Abstract
Pseudomembranous colitis is an uncommon complication in patients with cystic fibrosis, despite the use of multiple high‐dose antibiotic regimens and the frequency of hospital admissions. Four patients from a total of 137 patients with cystic fibrosis undergoing lung transplantation are described who developed fulminant pseudomembranous colitis. Initial presentation was variable and the mortality rate was 50% despite urgent colectomy. In one case the presenting abdominal distension was thought to be due to meconium ileus equivalent. It is concluded that Clostridium difficile colitis may be a difficult diagnosis in patients with cystic fibrosis and follows a fulminant course after lung transplantation.
Clostridium difficile colitis is rare in patients with cystic fibrosis (CF), although asymptomatic carriage has been reported to occur in up to 50%.1,2 We describe four patients who developed severe pseudomembranous colitis with variable presentation following lung transplantation with significant morbidity and mortality. The role of CT scanning in diagnosis is discussed.
Case reports
Case 1
Two months after lung transplantation, a 22‐year‐old man with CF was treated for acute allograft rejection and pulmonary infection with steroids and antibiotics. He initially received piperacillin‐tazobactam which was subsequently changed to flucloxacillin after 48 h following culture of Staphylococcus aureus from bronchoalveolar lavage fluid. His respiratory status improved over the following days but he developed abdominal pain, constipation and a palpable mass in the right iliac fossa. A clinical diagnosis of meconium ileus equivalent was made, a condition he had experienced previously. He was treated with gastrograffin and had a good bowel movement later that day. Although his symptoms initially settled, he developed further pain 3 days later with associated diarrhoea and a neutrophil leucocytosis. An abdominal radiograph showed thickened large bowel with very little luminal gas. A CT scan of the abdomen confirmed gross thickening of the entire colon consistent with severe pseudomembranous colitis (fig 1). Stool analysis confirmed C difficile toxin. Metronidazole was commenced but, because of the high risk of perforation, a subtotal colectomy was performed. Severe pseudomembranous colitis was confirmed on histopathological examination. He made an uneventful recovery.
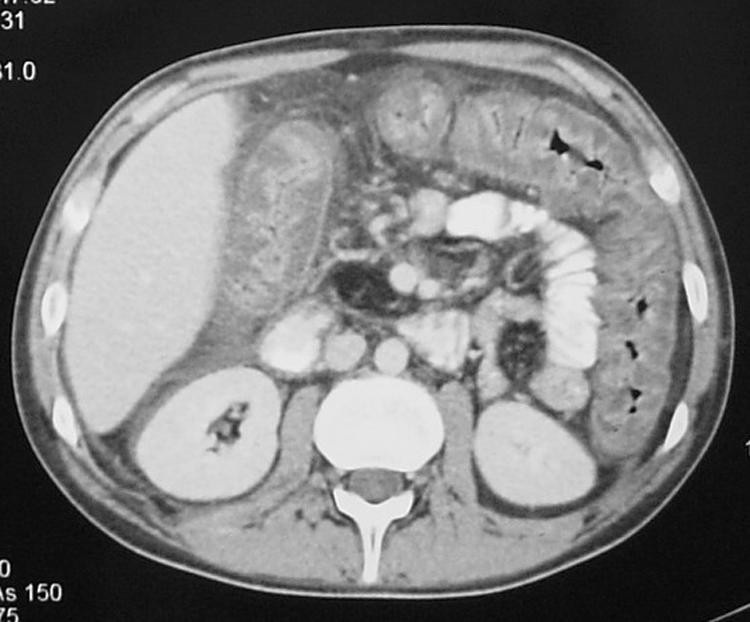
Figure 1 CT scan of the abdomen showing gross thickening of the large bowel wall and obliteration of the lumen.
Case 2
Ten years after lung transplantation for CF, a 32‐year‐old woman developed neutropenic sepsis and renal failure. She was commenced on intravenous piperacillin‐tazobactam and continuous venovenous haemofiltration. Although she had diarrhoea, the stool was negative for C difficile toxin. A CT scan of the abdomen showed thickening of the colon. Flexible sigmoidoscopy with biopsies failed to show any evidence of infection or colitis. She improved over the next 3 days but then developed profuse diarrhoea and a neutrophil leucocytosis. A presumptive diagnosis of pseudomembranous colitis was made and metronidazole commenced. Stool analysis subsequently confirmed C difficile and she gradually improved.
