Abstract
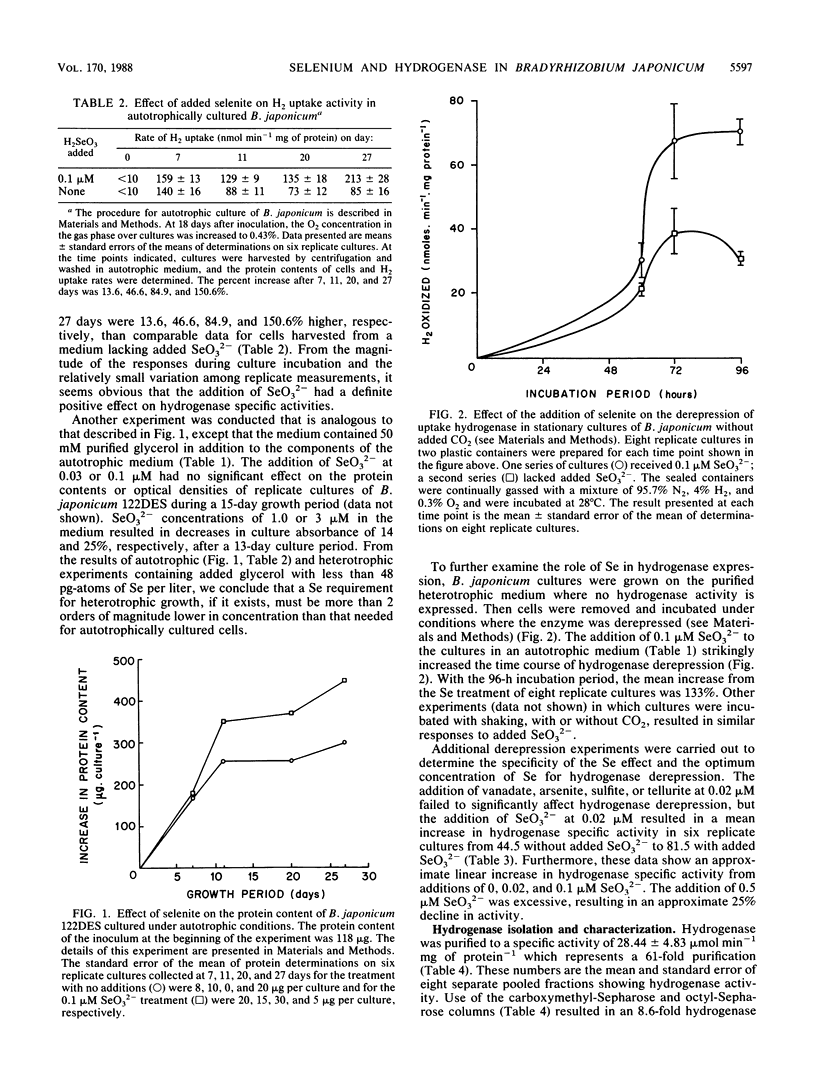
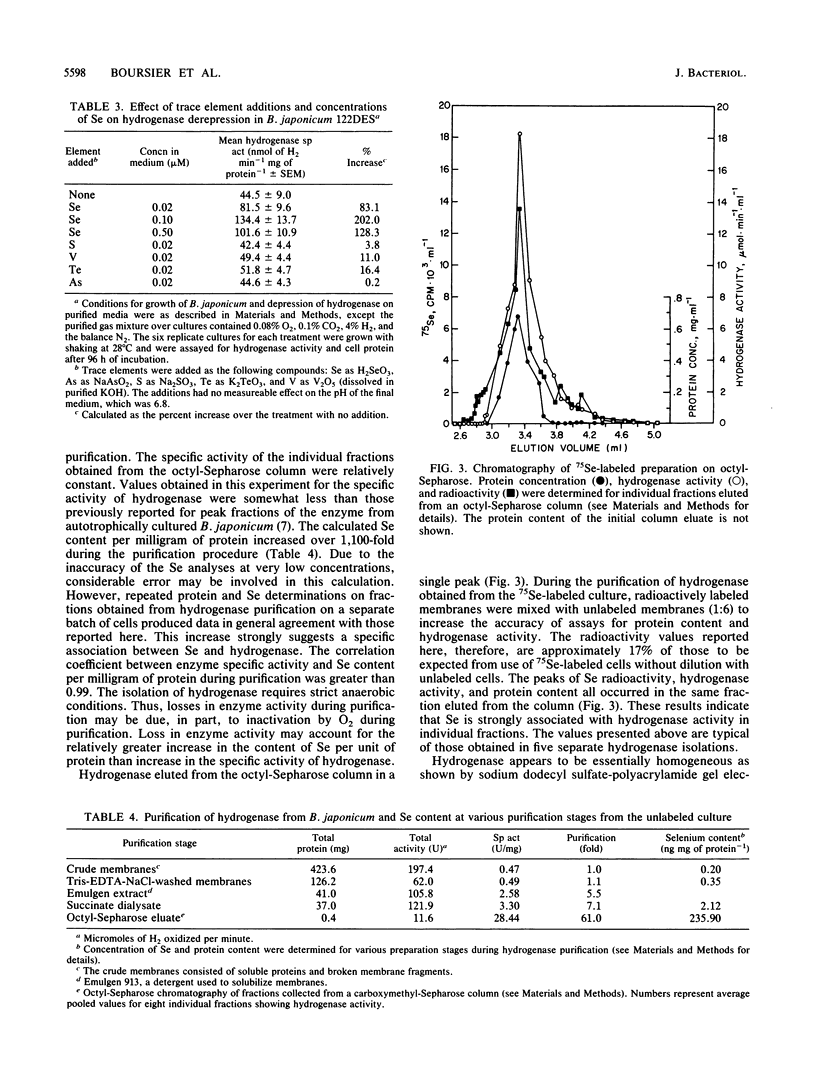
We have investigated the effect of added selenite on autotrophic growth and the time course of hydrogen oxidation derepression in Bradyrhizobium japonicum 122DES cultured in a medium purified to remove selenium compounds. In addition, hydrogenase was purified to near homogeneity and examined for the specific incorporation of Se into the enzyme. The addition of Se at 0.1 microM significantly increased total cell protein and hydrogenase specific activity of harvested cells. Also, the addition of SeO3(2-) enhanced the time course of hydrogenase derepression by 133%, whereas VO3, AsO2(2-), SO2(2-), and TeO3(2-) failed to substantially affect hydrogenase derepression. During the final chromatographic purification of hydrogenase, a striking coincidence in peaks of protein content, Se radioactivity, and hydrogenase activity of fractions was obtained. The total Se content expressed per milligram of protein increased manyfold during the purification procedure. The mean Se content of the purified hydrogenase was 0.56 +/- 0.13 mol of Se per mol of enzyme. These results indicate that Se is an important element in the H2 metabolism of B. japonicum and that hydrogenase from B. japonicum is a seleno protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broyer T. C., Lee D. C., Asher C. J. Selenium nutrition of green plants. Effect of selenite supply on growth and selenium content of alfalfa and subterranean clover. Plant Physiol. 1966 Nov;41(9):1425–1428. doi: 10.1104/pp.41.9.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone J. E., del Río R. M., Stadtman T. C. Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J Biol Chem. 1977 Aug 10;252(15):5337–5344. [PubMed] [Google Scholar]
- Dilworth G. L. Properties of the selenium-containing moiety of nicotinic acid hydroxylase from Clostridium barkeri. Arch Biochem Biophys. 1982 Nov;219(1):30–38. doi: 10.1016/0003-9861(82)90130-8. [DOI] [PubMed] [Google Scholar]
- Harker A. R., Xu L. S., Hanus F. J., Evans H. J. Some properties of the nickel-containing hydrogenase of chemolithotrophically grown Rhizobium japonicum. J Bacteriol. 1984 Sep;159(3):850–856. doi: 10.1128/jb.159.3.850-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon N. K., Peck H. D., Jr, Gall J. L., Przybyla A. E. Cloning and sequencing of the genes encoding the large and small subunits of the periplasmic (NiFeSe) hydrogenase of Desulfovibrio baculatus. J Bacteriol. 1987 Dec;169(12):5401–5407. doi: 10.1128/jb.169.12.5401-5407.