Abstract
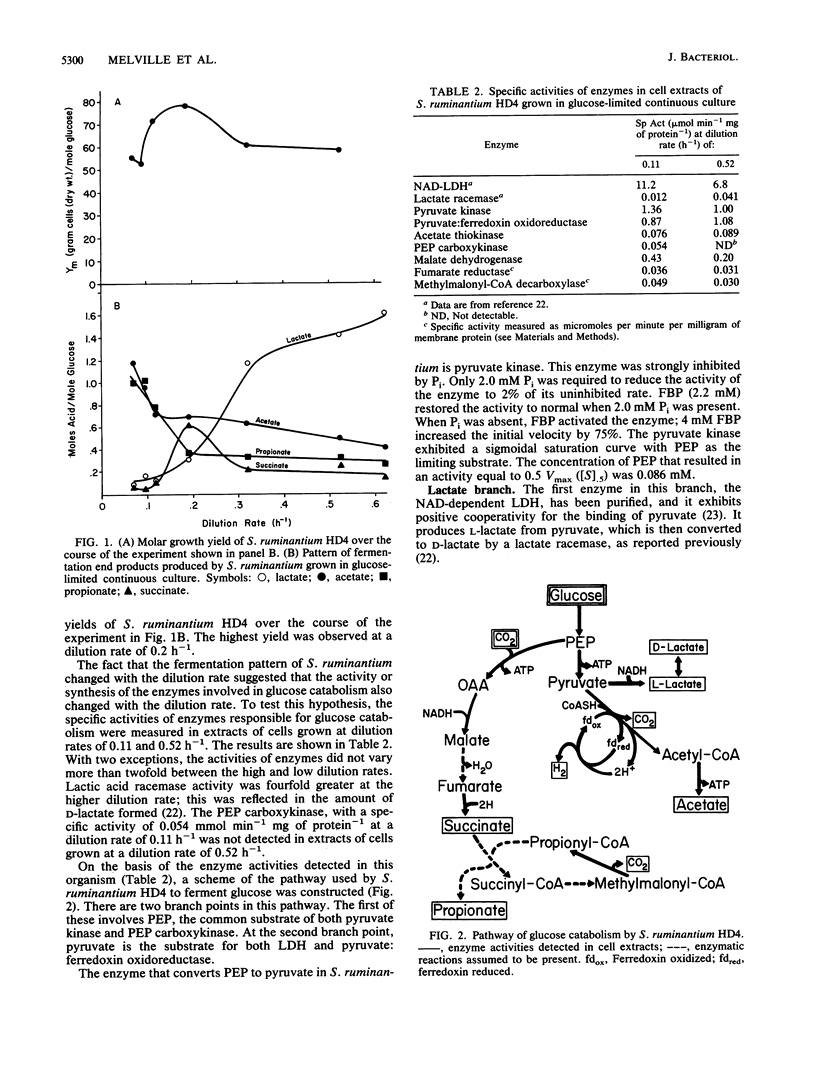
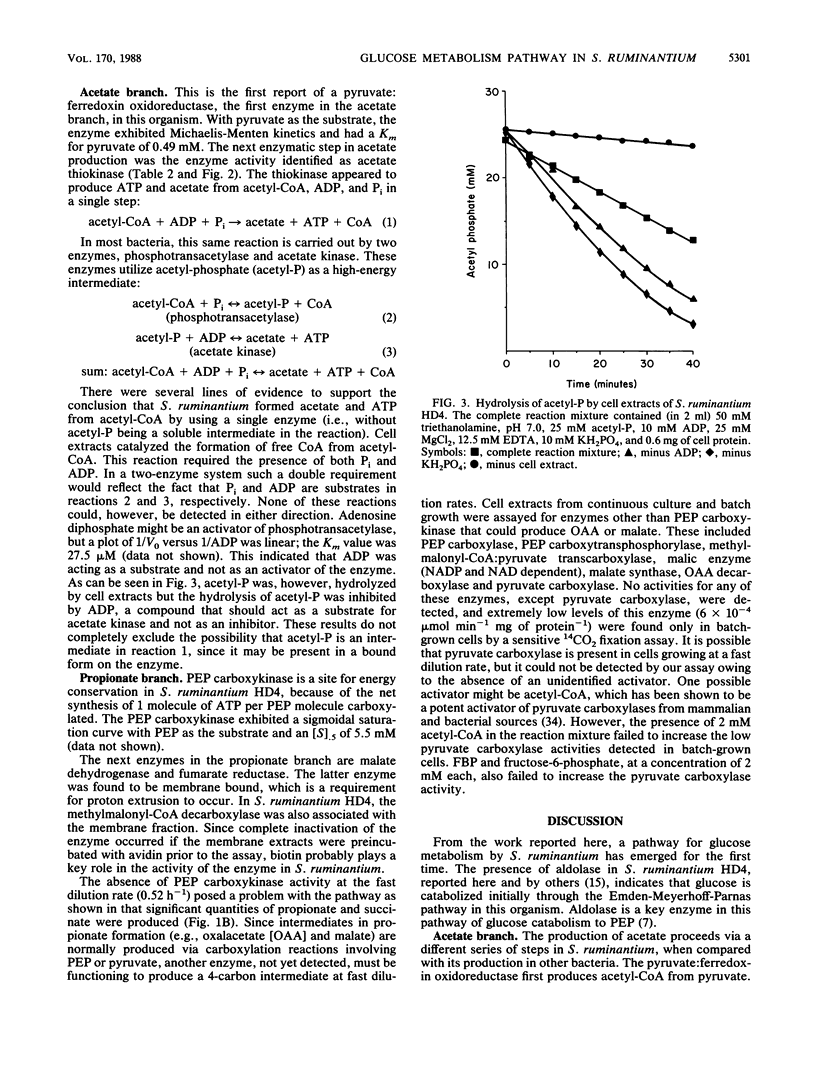
On the basis of enzyme activities detected in extracts of Selenomonas ruminantium HD4 grown in glucose-limited continuous culture, at a slow (0.11 h-1) and a fast (0.52 h-1) dilution rate, a pathway of glucose catabolism to lactate, acetate, succinate, and propionate was constructed. Glucose was catabolized to phosphoenol pyruvate (PEP) via the Emden-Meyerhoff-Parnas pathway. PEP was converted to either pyruvate (via pyruvate kinase) or oxalacetate (via PEP carboxykinase). Pyruvate was reduced to L-lactate via a NAD-dependent lactate dehydrogenase or oxidatively decarboxylated to acetyl coenzyme A (acetyl-CoA) and CO2 by pyruvate:ferredoxin oxidoreductase. Acetyl-CoA was apparently converted in a single enzymatic step to acetate and CoA, with concomitant formation of 1 molecule of ATP; since acetyl-phosphate was not an intermediate, the enzyme catalyzing this reaction was identified as acetate thiokinase. Oxalacetate was converted to succinate via the activities of malate dehydrogenase, fumarase and a membrane-bound fumarate reductase. Succinate was then excreted or decarboxylated to propionate via a membrane-bound methylmalonyl-CoA decarboxylase. Pyruvate kinase was inhibited by Pi and activated by fructose 1,6-bisphosphate. PEP carboxykinase activity was found to be 0.054 mumol min-1 mg of protein-1 at a dilution rate of 0.11 h-1 but could not be detected in extracts of cells grown at a dilution rate of 0.52 h-1. Several potential sites for energy conservation exist in S. ruminantium HD4, including pyruvate kinase, acetate thiokinase, PEP carboxykinase, fumarate reductase, and methylmalonyl-CoA decarboxylase. Possession of these five sites for energy conservation may explain the high yields reported here (56 to 78 mg of cells [dry weight] mol of glucose-1) for S. ruminantium HD4 grown in glucose-limited continuous culture.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P. The characteristics of strains of Selenomonas isolated from bovine rumen contents. J Bacteriol. 1956 Aug;72(2):162–167. doi: 10.1128/jb.72.2.162-167.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L. B., Thomas T. D. Pyruvate kinase of Streptococcus lactis. J Bacteriol. 1974 Oct;120(1):52–58. doi: 10.1128/jb.120.1.52-58.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENNIS D., KAPLAN N. O. LACTIC ACID RACEMIZATION IN CLOSTRIDIUM BUTYLICUM. Biochem Z. 1963;338:485–495. [PubMed] [Google Scholar]
