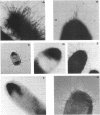
Abstract
The influence of genetic manipulation of the structural genes coding for major P-fimbrial subunits on the formation of fimbriae in Escherichia coli was studied. Deletion of two regions that code for hypervariable parts of the P fimbrillin resulted in strong reduction or total absence of fimbria production. Replacement of deleted amino acids by other amino acid residues restored the formation of fimbriae. The hypervariable regions may be important for biogenesis of fimbriae by imposing correct spacing between conserved regions of the protein. The potential for substituting amino acids in the P-fimbrial subunit opens interesting possibilities for use of fimbriae as carriers of foreign antigenic determinants. An antigenic determinant of foot-and-mouth disease virus (FMDV) was incorporated in the F11 fimbrial subunit. Hybrid fimbriae, recognized by an FMDV-specific neutralizing monoclonal antibody directed against FMDV, were formed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agterberg M., Adriaanse H., Tommassen J. Use of outer membrane protein PhoE as a carrier for the transport of a foreign antigenic determinant to the cell surface of Escherichia coli K-12. Gene. 1987;59(1):145–150. doi: 10.1016/0378-1119(87)90275-7. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Boulain J. C., Ryter A., Hofnung M. Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J. 1986 Nov;5(11):3029–3037. doi: 10.1002/j.1460-2075.1986.tb04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Korhonen T. K., Rhen M. Bacterial fimbriae as vaccines. Ann Clin Res. 1982 Dec;14(5-6):272–277. [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Svenson S., Möllby R., Cedergren B., Hultberg H., Winberg J. Structure of carbohydrate part of receptor on human uroepithelial cells for pyelonephritogenic Escherichia coli. Lancet. 1981 Sep 19;2(8247):604–606. doi: 10.1016/s0140-6736(81)92743-4. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Meloen R. H., Puyk W. C., Meijer D. J., Lankhof H., Posthumus W. P., Schaaper W. M. Antigenicity and immunogenicity of synthetic peptides of foot-and-mouth disease virus. J Gen Virol. 1987 Feb;68(Pt 2):305–314. doi: 10.1099/0022-1317-68-2-305. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F. Escherichia coli in extra-intestinal infections. J Hyg (Lond) 1985 Dec;95(3):551–575. doi: 10.1017/s0022172400060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegman N., van Die I., Leunissen J., Hoekstra W., Bergmans H. Biogenesis of F7(1) and F7(2) fimbriae of uropathogenic Escherichia coli: influence of the FsoF and FstFG proteins and localization of the Fso/FstE protein. Mol Microbiol. 1988 Jan;2(1):73–80. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Die I., Van Megen I., Zuidweg E., Hoekstra W., De Ree H., Van den Bosch H., Bergmans H. Functional relationship among the gene clusters encoding F7(1), F7(2), F9, and F11 fimbriae of human uropathogenic Escherichia coli. J Bacteriol. 1986 Jul;167(1):407–410. doi: 10.1128/jb.167.1.407-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Elo J., Tallgren L. G., Siitonen A., Mäkelä P. H., Svanborg-Edén C., Källenius G., Svenson S. B., Hultberg H., Korhonen T. Mannose-resistant haemagglutination and P antigen recognition are characteristic of Escherichia coli causing primary pyelonephritis. Lancet. 1981 Dec 19;2(8260-61):1366–1369. doi: 10.1016/s0140-6736(81)92796-3. [DOI] [PubMed] [Google Scholar]
- de Ree J. M., Schwillens P., van den Bosch J. F. Monoclonal antibodies that recognize the P fimbriae F71, F72, F9, and F11 from uropathogenic Escherichia coli. Infect Immun. 1985 Dec;50(3):900–904. doi: 10.1128/iai.50.3.900-904.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I., Hoekstra W., Bergmans H. Analysis of the primary structure of P-fimbrillins of uropathogenic Escherichia coli. Microb Pathog. 1987 Sep;3(3):149–154. doi: 10.1016/0882-4010(87)90091-x. [DOI] [PubMed] [Google Scholar]
- van der Ley P., Amesz H., Tommassen J., Lugtenberg B. Monoclonal antibodies directed against the cell-surface-exposed part of PhoE pore protein of the Escherichia coli K-12 outer membrane. Eur J Biochem. 1985 Mar 1;147(2):401–407. doi: 10.1111/j.1432-1033.1985.tb08764.x. [DOI] [PubMed] [Google Scholar]