Abstract
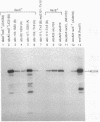
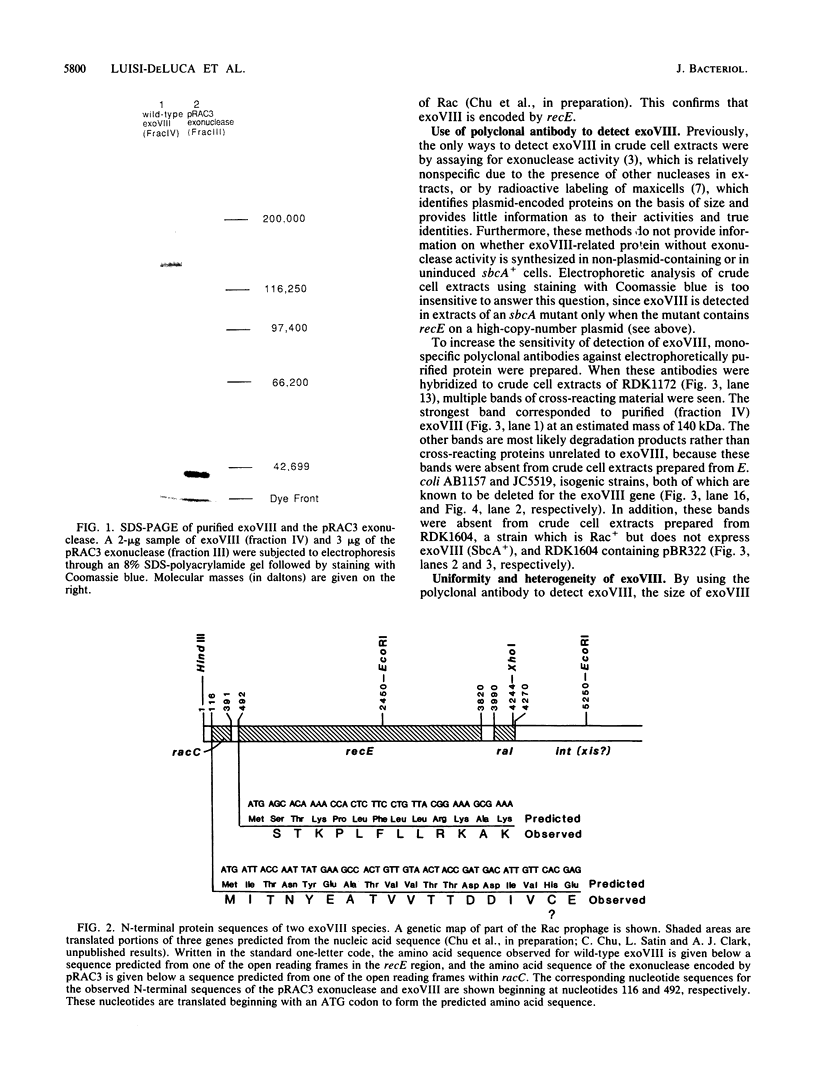
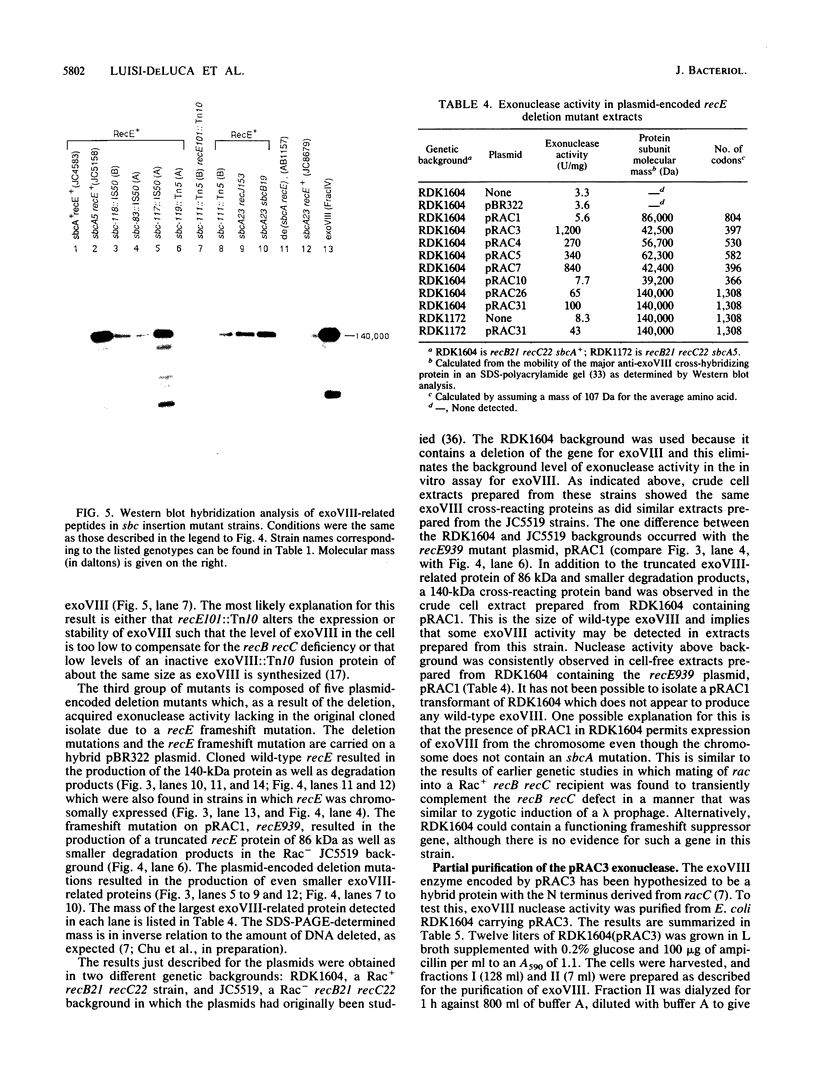
Exonuclease VIII (exoVIII) of Escherichia coli has been purified from a strain carrying a plasmid-encoded recE gene by using a new procedure. This procedure yielded 30 times more protein per gram of cells, and the protein had a twofold higher specific activity than the enzyme purified by the previously published procedure (J. W. Joseph and R. Kolodner, J. Biol. Chem. 258:10411-10417, 1983). The sequence of the 12 N-terminal amino acids was also obtained and found to correspond to one of the open reading frames predicted from the nucleic acid sequence of the recE region of Rac (C. Chu, A. Templin, and A. J. Clark, manuscript in preparation). Polyclonal antibodies directed against purified exoVIII were also prepared. Cell-free extracts prepared from strains containing a wide range of chromosomal- or plasmid-encoded point, insertion, and deletion mutations which result in expression of exoVIII were examined by Western blot (immunoblot) analysis. This analysis showed that two point sbcA mutations (sbcA5 and sbcA23) and the sbc insertion mutations led to the synthesis of the 140-kilodalton (kDa) polypeptide of wild-type exoVIII. Plasmid-encoded partial deletion mutations of recE reduced the size of the cross-reacting protein(s) in direct proportion to the size of the deletion, even though exonuclease activity was still present. The analysis suggests that 39 kDa of the 140-kDa exoVIII subunit is all that is essential for exonuclease activity. One of the truncated but functional exonucleases (the pRAC3 exonuclease) has been purified and confirmed to be a 41-kDa polypeptide. The first 18 amino acids from the N terminus of the 41-kDa pRAC3 exonuclease were sequenced and fond to correspond to one of the translational start signals predicted from the nucleotide sequence of radC (Chu et al., in preparation).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Nagaishi H., Templin A., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. II. Rec+ revertants caused by indirect suppression of rec- mutations. Proc Natl Acad Sci U S A. 1970 Sep;67(1):128–135. doi: 10.1073/pnas.67.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouché J. P., Gélugne J. P., Louarn J., Louarn J. M., Kaiser K. Relationships between the physical and genetic maps of a 470 x 10(3) base-pair region around the terminus of Escherichia coli K12 DNA replication. J Mol Biol. 1982 Jan 5;154(1):21–32. doi: 10.1016/0022-2836(82)90414-4. [DOI] [PubMed] [Google Scholar]
