Abstract
Expression of peroxisome proliferator-activated receptor α (PPARα) and enzymes of fatty acid (FA) oxidation is markedly reduced in the fat-laden, dysfunctional islets of obese, prediabetic Zucker diabetic fatty (fa/fa) rats with mutated leptin receptors (OB-R). Leptin, PPARα/retinoid x receptor ligands, and FA all up-regulate PPARα and enzymes of FA oxidation and stimulate [3H]-palmitate oxidation in normal islets but not in islets from fa/fa rats. Overexpression of normal OB-R in islets of fa/fa rats corrects all of the foregoing abnormalities and reverses the diabetic phenotype. PPARα is a OB-R-dependent factor required for normal fat homeostasis in islet cells.
Early in the next century more than 100,000,000 Americans will be obese. Of these, more than 15,000,000 will become diabetic, making obesity-dependent diabetes one of the most common American diseases (1). The mechanisms by which obesity causes diabetes remain obscure. It is well known that obesity is associated with insulin resistance, and that effective compensatory hyperinsulinemia initially maintains blood glucose levels within the normal range (2). Ultimately, however, the ability of the pancreatic β cells to compensate for increasing insulin resistance may flag, in which case hyperglycemia appears. Understanding the mechanism of this obesity-related β-cell failure is critical to the prevention of adipogenic diabetes.
Current understanding of mechanisms of β-cell failure in obesity is based almost entirely on studies of rodent models of the disease. Of these, the Zucker diabetic fatty (ZDF)-drt (fa/fa) rat has been the most extensively characterized both genetically and phenotypically (3–6). The fa mutation is a Gln-269 → Pro substitution in the leptin receptor (OB-R) (7, 8). β-cell failure is correlated with excessive accumulation of fat within the pancreatic islets (9). This triglyceride overload appears to be the result of increased expression and activity of lipogenic enzymes, coupled with reduced expression and activity of enzymes of fatty acid (FA) oxidation (9, 10). The overabundance of islet fat is associated with a constellation of islet derangements that have been referred to collectively as lipotoxicity (9–11). They include decreased β-cell GLUT-2 expression (3), enhanced nitric oxide (NO) formation (12), impaired β-cell function (3), and apoptosis of a substantial subgroup of β cells (13). These abnormalities can be prevented by reducing the islet fat content (14) or by overexpressing the b isoform of the wild-type OB-R in islets of ZDF rats in which the only known genetic defect (fa/fa) is the mutated OB-R (15). Thus, this mutation appears to be the proximal cause of the entire phenotype.
The enzymes of FA metabolism that are underexpressed in islets of ZDF rats (10) are regulated in other tissues by members of the peroxisome proliferator-activated receptor (PPAR) family (16–21). Genes encoding enzymes of β oxidation, carnitine palmitoyl transferase-1 (CPT-1) and acyl CoA oxidase (ACO), and those encoding the lipogenic enzymes, acetyl CoA synthetase, FA synthase, and fatty acyl CoA synthase, all have PPAR response elements (16–21). Although PPAR isoforms are expressed in islets (22), their role in islet cell function is unknown. We suspected that an abnormality of a crucial PPAR isoform in these islets might account for the lipid abnormalities. This suspicion was heightened by our finding that troglitazone, a ligand activator of PPARγ (23), ameliorates the abnormalities in FA metabolism of ZDF islets (24) while correcting the β-cell dysfunction (25–27). This study was designed to test this possibility.
MATERIALS AND METHODS
Animals.
Lean wild-type (+/+) male ZDF rats and obese homozygous (fa/fa) male ZDF rats were bred in our laboratory from [ZDF-Drt-fa(F10)] rats purchased from R. Peterson (University of Indiana School of Medicine, Indianapolis).
Islet Isolation and Culture.
Pancreatic islets were isolated by the method of Naber et al. (28) with modifications (12). Isolated islets were cultured as described previously (12). In some experiments, islets were cultured with or without 2 mM long-chain free fatty acids (FFA) (2:1 oleate/palmitate, Sigma) in the absence and presence of 20 ng/ml of recombinant human leptin (kindly provided by Hector BeltrandelRio and Gayle Yamamoto, Zymogenetics, Seattle, WA), and in others with 1 mM clofibrate (CF) plus 1 mM 9-cis-retinoic acid (RA) (Sigma).
