Abstract
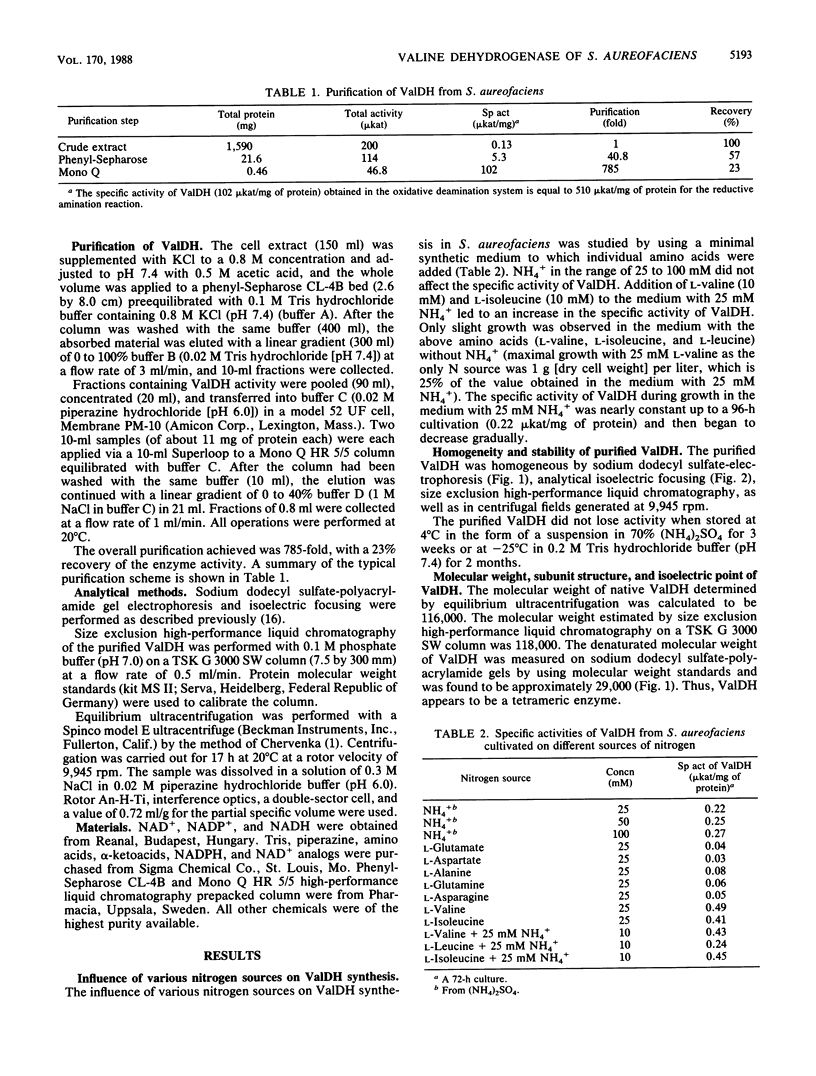
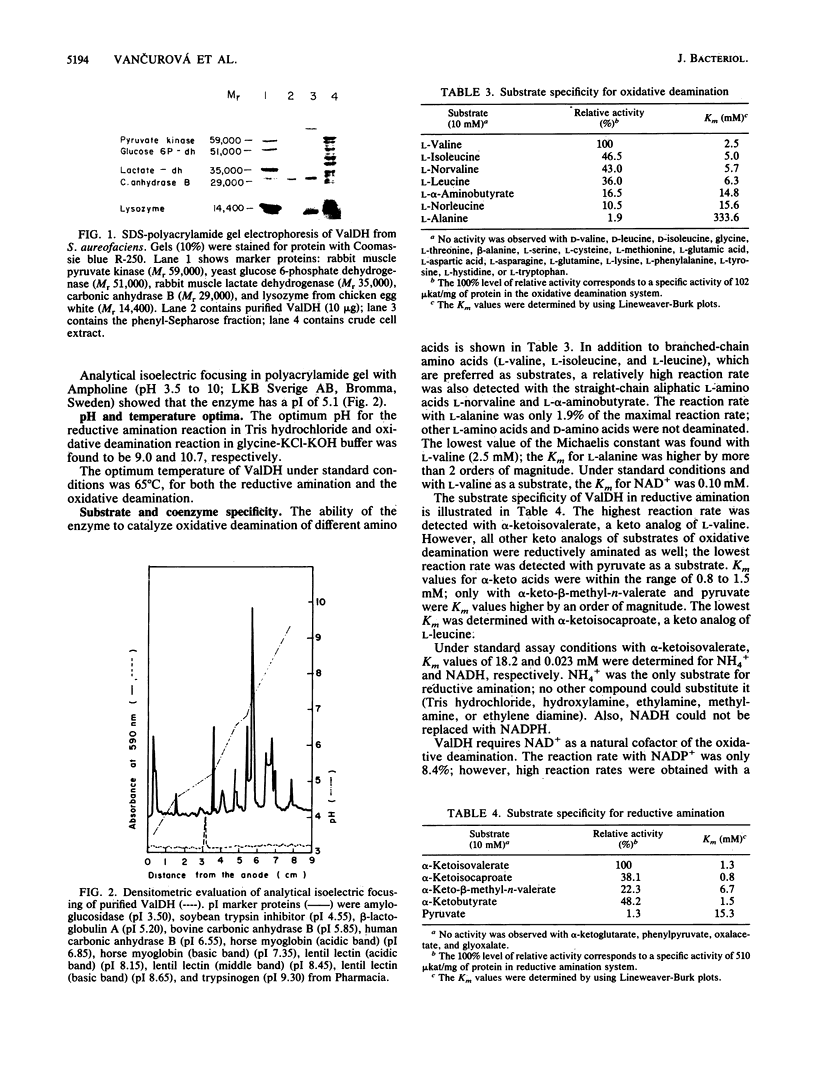
Valine dehydrogenase was purified to homogeneity from the crude extracts of Streptomyces aureofaciens. The molecular weight of the native enzyme was 116,000 by equilibrium ultracentrifugation and 118,000 by size exclusion high-performance liquid chromatography. The enzyme was composed of four subunits with molecular weights of 29,000. The isoelectric point was 5.1. The enzyme required NAD+ as a cofactor, which could not be replaced by NADP+. Sulfhydryl reagents inhibited the enzyme activity. The pH optimum was 10.7 for oxidative deamination of L-valine and 9.0 for reductive amination of alpha-ketoisovalerate. The Michaelis constants were 2.5 mM for L-valine and 0.10 mM for NAD+. For reductive amination the Km values were 1.25 mM for alpha-ketoisovalerate, 0.023 mM for NADH, and 18.2 mM for NH4Cl.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nitta Y., Yasuda Y., Tochikubo K., Hachisuka Y. L-amino acid dehydrogenases in Bacillus subtilis spores. J Bacteriol. 1974 Feb;117(2):588–592. doi: 10.1128/jb.117.2.588-592.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier N., Poralla K. Some physiological functions of the L-leucine dehydrogenase in Bacillus subtilis. Arch Microbiol. 