- Carter D. M., Radding C. M. The role of exonuclease and beta protein of phage lambda in genetic recombination. II. Substrate specificity and the mode of action of lambda exonuclease. J Biol Chem. 1971 Apr 25;246(8):2502–2512. [PubMed] [Google Scholar]
- Clark A. J., Sandler S. J., Willis D. K., Chu C. C., Blanar M. A., Lovett S. T. Genes of the RecE and RecF pathways of conjugational recombination in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1984;49:453–462. doi: 10.1101/sqb.1984.049.01.051. [DOI] [PubMed] [Google Scholar]
- Cohen A., Laban A. Plasmidic recombination in Escherichia coli K-12: the role of recF gene function. Mol Gen Genet. 1983;189(3):471–474. doi: 10.1007/BF00325911. [DOI] [PubMed] [Google Scholar]
- Evans R., Seeley N. R., Kuempel P. L. Loss of rac locus DNA in merozygotes of Escherichia coli K12. Mol Gen Genet. 1979 Oct 1;175(3):245–250. doi: 10.1007/BF00397223. [DOI] [PubMed] [Google Scholar]
- Fishel R. A., James A. A., Kolodner R. recA-independent general genetic recombination of plasmids. Nature. 1981 Nov 12;294(5837):184–186. doi: 10.1038/294184a0. [DOI] [PubMed] [Google Scholar]
- Fouts K. E., Wasie-Gilbert T., Willis D. K., Clark A. J., Barbour S. D. Genetic analysis of transposon-induced mutations of the Rac prophage in Escherichia coli K-12 which affect expression and function of recE. J Bacteriol. 1983 Nov;156(2):718–726. doi: 10.1128/jb.156.2.718-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen J. R., Karu A. E., Nagaishi H., Clark A. J. Characterization of the deoxyribonuclease determined by lambda reverse as exonuclease VIII of Escherichia coli. J Mol Biol. 1977 Jun 15;113(1):27–41. doi: 10.1016/0022-2836(77)90039-0. [DOI] [PubMed] [Google Scholar]
- Gillen J. R., Willis D. K., Clark A. J. Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):521–532. doi: 10.1128/jb.145.1.521-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Gottesman M. E., Gottesman S., Gellert M. Characterization of bacteriophage lambda reverse as an Escherichia coli phage carrying a unique set of host-derived recombination functions. J Mol Biol. 1974 Sep 15;88(2):471–487. doi: 10.1016/0022-2836(74)90496-3. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Halling S. M., Simons R. W., Way J. C., Walsh R. B., Kleckner N. DNA sequence organization of IS10-right of Tn10 and comparison with IS10-left. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2608–2612. doi: 10.1073/pnas.79.8.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- James A. A., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982 Sep 25;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Joseph J. W., Kolodner R. Exonuclease VIII of Escherichia coli. I. Purification and physical properties. J Biol Chem. 1983 Sep 10;258(17):10411–10417. [PubMed] [Google Scholar]