Semiquantification of mRNA.
mRNA of each PPAR isoform, ACO, and CPT-1 were measured by reverse transcription-PCR (RT-PCR) and normalized with β actin as described previously (29). Total RNA was extracted by the TRIzol isolation with Rnase-free DNase (Promega), and first-strand cDNA was generated from 1 μg of RNA in a 20-μl volume using oligo(dT) primer in the first-strand cDNA synthesis kit (CLONTECH). The following primers were used: PPARα, 5′-AAGCCATCTTCACGATGCTG-3′ (sense, 1321–1340) and 5′-TCAGAGGTCCCTGAACAGTG-3′ (antisense, 1811–1830) (GenBank M88592); PPARβ, 5′-CTTCAGTGA CATCATTGAGC-3′ (sense, 1221–1240) and 5′-GACAGCATGAACAGGAAGTG-3′ (antisense, 1751–1760) (GenBank U40064); PPARγ, 5′-TCCGTGATGGAAGACCACTC-3′ (sense, 190–209) and 5′-CCCTTGCATCCTTCACAAGC-3′ (antisense, 502–521) (GenBank U09138); ACO, 5′-GCCCTCAGCTATGGTATTAC-3′ (sense, 2891–2910) and 5′-AGGAACTGCTCTCACAATGC-3′ (antisense, 3505–3524) (GenBank J02752); CPT-I, 5′-TATGTGAGGATGCTGCTTCC-3′ (sense, 3094–3113) and 5′-CTCGGAGAGCTAAGCTTGTC-3′ (antisense 3703–3722) (GenBank L07736), and β actin, 5′-TTGTAACCAACTGGGACGATATGG-3′ (sense, 1552–1575) and 5′-GATCTTGATCTTCATGGTGCTAGG-3′ (antisense, 2967–2991) (GenBank J00691). Linearity of the PCR was tested by amplification of 200 ng per reaction from 20–50 cycles. The linear range was found to be between 20 and 40 cycles. In no case did the amount of total RNA used for PCR exceed 200 ng per reaction. Two microliters of first-strand cDNA was amplified for 30 cycles (PPARα, β, and γ), 28 cycles (ACO and CPT-1), and 25 cycles (β actin) by using the following parameters: 92°C for 45 sec, 55°C for 45 sec, and 72°C for 1 min. The PCR products were electrophoresed on a 1.5% agarose gel, and Southern blotting was carried out. Radiolabeled probes for amplified fragments (5′-ACTCGGTCTTCTTGATGACCTGCACGAGCT-3′ for PPARα; 5′-CTGCGTGCAGCCTTAGTACATGTCCTTGTA-3′ for PPARβ; 5′-AGAGCTGATTCCGAAGTTGGTGGGCCAGAA-3′ for PPARγ; 5′-GCCTGCACTTTCTTCAGCCATCTTCAACGA-3′ for ACO; 5′-ACTCTGGTTGGAATCTGACTGGGTGGGATT-3′ for CPT-I, and 5′-GGTCAGGATCTTCATGAGGTAGTCTGTCAG-3′ for β actin) were hybridized to the nylon membrane and quantitated by Molecular Imager (GS-363, Bio-Rad). Levels of mRNA expression were expressed as the ratio of signal intensity for the mRNA of interest relative to that for β-actin mRNA.
[3H]-Palmitate Oxidation.
Palmitate oxidation also was measured by adding 9–10-[3H] palmitate to the culture media as described previously (10). After 3 days culture, islets were collected, and palmitate oxidation was assessed by measuring 3H2O in the medium. Excess [3H] palmitate in the medium was removed by precipitating twice with an equal volume of 10% trichloroacetic acid with 2% BSA. Supernatant was transferred to a 1.5-ml microcentrifuge tube, placed in a scintillation vial containing 0.5 ml of unlabeled water, and incubated at 50°C for 18 hr. To determine the equilibrium coefficient, 10 μl of 3H2O was added to 490 μl of unlabeled water into 1.5-ml microfuge tubes in parallel with the experiment samples.
Induction of Hyperleptinemia in Vivo.
Hyperleptinemia was induced by infusing recombinant adenovirus containing the leptin cDNA (AdCMV-leptin) as described previously (30). Recombinant adenovirus containing the bacterial β-galactosidase gene (AdCMV-β-gal) was infused as a control. Two milliliters of either AdCMV-leptin or AdCMV-β-gal containing a total of 1012 plaque-forming units was infused into homozygous (+/+) or (fa/fa) ZDF rats. Animals were studied in individual metabolic cages, and food intake and body weight were measured daily. Plasma leptin was assayed by using the Linco leptin assay kit (Linco Research Immunoassay, St. Charles, MO).