Case 3
A 28‐year‐old man with CF underwent lung transplantation and received aztreonam and clindamycin. His initial postoperative course was complicated by reperfusion injury requiring reintubation and renal failure. He developed abdominal distension and initially a clinical diagnosis of meconium ileus equivalent was made. Abdominal radiography showed a grossly dilated large bowel and a manual evacuation was performed. The following day he became septic and hypotensive and antimicrobial treatment was changed to piperacillin‐tazobactam and fluconazole in the light of bronchoalveolar lavage culture. A laparotomy was performed on the suspicion of perforation. No perforation was found but a caecostomy was fashioned to decompress his bowel. At 37 days after transplantation he remained dependent on a ventilator and dialysis but without further bowel problems and off antibiotics. He developed right upper abdominal pain; ultrasonography revealed gallbladder sludge but also a thickened colon suggestive of colitis. A CT scan confirmed a grossly thickened large bowel at risk of perforation despite minimal diarrhoea rectally and only soft stool from his stoma. Piperacillin‐tazobactam, metronidazole and caspofungin were commenced. Colectomy was delayed as he initially refused consent but by this time he was deteriorating rapidly with sepsis. He died 1 week following colectomy from multiple organ failure. Histological examination of the resected colon showed severe pseudomembranous colitis.
Case 4
A 38‐year‐old woman with CF underwent lung transplantation, complicated by early haemodynamic instability followed by renal failure and failed extubation. She then developed abdominal distension and diarrhoea, negative for C difficile toxin. Despite continuing episodes of diarrhoea and distension, stool toxin remained persistently negative. Bronchoalveolar lavage isolated Pseudomonas spp and she was commenced on ciprofloxacin and aztreonam. Diarrhoea, abdominal pain and distension remained problematic, and a CT scan showed thickened large bowel consistent with pseudomembranous colitis. A further stool specimen tested positive for C difficile. Metronidazole was commenced with resolution of diarrhoea and negative stool toxin after 5 days. However, her clinical condition remained critical and she died 42 days after transplantation from multiorgan failure.
Discussion
Clostridium difficile is a spore‐forming Gram positive bacillus. It is a well recognised cause of antibiotic‐associated diarrhoea.3 Asymptomatic carriage is rare in healthy adults but it is more frequent during hospitalisation, occurring in up to 25% of inpatients.2,4Clostridium difficile colitis results from a chain of events: first a disruption of bowel flora, followed by colonisation and finally toxin‐mediated mucosal damage and inflammation.4 The clinical symptoms may vary from a mild diarrhoea to fulminant colitis with severe septic shock (seen in 1–3%).5 Diagnosis is made by the detection of toxins in the stool. Pseudomembranous colitis, the most severe manifestation of the disease, presents with diarrhoea, abdominal cramping and tenderness, with systemic symptoms that may lead to haemodynamic collapse. Treatment with metronidazole or vancomycin should be promptly initiated and improvement is generally seen within 48–72 h.4 Surgical intervention is mandatory in perforation and may be required in severe cases where medical treatment is not sufficient or where improvement is not seen.
Carriage rates of C difficile in patients with CF have been reported to be up to 50%, but despite this and the numerous courses of antibiotics and hospital admissions these patients undergo, pseudomembranous colitis remains rare.1,2,6 The first case was described in 1992 with only a few subsequent reports.2,6,7 A genotype link (N1303K mutation) has been described between CF and C difficile,7 although none of our patients possessed this genotype. To date, although various hypotheses have been suggested, there is no conclusive reason to explain the low rates of C difficile in CF.7,8 Pseudomembranous colitis has been described with a high incidence in patients following lung transplantation by Dallal et al.3 Their series of 2334 patients with C difficile over an 11 year period included 78 of 250 lung transplants causing significant morbidity and mortality.