1976 Aug;109(1-2):59–63. doi: 10.1007/BF00425113. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Misono H., Soda K. Properties of crystalline leucine dehydrogenase from Bacillus sphaericus. J Biol Chem. 1978 Aug 25;253(16):5719–5725. [PubMed] [Google Scholar]
- Omura S., Tanaka Y., Mamada H., Masuma R. Ammonium ion suppresses the biosynthesis of tylosin aglycone by interference with valine catabolism in Streptomyces fradiae. J Antibiot (Tokyo) 1983 Dec;36(12):1792–1794. doi: 10.7164/antibiotics.36.1792. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., ZINK M. W. L-Leucine dehydrogenase of Bacillus cereus. Arch Biochem Biophys. 1961 Sep;94:430–435. doi: 10.1016/0003-9861(61)90070-4. [DOI] [PubMed] [Google Scholar]
- Vancurová I., Volc J., Flieger M., Neuzil J., Novotná J., Vlach J., Behal V. Isolation of pure anhydrotetracycline oxygenase from Streptomyces aureofaciens. Biochem J. 1988 Jul 1;253(1):263–267. doi: 10.1042/bj2530263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J. R., Granum P. E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem. 1980 Nov 15;109(1):156–159. doi: 10.1016/0003-2697(80)90024-x. [DOI] [PubMed] [Google Scholar]