Overexpression of OB-Rb.
As described previously (15), pancreata of ≈10-week-old male ZDF (fa/fa) rats were perfused with 1 × 1012 plaque-forming units of recombinant adenovirus containing either the full-length OB-Rb cDNA (AdCMV-OB-Rb) or the β-galactosidase gene (AdCMV-β-gal) in Krebs–Ringer bicarbonate buffer with 4.5% Dextran T70, 1% BSA, 5.6 mM glucose, and 5 mM each of sodium pyruvate, sodium glutamate, and sodium fumarate. Pancreatic islets then were isolated and maintained for 2 days in suspension culture in 60-mm Petri dishes at 37°C in a humidified atmosphere of 5% CO2 and 95% air as described previously (15). OB-Rb mRNA expression was determined by RT-PCR and protein by immunoblotting as described (15).
Statistical Analyses.
All values shown are expressed as mean ±SEM. Statistical analysis was performed by unpaired Student’s t test or by one-way ANOVA.
RESULTS
PPAR Expression in Pancreatic Islets.
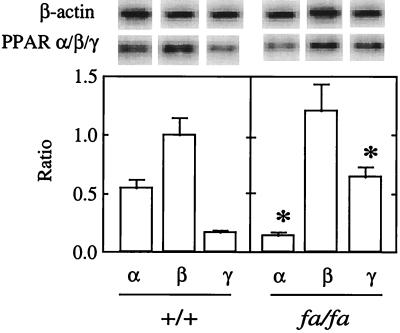
Because little was known about PPAR in islets, we first determined which of the known PPAR isoforms normally are expressed in the endocrine pancreas. We semiquantified by RT-PCR the mRNA of all PPAR isoforms in islets isolated from normal 7-week-old lean, wild-type (+/+) ZDF rats. As shown in Fig. 1A, the α, β/δ, and γ isoforms were expressed in wild-type islets. PPARβ/δ was the most abundant, confirming a previous report by Braissant et al. (22). In islets of age-matched obese (fa/fa) ZDF rats, however, the expression of PPARα was only 25% of lean +/+ islets, whereas PPARγ mRNA was 3.5 times normal. PPARβ/δ expression was the same in islets from the two groups (Fig. 1). The substantial reduction in PPARα mRNA in the fat-laden islets of the obese rats raised the possibility that the decreased expression of enzymes of FA oxidation might be secondary to deficient PPARα. Indeed, the livers of PPARα-null mice reportedly underexpress genes encoding enzymes of mitochondrial and peroxisomal FA β oxidation (31).
Figure 1.
Representative blot of PPARα, β/δ, γ, and β-actin mRNA in normal islets (+/+) and in islets of ZDF rats (fa/fa). Bars represent the mean of PPAR/β-actin ratios (±SEM) of 3–5 experiments. ∗, P < 0.01 vs. +/+.
Activities of PPARα Ligand CF and RA in +/+ and fa/fa Islets.
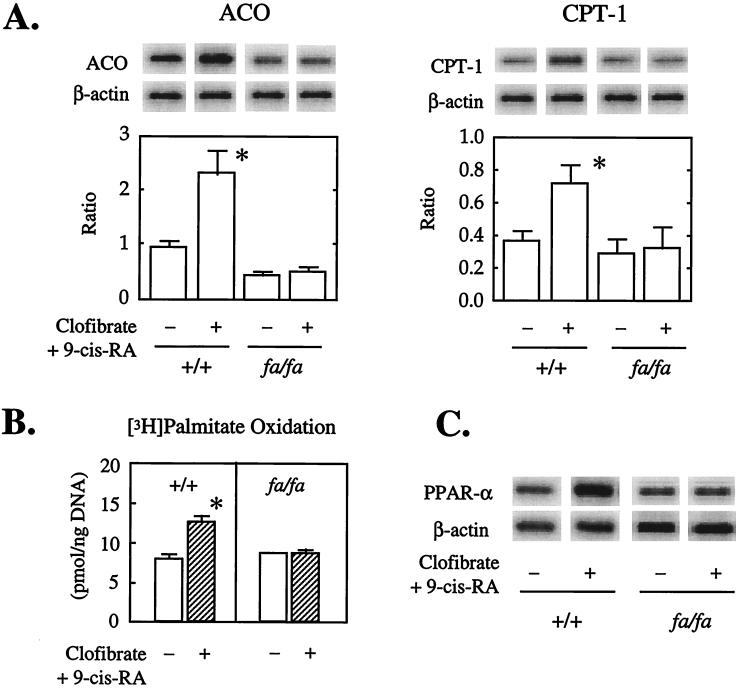
If PPARα is, in fact, an important transcriptional regulator of the enzymes CPT-1 and ACO that catalyze β oxidation of long-chain FAs in normal islets, then the PPARα ligand, CF, together with RA, the ligand for its dimerization partner, retinoid x receptor, should up-regulate them. Moreover, if a PPARα deficiency and/or defect was responsible for the underexpression of CPT-1 and ACO in ZDF islets, these ligands should have little or no effect on their expression in these animals. Islets from +/+ and fa/fa rats therefore were cultured in the presence and absence of CF/RA. In the islets of +/+ rats cultured with CF/RA both the ACO/β actin and the CPT-1/β actin mRNA ratios were at least twice as high as the controls (Fig. 2A). However, in islets of fa/fa rats CF/RA was without effect on the expression of either enzyme. Thus, PPARα ligands up-regulate the enzymes of FA oxidation in islets with a normal PPARα expression but have no effect in fa/fa islets with reduced expression of the nuclear receptor. The lack of even a small effect in these islets implies that if the underexpressed PPARα mRNA is translated in fa/fa islets the protein is not functional.