We describe four cases of severe C difficile colitis in patients with CF following lung transplantation and illustrate a number of important points in the diagnosis and management of these patients. First, the presentation was varied and—particularly in case 1—was associated initially with an absence of diarrhoea and signs far more in keeping with meconium ileus equivalent. Case 3 underwent caecostomy and, despite grossly abnormal CT appearances of his large bowel, he did not exhibit significant diarrhoea. Both of these patients went on to have a subtotal colectomy, early in the course of the illness in case 1 and delayed following failed medical treatment in case 3. The two other cases with diarrhoea responded rapidly to metronidazole. CT scanning played an important role in the management of these patients in assisting with diagnosis, excluding meconium ileus equivalent and giving prognostic information regarding the severity of the disease.
The importance of toxin‐negative stool analysis despite obvious symptoms and extensive radiographic abnormality is highlighted in cases 2 and 4. Indeed, in case 2, even sigmoidoscopy after CT scanning revealed negative findings. A high index of clinical suspicion with close monitoring of the patient's condition is required as symptoms and signs may be misleading and deterioration with haemodynamic collapse sudden.
Immunosuppression may have played a role in the variable presentation of the condition and is likely to be important in the pathogenesis of disease in our cases by increasing the risk of colonisation, as highlighted by the series of Dallal et al.3 However, the low overall rate of C difficile in our institution mitigates against this being the sole reason.
Diagnosis is often made difficult in the intubated ventilated patient because of difficulty in communication. The two patients on a ventilator both had an early tracheostomy performed with a speaking valve.
Enteral nutritional support is of great importance in any critically ill patient for energy provision and maintenance of enterocyte integrity. All four of our patients received enteral feeding throughout their illness with either nasogastric feeding or oral supplementation.
Antibiotics had been administered in the preceding days to weeks in all patients. Three of the four patients had been treated with piperacillin‐tazobactam shortly before presentation. Piperacillin‐tazobactam is not listed as a common cause of C difficile colonisation and, in fact, its use in the treatment of hospital‐acquired infections is credited with lowering the incidence of C difficile.9
In conclusion, we present a series of four patients with CF who developed severe C difficile colitis following lung transplantation. Two patients died, two required a colectomy, and the disease was certainly a significant event in the decline of a further patient. In one case the presentation of the disease was mistaken for meconium ileus equivalent and in a further case a diagnosis took several days to secure, highlighting the need for a high level of clinical suspicion. CT scanning revealed colitis in all patients; it not only suggested the diagnosis but gave an important indicator to the severity of the disease. Clostridium difficile colitis is an uncommon but important diagnosis in patients with CF following lung transplantation. The persistent carriage of C difficile in the bowel of prospective transplant recipients with CF may represent a relative contraindication, particularly patients with a prior history of pseudomembranous colitis, and we recommend all lung transplant centres to be vigilant for this problem.
Footnotes
Conflicts of interest: None.
References
- 1.Peach S L, Borriello S P, Gaya H.et al Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol 1986391013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pokorny C S, Bye P T, MacLeod C.et al Antibiotic‐associated colitis and cystic fibrosis. Dig Dis Sci 1992371464–1468. [DOI] [PubMed] [Google Scholar]
- 3.Dallal R M, Harbrecht B G, Boujoukas A J.et al Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002235363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly C P, Pothoulakis C, LaMont J T. Clostridium difficile colitis. N Engl J Med 1994330257–262. [DOI] [PubMed] [Google Scholar]
- 5.Mylonakis E, Ryan E T, Calderwood S B. Clostridium difficile‐associated diarrhea: a review. Arch Intern Med 2001161525–533. [DOI] [PubMed] [Google Scholar]
- 6.Binkovitz L A, Allen E, Bloom D.et al Atypical presentation of Clostridium difficile colitis in patients with cystic fibrosis. AJR Am J Roentgenol 1999172517–521. [DOI] [PubMed] [Google Scholar]
- 7.Rivlin J, Lerner A, Augarten A.et al Severe Clostridium difficile‐associated colitis in young patients with cystic fibrosis. J Pediatr 1998132177–179. [DOI] [PubMed] [Google Scholar]
- 8.Chaun H. Colonic disorders in adult cystic fibrosis. Can J Gastroenterol 200115586–590. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox M H, Freeman J, Fawley W.et al Long‐term surveillance of cefotaxime and piperacillin‐tazobactam prescribing and incidence of Clostridium difficile diarrhoea. J Antimicrob Chemother 200454168–172. [DOI] [PubMed] [Google Scholar]


