studies. Photochem Photobiol. 1984 Jul;40(1):63–67. doi: 10.1111/j.1751-1097.1984.tb04555.x. [DOI] [PubMed] [Google Scholar]
- Moss S. H., Smith K. C. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Feb;33(2):203–210. doi: 10.1111/j.1751-1097.1981.tb05325.x. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Simonitch T. A., Harrison-Lavoie K. J., Liu S. T. Cloning and regulation of Erwinia herbicola pigment genes. J Bacteriol. 1986 Nov;168(2):607–612. doi: 10.1128/jb.168.2.607-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyftmann J. P., Kagan J., Santus R., Morliere P. Excited state properties of alpha-terthienyl and related molecules. Photochem Photobiol. 1985 Jan;41(1):1–7. doi: 10.1111/j.1751-1097.1985.tb03439.x. [DOI] [PubMed] [Google Scholar]
- Roth M. M. Carotenoid pigments and photokilling by acridine orange. J Bacteriol. 1967 Jan;93(1):506–507. doi: 10.1128/jb.93.1.506-507.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Thomas S. A., Sargent M. L., Tuveson R. W. Inactivation of normal and mutant Neurospora crassa conidia by visible light and near-UV: role of 1O2, carotenoid composition and sensitizer location. Photochem Photobiol. 1981 Mar;33(3):349–354. doi: 10.1111/j.1751-1097.1981.tb05428.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Jonas R. B. Genetic control of near-UV (300-400 NM) sensitivity independent of the recA gene in strains of Escherichia coli K12. Photochem Photobiol. 1979 Dec;30(6):667–676. doi: 10.1111/j.1751-1097.1979.tb07197.x. [DOI] [PubMed] [Google Scholar]
- Vedaldi D., Dall'Acqua F., Gennaro A., Rodighiero G. Photosensitized effects of furocoumarins: the possible role of singlet oxygen. Z Naturforsch C. 1983 Sep-Oct;38(9-10):866–869. doi: 10.1515/znc-1983-9-1033. [DOI] [PubMed] [Google Scholar]