Figure 2.
Effect of the PPARα/retinoid x receptor ligand pair, CF, and RA, on (A) the ratio of ACO, CPT-1 mRNA to β-actin mRNA (n = 3); (B) oxidation of [3H]-palmitate (n = 3); (C) mRNA of PPARα (n = 3). Representative blots are displayed. Bars in A indicate the mean (±SEM) of mRNA ratio of ACO/and CPT-1/β actin. Bars in B indicate mean (±SEM) of palmitate oxidation.
To determine whether the CF/RA-induced up-regulation of the mRNA encoding the enzymes of FA oxidation is associated with an increase in FA oxidation, we cultured +/+ and fa/fa islets in [3H] palmitate for 48 hr with and without CF/RA and measured the accumulation of 3H2O. There was a 42% rise in oxidation in the +/+ islets but no change in the fa/fa islets (Fig. 2B), again implying that the underexpressed PPARα is functionally inactive.
Effect of CF/RA on PPARα Expression.
It is reported that in liver PPARα ligands not only activate the receptor, but also increase its expression (32, 33). To determine whether CF/RA increases expression of PPARα in normal islets, the PPARα/β actin mRNA in ratio was measured in islets of both the CF/RA-responsive +/+ rats and the obese fa/fa rats. It rose 3.2-fold in +/+ islets but was unchanged in fa/fa islets (Fig. 2C). We interpret this as additional evidence that yet another functional activity of PPARα, its auto-up-regulation, is lacking in islets of fa/fa rats.
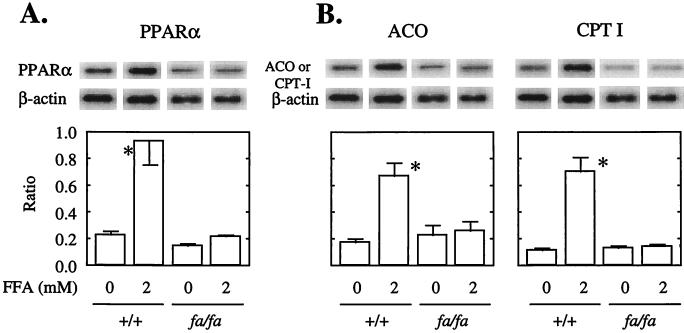
Effects of Long Chain FAs on PPARα, ACO, and CPT-1 Expression in +/+ and fa/fa Islets.
Long chain FAs in islets are considered to be endogenous PPARα activators (19). To determine whether, like CF/RA, FAs also up-regulate PPARα, ACO, and CPT-1 expression in normal islets, but not in islets from obese (fa/fa) ZDF rats, we cultured both groups of islets in medium containing 2 mM of a 2:1 oleate/palmitate mixture. In normal +/+ islets PPARα mRNA was more than 4.5 times more abundant in the presence of FFA than in its absence and ACO and CPT-1 both were approximately 3.5 times greater (Fig. 3A). In islets from fa/fa ZDF rats, by contrast, FFA had no effect on the depressed levels of PPARα, ACO, or CPT-1 mRNA. These findings indicate that in the presence of the wild-type OB-R FAs up-regulate expression of PPARα and the enzymes of FA oxidation. However, a mutated OB-R results in impaired function of underexpressed PPARα and in underexpression of the enzymes for which it is a transcription factor.
