Abstract
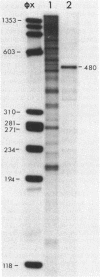
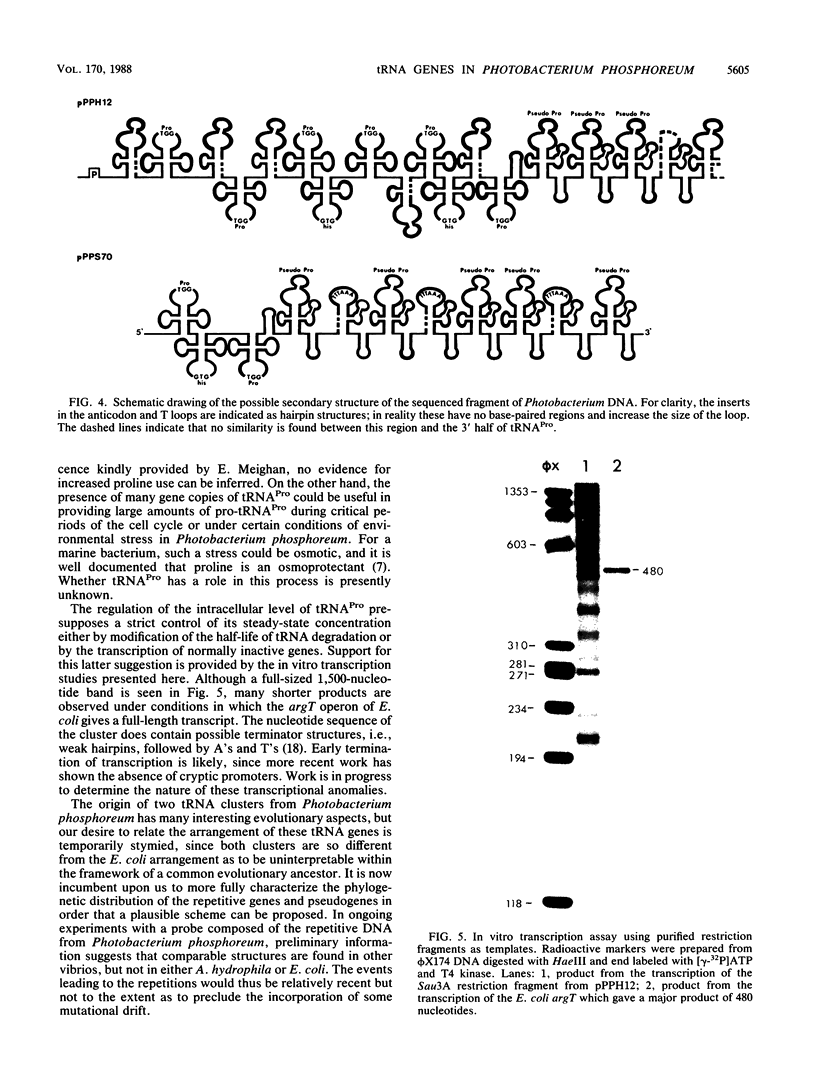
A DNA fragment comprising the four tRNA gene sequences of the Escherichia coli argT locus hybridized with two Sau3A-generated DNA fragments from the vibrio Photobacterium phosphoreum (ATCC 11040). Detailed sequence analysis of the longer fragment shows the following gene organization: 5'-promoter-tRNA(Pro)-tRNAPro-tRNA(Pro)-tRNA(His)-tRNA(Pro)-tRNA(Pro)- tRNA(His)-tRNA(Pro)-five pseudogenes derived from the upstream tRNAPro interspersed by putative Rho-independent terminators. This sequence demonstrates the presence of highly repetitive, tandem tRNA genes in a bacterial genome. Furthermore, a stretch of 304 nucleotides from this cluster was found virtually unchanged in the other (shorter) fragment which was previously sequenced. The two clusters together contain eight tRNA(Pro) pseudogenes and eight fully intact tRNA(Pro) genes, an unusually high number for a single eubacterial isoacceptor tRNA. These results show that the organization of some tRNA operons is highly variable in eubacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Bossi L., Smith D. M. Conformational change in the DNA associated with an unusual promoter mutation in a tRNA operon of Salmonella. Cell. 1984 Dec;39(3 Pt 2):643–652. doi: 10.1016/0092-8674(84)90471-9. [DOI] [PubMed] [Google Scholar]
- Bossi L. The hisR locus of Salmonella: nucleotide sequence and expression. Mol Gen Genet. 1983;192(1-2):163–170. doi: 10.1007/BF00327662. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cantatore P., Gadaleta M. N., Roberti M., Saccone C., Wilson A. C. Duplication and remoulding of tRNA genes during the evolutionary rearrangement of mitochondrial genomes. 1987 Oct 29-Nov 4Nature. 329(6142):853–855. doi: 10.1038/329853a0. [DOI] [PubMed] [Google Scholar]
- Csonka L. N. Regulation of cytoplasmic proline levels in Salmonella typhimurium: effect of osmotic stress on synthesis, degradation, and cellular retention of proline. J Bacteriol. 1988 May;170(5):2374–2378. doi: 10.1128/jb.170.5.2374-2378.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. M., Klee H. J., Zagorski J., Fournier M. J. Structure of an Escherichia coli tRNA operon containing linked genes for arginine, histidine, leucine, and proline tRNAs. J Bacteriol. 1984 Jun;158(3):934–942. doi: 10.1128/jb.158.3.934-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol. 1985 Jan;2(1):13–34. doi: 10.1093/oxfordjournals.molbev.a040335. [DOI] [PubMed] [Google Scholar]
- MacDonell M. T., Swartz D. G., Ortiz-Conde B. A., Last G. A., Colwell R. R. Ribosomal RNA phylogenies for the vibrio-enteric group of eubacteria. Microbiol Sci. 1986 Jun;3(6):172-5, 178. [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Nicoghosian K., Bigras M., Sankoff D., Cedergren R. Archetypical features in tRNA families. J Mol Evol. 1987;26(4):341–346. doi: 10.1007/BF02101153. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Palmer J. D. Comparative organization of chloroplast genomes. Annu Rev Genet. 1985;19:325–354. doi: 10.1146/annurev.ge.19.120185.001545. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Reed R. E., Altman S. Repeated sequences and open reading frames in the 3' flanking region of the gene for the RNA subunit of Escherichia coli ribonuclease P. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5359–5363. doi: 10.1073/pnas.80.17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. A Spiroplasma tRNA gene cluster. Isr J Med Sci. 1984 Sep;20(9):768–772. [PubMed] [Google Scholar]
- Rubnitz J., Subramani S. Extrachromosomal and chromosomal gene conversion in mammalian cells. Mol Cell Biol. 1986 May;6(5):1608–1614. doi: 10.1128/mcb.6.5.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Mechanisms of DNA reorganization in bacteria. Int Rev Cytol. 1985;93:25–56. doi: 10.1016/s0074-7696(08)61371-6. [DOI] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]