Abstract
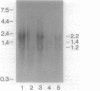
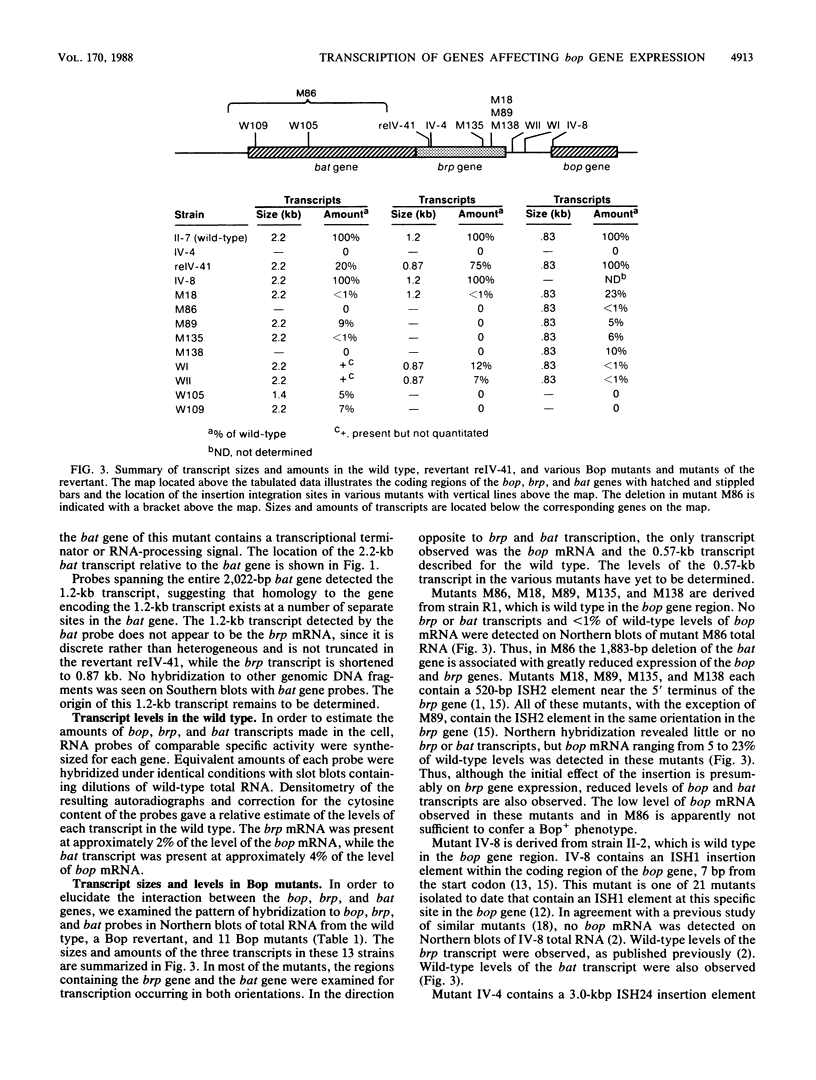
Recent studies on the regulation of the bacterio-opsin (bop) gene of the archaebacterium Halobacterium halobium suggest that the brp and putative bat genes are involved in bop gene expression or purple membrane assembly. These two genes are located 526 and 1,602 base pairs, respectively, upstream of the bop gene and are both transcribed in the opposite orientation to the bop gene. Transcription of the bop, brp, and putative bat genes was characterized in the wild type, 11 Bop mutants, and a Bop revertant by using a series of RNA probes. Quantitation of the relative mRNA levels for these three genes in the wild type revealed that the brp and bat transcripts are present at approximately 2 and 4%, respectively, of bop mRNA levels under the growth conditions used. Northern (RNA) blot analysis of Bop mutants indicated that insertions in the brp gene affect expression of the putative bat gene. In addition, deletion of most of the bat gene resulted in virtually undetectable levels of bop and brp mRNAs. These and other results lead us to propose that (i) brp gene expression can affect bat gene expression and (ii) the putative bat gene is involved in activating bop and brp gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betlach M., Friedman J., Boyer H. W., Pfeifer F. Characterization of a halobacterial gene affecting bacterio-opsin gene expression. Nucleic Acids Res. 1984 Oct 25;12(20):7949–7959. doi: 10.1093/nar/12.20.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DasSarma S., RajBhandary U. L., Khorana H. G. High-frequency spontaneous mutation in the bacterio-opsin gene in Halobacterium halobium is mediated by transposable elements. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2201–2205. doi: 10.1073/pnas.80.8.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassarma S., Rajbhandary U. L., Khorana H. G. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc Natl Acad Sci U S A. 1984 Jan;81(1):125–129. doi: 10.1073/pnas.81.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., McCoy J., Simsek M., Majumdar A., Chang S. H., Rajbhandary U. L., Khorana H. G. The bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6744–6748. doi: 10.1073/pnas.78.11.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Pfeifer F., Boyer H., Betlach M. Characterization of a second gene involved in bacterio-opsin gene expression in a halophilic archaebacterium. J Bacteriol. 1988 Oct;170(10):4903–4909. doi: 10.1128/jb.170.10.4903-4909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Phototrophic growth of halobacteria and its use for isolation of photosynthetically-deficient mutants. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):137–150. doi: 10.1016/s0769-2609(83)80101-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F. A., Boyer H. W., Betlach M. C. Restoration of bacterioopsin gene expression in a revertant of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):414–420. doi: 10.1128/jb.164.1.414-420.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Friedman J., Boyer H. W., Betlach M. Characterization of insertions affecting the expression of the bacterio-opsin gene in Halobacterium halobium. Nucleic Acids Res. 1984 Mar 12;12(5):2489–2497. doi: 10.1093/nar/12.5.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Characterization of plasmids in halobacteria. J Bacteriol. 1981 Jan;145(1):369–374. doi: 10.1128/jb.145.1.369-374.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer F., Weidinger G., Goebel W. Genetic variability in Halobacterium halobium. J Bacteriol. 1981 Jan;145(1):375–381. doi: 10.1128/jb.145.1.375-381.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek M., DasSarma S., RajBhandary U. L., Khorana H. G. A transposable element from Halobacterium halobium which inactivates the bacteriorhodopsin gene. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7268–7272. doi: 10.1073/pnas.79.23.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Biogenesis of purple membrane: regulation of bacterio-opsin synthesis. FEBS Lett. 1976 Oct 15;69(1):149–152. doi: 10.1016/0014-5793(76)80673-4. [DOI] [PubMed] [Google Scholar]
- Sumper M., Herrmann G. Biosynthesis of purple membrane: control of retinal synthesis by bacterio-opsin. FEBS Lett. 1976 Dec 1;71(2):333–336. doi: 10.1016/0014-5793(76)80964-7. [DOI] [PubMed] [Google Scholar]