Figure 3.
Effect of a 2 mM mixture of oleate/palmitate (2:1) on PPARα, ACO, and CPT-1 mRNA/β-actin mRNA ratios of islets isolated from wild-type (+/+) and obese (fa/fa) ZDF rats (N+3). ∗, P < 0.01 vs. CF + RA.
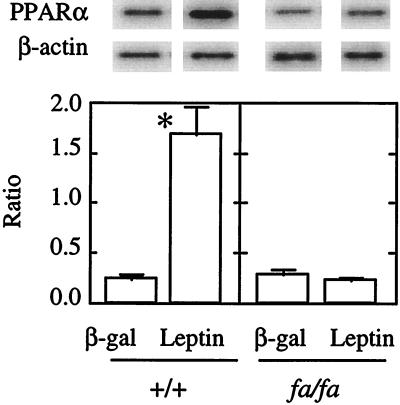
Effects of in Vivo Hyperleptinemia on PPARα Expression in +/+ and fa/fa Rats.
Based on these findings we suggest that in islets with a normal OB-R the triglyceride content is maintained at its normal level by regulation of the expression of islet CPT-1 and ACO so as to assure oxidation of any excess FAs entering the islets. In fa/fa rat islets, by contrast, fat accumulates because of the lack of sufficient leptin-induced FA oxidation. If this is true, hyperleptinemia should up-regulate PPARα, CPT-1, and ACO in normal islets but not in islets of fa/fa rats. We therefore induced hyperleptinemia in lean +/+ and obese fa/fa rats by infusing AdCMV-leptin. Control rats were infused with AdCMV-βgal. All AdCMV-leptin-infused rats developed hyperleptinemia in excess of 18 ng/ml, whereas in the AdCMV-βgal controls leptin was less than 1 ng/ml. As shown in Fig. 4, in +/+ islets, PPARα expression was seven times greater than in controls, and expression of ACO and CPT-1 was increased 2.5- and 2-fold (data not shown), respectively, confirming previous data (20). In fa/fa islets, in contrast, the levels of PPARα, ACO, and CPT1 expression were not altered by the increase in their hyperleptinemia (Fig. 4).
Figure 4.
In vivo effect of hyperleptinemia on PPARα/β-actin mRNA ratio. β-gal and leptin refer to islets isolated from rats infused with AdCMV-βgal or AdCMV-leptin. Bars represent mean (±SEM) of three experiments. ∗, P < 0.01 vs. β-gal.
Effects of Leptin in Vitro on PPARα Expression in +/+ and fa/fa Islets.
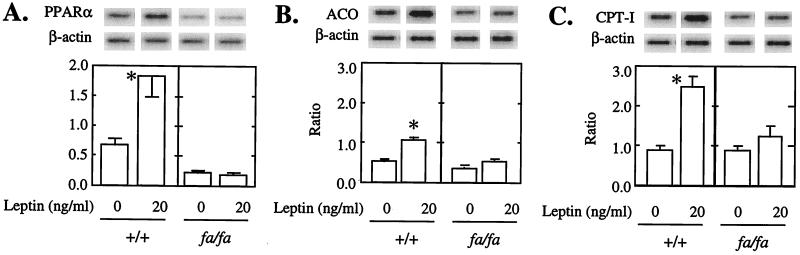
To determine whether the effects of hyperleptinemia were exerted via direct action of leptin on islets, in addition to actions via the hypothalamus, we cultured +/+ and fa/fa islets for 3 days with or without 20 ng/ml of recombinant leptin added 24 hr after the start of culture. In +/+ islets, PPARα was three times higher in the presence of leptin than in its absence (Fig. 5A). In contrast, in islets of fa/fa rats the initially low PPARα mRNA level was unaffected by leptin. Leptin also significantly increased ACO (Fig. 5B) and CPT-1 mRNA (Fig. 5C) in +/+ but not in fa/fa islets.
Figure 5.
An in vitro effect of recombinant leptin on (A) PPARα, (B) ACO, and (C) CPT-1 mRNA/β-actin mRNA. Islets were cultured for 48 hr either with or without 20 ng/ml of human leptin. Bars represent mean (±SEM) of three experiments. ∗, P < 0.01 vs. 0 ng/ml of leptin.
Effects of OB-Rb Overexpression in fa/fa Islets on PPARα Underexpression.