- Joseph J. W., Kolodner R. Exonuclease VIII of Escherichia coli. II. Mechanism of action. J Biol Chem. 1983 Sep 10;258(17):10418–10424. [PubMed] [Google Scholar]
- Kaiser K., Murray N. E. On the nature of sbcA mutations in E. coli K 12. Mol Gen Genet. 1980;179(3):555–563. doi: 10.1007/BF00271745. [DOI] [PubMed] [Google Scholar]
- Kaiser K., Murray N. E. Physical characterisation of the "Rac prophage" in E. coli K12. Mol Gen Genet. 1979 Sep;175(2):159–174. doi: 10.1007/BF00425532. [DOI] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Clark A. J. Isolation of exonuclease VIII: the enzyme associated with sbcA indirect suppressor. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3593–3597. doi: 10.1073/pnas.71.9.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laban A., Cohen A. Interplasmidic and intraplasmidic recombination in Escherichia coli K-12. Mol Gen Genet. 1981;184(2):200–207. doi: 10.1007/BF00272905. [DOI] [PubMed] [Google Scholar]
- Little J. W. An exonuclease induced by bacteriophage lambda. II. Nature of the enzymatic reaction. J Biol Chem. 1967 Feb 25;242(4):679–686. [PubMed] [Google Scholar]
- Lovett S. T., Clark A. J. Genetic analysis of the recJ gene of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):190–196. doi: 10.1128/jb.157.1.190-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. Restoration by the rac locus of recombinant forming ability in recB - and recC - merozygotes of Escherichia coli K-12. Mol Gen Genet. 1973 Apr 12;122(2):119–130. doi: 10.1007/BF00435185. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington L. S., Morrison P., Kolodner R. Intramolecular recombination of linear DNA catalyzed by the Escherichia coli RecE recombination system. J Mol Biol. 1985 Dec 5;186(3):515–525. doi: 10.1016/0022-2836(85)90126-3. [DOI] [PubMed] [Google Scholar]
- Templin A., Kushner S. R., Clark A. J. Genetic analysis of mutations indirectly suppressing recB and recC mutations. Genetics. 1972 Oct;72(2):105–115. [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Willis D. K., Fouts K. E., Barbour S. D., Clark A. J. Restriction nuclease and enzymatic analysis of transposon-induced mutations of the Rac prophage which affect expression and function of recE in Escherichia coli K-12. J Bacteriol. 1983 Nov;156(2):727–736. doi: 10.1128/jb.156.2.727-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. K., Satin L. H., Clark A. J. Mutation-dependent suppression of recB21 recC22 by a region cloned from the Rac prophage of Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1166–1172. doi: 10.1128/jb.162.3.1166-1172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]