- Dimroth P. Biotin-dependent decarboxylases as energy transducing systems. Ann N Y Acad Sci. 1985;447:72–85. doi: 10.1111/j.1749-6632.1985.tb18426.x. [DOI] [PubMed] [Google Scholar]
- Dimroth P. Characterization of a membrane-bound biotin-containing enzyme: oxaloacetate decarboxylase from Klebsiella aerogenes. Eur J Biochem. 1981 Apr;115(2):353–358. doi: 10.1111/j.1432-1033.1981.tb05245.x. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Henderson C. The influence of extracellular hydrogen on the metabolism of Bacteroides ruminicola, Anaerovibrio lipolytica and Selenomonas ruminantium. J Gen Microbiol. 1980 Aug;119(2):485–491. doi: 10.1099/00221287-119-2-485. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Dimroth P. Purification and characterization of a new sodium-transport decarboxylase. Methylmalonyl-CoA decarboxylase from Veillonella alcalescens. Eur J Biochem. 1983 May 16;132(3):579–587. doi: 10.1111/j.1432-1033.1983.tb07403.x. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Schink B., Dimroth P. Life by a new decarboxylation-dependent energy conservation mechanism with Na as coupling ion. EMBO J. 1984 Aug;3(8):1665–1670. doi: 10.1002/j.1460-2075.1984.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P. N., Wallace R. J. Microbial ecology and activities in the rumen: Part II. Crit Rev Microbiol. 1982 May;9(4):253–320. doi: 10.3109/10408418209104492. [DOI] [PubMed] [Google Scholar]
- Isaacson H. R., Hinds F. C., Bryant M. P., Owens F. N. Efficiency of energy utilization by mixed rumen bacteria in continuous culture. J Dairy Sci. 1975 Nov;58(11):1645–1659. doi: 10.3168/jds.S0022-0302(75)84763-1. [DOI] [PubMed] [Google Scholar]
- Joyner A. E., Jr, Baldwin R. L. Enzymatic studies of pure cultures of rumen microorganisms. J Bacteriol. 1966 Nov;92(5):1321–1330. doi: 10.1128/jb.92.5.1321-1330.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A., Dadák V., Klingenberg M., Diemer F. On the role of quinones in bacterial electron transport. Differential roles of ubiquinone and menaquinone in Proteus rettgeri. Eur J Biochem. 1971 Aug 16;21(3):322–333. doi: 10.1111/j.1432-1033.1971.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville S. B., Michel T. A., Macy J. M. Regulation of carbon flow in Selenomonas ruminantium grown in glucose-limited continuous culture. J Bacteriol. 1988 Nov;170(11):5305–5311. doi: 10.1128/jb.170.11.5305-5311.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Biochemistry of protozoan microbodies: peroxisomes, alpha-glycerophosphate oxidase bodies, hydrogenosomes. Annu Rev Microbiol. 1975;29:467–483. doi: 10.1146/annurev.mi.29.100175.002343. [DOI] [PubMed] [Google Scholar]