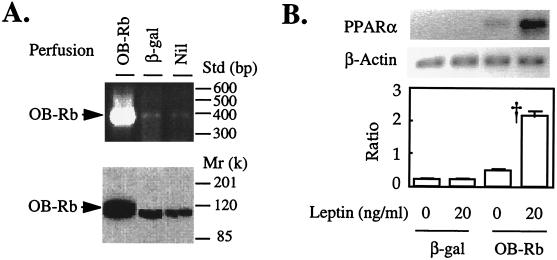
The foregoing results imply that overexpression of wild-type OB-Rb in islets of fa/fa with mutated OB-R would up-regulate the underexpressed PPARα and restore the ability of leptin to up-regulate PPAR and the enzymes of oxidation. To determine whether this is the case, we perfused pancreata of fa/fa rats with AdCMV-OB-Rb, the full-length OB-R isoform or AdCMV-βgal. We then isolated and cultured the islets for 3 days either with or without 20 ng/ml of leptin added 24 hr after the start of the culture. Islets from pancreata that had been perfused with AdCMV-OB-R expressed OB-R and protein at a level six times the AdCMV-βgal-perfused controls (Fig. 6A). As shown in Fig. 6B, PPARα rose more than 2-fold in OB-R-overexpressing fa/fa islets cultured without leptin and more than 5-fold in the presence of 20 ng/ml of leptin. There was no increase in PPARα in AdCMV-βgal-perfused control islets either with or without leptin in the culture medium. Thus OB-Rb overexpression in fa/fa islets confers ligand-independent up-regulation of PPARα, but the presence of leptin induces further up-regulation.
Figure 6.
(A) The effect of perfusion of recombinant adenovirus containing either wild-type OB-Rb cDNA or β-gal cDNA into pancreata from fa/fa ZDF rats on wild-type OB-Rb mRNA and protein in islets isolated immediately after perfusion and cultured for 3 days. Both OB-Rb mRNA and protein are intensely overexpressed. (B) Effect of overexpression of OB-Rb on PPARα mRNA of cultured fa/fa islets in the absence or presence of 20 ng/ml of recombinant leptin. Leptin was added 24 hr after the start of the 3-day culture period. Bars represent mean (±SEM) of three experiments. ∗, P < 0.01 vs. 0 ng/ml of leptin.
DISCUSSION
Although the mechanism of the PPARα up-regulation observed here cannot be firmly established from these data, others have found that phosphorylated signal transducer and activation of transcription-3 (STAT-3) is a major mediator of leptin action (36). STAT-3 is low in fa/fa islets but increases to normal unstimulated levels when OB-R is overexpressed islets in the absence of leptin treatment; the addition of leptin increases it to still higher levels (unpublished observations). Thus, it is possible that ligand-independent OB-R activation of STAT-3 maintains a tonic effect on expression of enzymes of FA oxidation such that islet triglyceride content remains within a normal range. In obesity the flux of FFAs from adipocytes into islets is greatly enhanced in proportion to the obesity. Because leptin levels also rise proportionately to the obesity (34, 35), we suggest that they stimulate fatty oxidation in nonadipacytes, thereby preventing excessive accumulation of triglycerides and lipotoxicity. However, if the islet leptin receptors are defective, as in ZDF rats, or if the sensitivity of islets to leptin is substantially diminished by postreceptor defects, islet fat content in obesity may rise to a lipotoxic level, in which case adipogenic diabetes will ensue. In ZDF rats the lipotoxic level of fat content is more than 50 times the normal fat content. We postulate that this may be the cause of β-cell failure in obesity (9, 36).
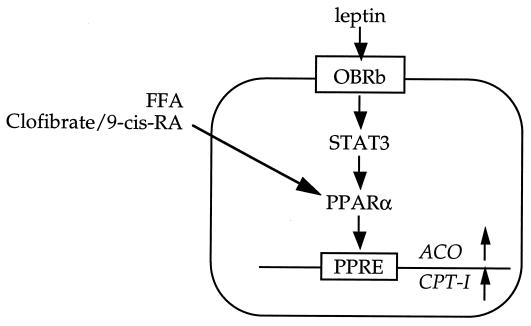
These findings are consistent with a hitherto unrecognized pathway of intracellular fat homeostasis, the integrity of which appears to be critical for normal β-cell function. PPARα appears to play a central role in this pathway (Fig. 7). On one hand, depletion of islet fat to levels below 5 ng/islet seems to “paralyze” the β cells, rendering them incapable of secreting insulin in response to glucose and amino acids (37). On the other hand, overabundance of islet fat induces a spectrum of important changes: a modest increase in triglyceride content causes hyperinsulinemia that is proportional to the level of obesity and obesity-induced insulin resistance, and therefore plays a compensatory role; more severe fat overload lowers compensatory hyperinsulinemia and elicits ceramide-mediated overproduction of NO, which causes β-cell apoptosis (12, 13). Thus, normal leptin function is required to maintain normal triglyceride homeostasis in β cells. Further, in contrast to the pathway of leptin regulation of appetite and body composition, which seems to be largely, if not entirely, mediated via the hypothalamus (38, 39), its lipopenic effects on islets are, at least in part, direct (40).