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON E. L., MILSTREY R., STOKSTAD E. L. Effect of selenium in preventing exudative diathesis in chicks. Proc Soc Exp Biol Med. 1957 Aug-Sep;95(4):617–620. doi: 10.3181/00379727-95-23307. [DOI] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Mayer R. Dense autotrophic cultures of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):592–597. doi: 10.1128/aem.32.4.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Repaske A. C. Quantitative requirements for exponential growth of Alcaligenes eutrophus. Appl Environ Microbiol. 1976 Oct;32(4):585–591. doi: 10.1128/aem.32.4.585-591.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotruck J. T., Pope A. L., Ganther H. E., Swanson A. B., Hafeman D. G., Hoekstra W. G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973 Feb 9;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- SCHUBERT J. R., MUTH O. H., OLDFIELD J. E., REMMERT L. F. Experimental results with selenium in white muscle disease of lambs and calves. Fed Proc. 1961 Jul;20:689–694. [PubMed] [Google Scholar]
- Schink B., Schlegel H. G. The membrane-bound hydrogenase of Alcaligenes eutrophus. I. Solubilization, purification, and biochemical properties. Biochim Biophys Acta. 1979 Apr 12;567(2):315–324. doi: 10.1016/0005-2744(79)90117-7. [DOI] [PubMed] [Google Scholar]
- Seefeldt L. C., Arp D. J. Purification to homogeneity of Azotobacter vinelandii hydrogenase: a nickel and iron containing alpha beta dimer. Biochimie. 1986 Jan;68(1):25–34. doi: 10.1016/s0300-9084(86)81064-1. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium-dependent enzymes. Annu Rev Biochem. 1980;49:93–110. doi: 10.1146/annurev.bi.49.070180.000521. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Specific occurrence of selenium in enzymes and amino acid tRNAs. FASEB J. 1987 Nov;1(5):375–379. doi: 10.1096/fasebj.1.5.2445614. [DOI] [PubMed] [Google Scholar]
- Teixeira M., Moura I., Fauque G., Czechowski M., Berlier Y., Lespinat P. A., Le Gall J., Xavier A. V., Moura J. J. Redox properties and activity studies on a nickel-containing hydrogenase isolated from a halophilic sulfate reducer Desulfovibrio salexigens. Biochimie. 1986 Jan;68(1):75–84. doi: 10.1016/s0300-9084(86)81071-9. [DOI] [PubMed] [Google Scholar]
- Wagner R., Andreesen J. R. Selenium requirement for active xanthine dehydrogenase from Clostridium acidiurici and Clostridium cylindrosporum. Arch Microbiol. 1979 Jun;121(3):255–260. doi: 10.1007/BF00425064. [DOI] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]



