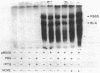
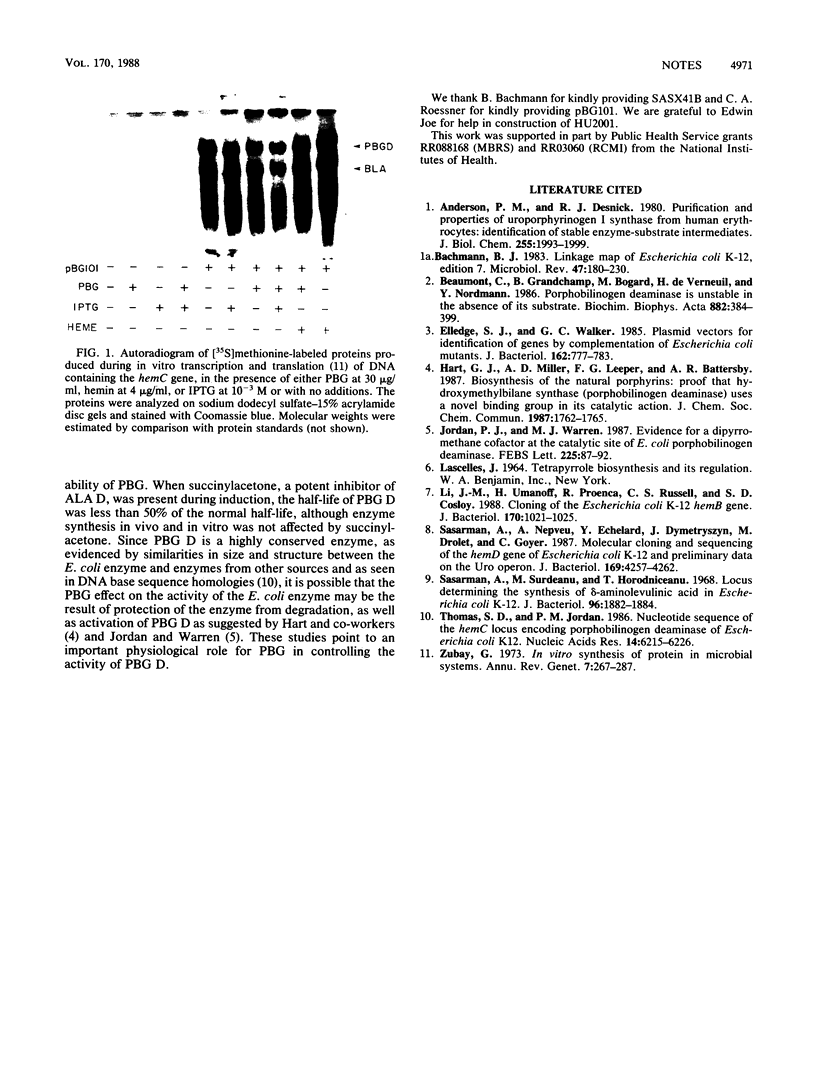
Abstract
A hemin-permeable hemB mutant had no 5-aminolevulinate dehydratase (ALA D) and extremely low porphobilinogen deaminase (PBG D) activity. When the structural gene for hemB was introduced into this strain on a single-copy plasmid, both activities were observed. When the mutant was grown on PBG, normal PBG D activity was observed. Moreover, a hemA mutant had little or no PBG D activity unless it was grown on ALA or PBG. Neither hemin nor PBG affected the level of PBG D protein produced from in vitro transcription and translation of a plasmid harboring the hemC gene as an insert. We conclude that, in Escherichia coli, PBG availability controls the activity of PBG D at some posttranscriptional level.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C., Grandchamp B., Bogard M., de Verneuil H., Nordmann Y. Porphobilinogen deaminase is unstable in the absence of its substrate. Biochim Biophys Acta. 1986 Jul 16;882(3):384–388. doi: 10.1016/0304-4165(86)90262-x. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Phasmid vectors for identification of genes by complementation of Escherichia coli mutants. J Bacteriol. 1985 May;162(2):777–783. doi: 10.1128/jb.162.2.777-783.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Warren M. J. Evidence for a dipyrromethane cofactor at the catalytic site of E. coli porphobilinogen deaminase. FEBS Lett. 1987 Dec 10;225(1-2):87–92. doi: 10.1016/0014-5793(87)81136-5. [DOI] [PubMed] [Google Scholar]
- Li J. M., Umanoff H., Proenca R., Russell C. S., Cosloy S. D. Cloning of the Escherichia coli K-12 hemB gene. J Bacteriol. 1988 Feb;170(2):1021–1025. doi: 10.1128/jb.170.2.1021-1025.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]