Figure 7.
A proposed pathway of triglyceride homeostasis and prevention of overaccumulation of fat in normal islets.
The implications of these findings in rodents for the human adipogenic diabetes cannot as yet be determined, but certain predictions can be made. First, obesity-dependent diabetes in humans will involve a heterogeneity of mechanisms rather than the monogenic etiology of ZDF rats. Second, because the natural history of the human disorder is measured in decades rather than in weeks as in ZDF rats, the rate of fat accumulation will be extremely slow in comparison to the rodent model. Third, the inaccessibility of human islets will prevent careful study of islet fat content at the onset of diabetes and thus make it extremely difficult to determine whether lipotoxic diabetes of ZDF rats has a human counterpart.
Although diet-induced leptin resistance appears to be very common in humans (41), presumably the result of a postreceptor defect, it has not been determined whether this form of leptin resistance involves the islets. Nevertheless, troglitazone, a PPARγ ligand (23), lowers islet fat content of ZDF rats (12, 24); the fact that it ameliorates diabetes in both obese humans (42–45) and obese rodents (25, 26) is perhaps consistent with a common cause of the β-cell functional abnormality.
Acknowledgments
Michael McPhaul, M.D., provided the initial impetus for this study. We wish to thank David Mangelsdorf, Ph.D., and Daniel Foster, M.D., for careful review of this paper. We thank Kay McCorkle and Tagan Ferguson for excellent technical work and Tess Perico for outstanding secretarial assistance. This work was supported by Veterans Administration Institutional Research Support Grant SMI 821-109 and National Institutes of Health Grant DK-02700-37, National Institutes of Health/Juvenile Diabetes Foundation International Diabetes Interdisciplinary Research program, and grants from Novo Nordisk, Copenhagen, and Sankyo, Tokyo (R.H.U.).
ABBREVIATIONS
- PPAR
peroxisome proliferator-activated receptor
- FA
fatty acid
- FFA
free fatty acid
- ZDF
Zucker diabetic fatty
- OB-R
leptin receptor
- CPT-I
carnitine palmitoyl transferase-I
- ACO
acyl CoA oxidase
- RT-PCR
reverse transcriptase–PCR
- CF
clofibrate
- RA
9-cis-retinoic acid
- AdCMV-leptin
adenovirus containing the leptin cDNA
- AdCMV-β-gal
adenovirus containing bacterial β-galactosidase gene
References
- 1.Unger R H, Foster D W. In: William’s Textbook of Endocrinology. 9th Ed. Wilson JD, Foster H M, Kronenberg P R, Larsen P R, editors. Philadelphia: Saunders; 1998. pp. 973–1059. [Google Scholar]
- 2.Reaven G M. Diabetologia. 1995;38:3–13. doi: 10.1007/BF02369347. [DOI] [PubMed] [Google Scholar]
- 3.Johnson J H, Ogawa A, Chen L, Orci L, Newgard C B, Alam T, Unger R H. Science. 1990;250:546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- 4.Lee Y, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orci L, Ravazzola M, Baetens D, Inman L, Amherdt M, Peterson R G, Newgard C B, Johnson J H, Unger R H. Proc Natl Acad Sci USA. 1990;87:9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unger R H. Science. 1991;251:1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- 7.Iida M, Murakami T, Ishida K, Mizuno A, Kuwajima M, Shoma K. Biochem Biophys Res Commun. 1996;224:597–606. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- 8.Phillips M S, Liu Q Y, Hammond H A, Dugan V, Hey P J, Caskey C T, Hess J F. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Hirose H, Ohneda M, Johnson J H, McGarry J D, Unger R H. Proc Natl Acad Sci USA. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Hirose H, Zhou Y-T, Easer V, McGarry J D, Unger R H. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- 11.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 12.Shimabukuro M, Ohneda M, Lee Y, Unger R H. J Clin Invest. 1997;100:290–295. doi: 10.1172/JCI119534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimabukuro M, Zhou Y-T, Levi M, Unger R H. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohneda M, Inman L R, Unger R H. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- 15.Wang M-Y, Koyama K, Shimabukuro M, Newgard C B, Unger R H. Proc Natl Acad SciUSA. 1998;95:714–718. doi: 10.1073/pnas.95.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Proc Natl Acad Sci USA. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller H, Mahfoudi A, Dreyer C, Hihi A K, Medin J, Ozato K, Wahli W. Ann NY Acad Sci. 1993;684:157–173. doi: 10.1111/j.1749-6632.1993.tb32279.x. [DOI] [PubMed] [Google Scholar]