- Otto R., Sonnenberg A. S., Veldkamp H., Konings W. N. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5502–5506. doi: 10.1073/pnas.77.9.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Elsden S. R. Mechanism of propionate formation by Selenomonas ruminantium, a rumen micro-organism. J Gen Microbiol. 1970 Apr;61(1):1–7. doi: 10.1099/00221287-61-1-1. [DOI] [PubMed] [Google Scholar]
- Relationship of lactate dehydrogenase specificity and growth rate to lactate metabolism by Selenomonas ruminantium. Appl Microbiol. 1975 Dec;30(6):916–921. doi: 10.1128/am.30.6.916-921.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenkamp R., Reipen G., Hollenberg C. P. Mediation, by Saccharomyces cerevisiae translocation signals, of beta-lactamase transport through the Escherichia coli inner membrane and sensitive method for detection of signal sequences. J Bacteriol. 1986 Oct;168(1):467–469. doi: 10.1128/jb.168.1.467-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of substrate affinities among several rumen bacteria: a possible determinant of rumen bacterial competition. Appl Environ Microbiol. 1979 Mar;37(3):531–536. doi: 10.1128/aem.37.3.531-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanwal B. D. Regulatory characteristics of the diphosphopyridine nucleotide-specific malic enzyme of Escherichia coli. J Biol Chem. 1970 Mar 10;245(5):1212–1216. [PubMed] [Google Scholar]
- Sanwal B. D., Smando R. Malic enzyme of Escherichia coli. Diversity of the effectors controlling enzyme activity. J Biol Chem. 1969 Apr 10;244(7):1817–1823. [PubMed] [Google Scholar]
- Steinmüller W., Bock E. Enzymatic studies on autotrophically, mixotrophically and heterotrophically grown Nitrobacter agilis with special reference to nitrite oxidase. Arch Microbiol. 1977 Oct 24;115(1):51–54. doi: 10.1007/BF00427844. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. A reevaluation of the method for the determination of ATP production by measuring molar growth yields. Biochim Biophys Acta. 1973 Feb 12;301(1):53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- Ten Brink B., Konings W. N. Generation of an electrochemical proton gradient by lactate efflux in membrane vesicles of Escherichia coli. Eur J Biochem. 1980 Oct;111(1):59–66. doi: 10.1111/j.1432-1033.1980.tb06074.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. Control of lactate production by Selenomonas ruminantium: homotropic activation of lactate dehydrogenase by pyruvate. J Gen Microbiol. 1978 Jul;107(1):45–52. doi: 10.1099/00221287-107-1-45. [DOI] [PubMed] [Google Scholar]