- 18.Keller H, Wahli W. Trends Endocrinol Metab. 1993;4:291–296. doi: 10.1016/1043-2760(93)90048-j. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer A, Forman B M, Blumberg B, Ong E S, Borgmeyer U, Mangelsdorf D J, Umesono K, Evans R M. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kliewer S A, Sundseth S S, Jones S A, Brown P J, Wisely G B, Koble C S, Devchand P, Wahli W, Willson T M, Lenhard J M, Lehmann J M. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman B M, Chen J, Evans R M. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann J M, Moore L B, Smith-Oliver T A, Wilkison W O, Willson T M, Kliewer S A. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 24.Shimabukuro M, Zhou Y-T, Lee Y, Unger R H. J Biol Chem. 1998;273:3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- 26.Sreenans S, Sturis J, Pugh W, Burant C F, Polonsky K S. Am J Physiol. 1996;271:E742–E747. doi: 10.1152/ajpendo.1996.271.4.E742. [DOI] [PubMed] [Google Scholar]
- 27.Saltiel A R, Olefsky J M. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- 28.Naber S P, McDonald J M, Jarett L, McDaniel M L, Ludvigsen C W, Lacy P E. Diabetologia. 1980;19:439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y-T, Shimabukuro M, Koyama K, Lee Y, Wang M-Y, Trieu F, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:6386–6390. doi: 10.1073/pnas.94.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Koyama K, Yuan X, Lee Y, Zhou Y-T, O’Doherty R, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoyama T, Peters J M, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez F J. J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 32.Sterchele P F, Sun H, Peterson R E, Vanden Heuvel J P. Arch Biochem Biophys. 1996;326:281–289. doi: 10.1006/abbi.1996.0077. [DOI] [PubMed] [Google Scholar]
- 33.Gebel T, Arand M, Oesch F. FEBS Lett. 1992;309:37–40. doi: 10.1016/0014-5793(92)80734-x. [DOI] [PubMed] [Google Scholar]
- 34.Maffei M, Halaas J, Ravussin E, Pratley R E, Lee G H, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Nat Med. 1995;11:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 35.Considine R V, Sinha M K, Heiman M L, Kriauciunas A, Stephens T W, Nyce M R, Ohannesian J P, Marco C C, McKee L J, Bauer T L, et al. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 36.Darnell J E., Jr Proc Natl Acad Sci USA. 1996;93:6221–6224. doi: 10.1073/pnas.93.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirose H, Lee Y H, Inman L R, Nagasawa Y, Johnson J H, Unger R H. J Biol Chem. 1996;271:5633–5637. doi: 10.1074/jbc.271.10.5633. [DOI] [PubMed] [Google Scholar]
- 38.Koyama K, Chen G, Wang M-Y, Lee Y, Shimabukuro M, Newgard C B, Unger R H. Diabetes. 1997;46:1276–1280. doi: 10.2337/diab.46.8.1276. [DOI] [PubMed] [Google Scholar]
- 39.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 40.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 41.Shimabukuro M, Koyama K, Chen G, Wang M-Y, Trieu F, Lee Y, Newgard C B, Unger R H. Proc Natl Acad Sci USA. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caro J F, Sinha M K, Kolaczynski J W, Zhang P L, Considine R V. Diabetes. 1996;45:1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 43.Nolan J J, Ludvik B, Beerdsen P, Joyce M, Olefsky J. N Engl J Med. 1994;331:1188–1193. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 44.Iwamoto Y, Kuzuya T, Matsuda A, Awata T, Kumakura S, Inooka G, Shiraishi I. Diabetes Care. 1994;14:1083–1086. doi: 10.2337/diacare.14.11.1083. [DOI] [PubMed] [Google Scholar]
- 45.Saltiel A R, Olefsky J M. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]