Abstract
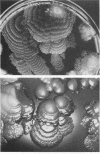
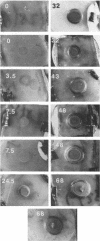
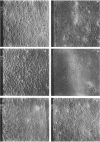
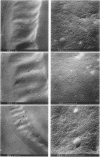
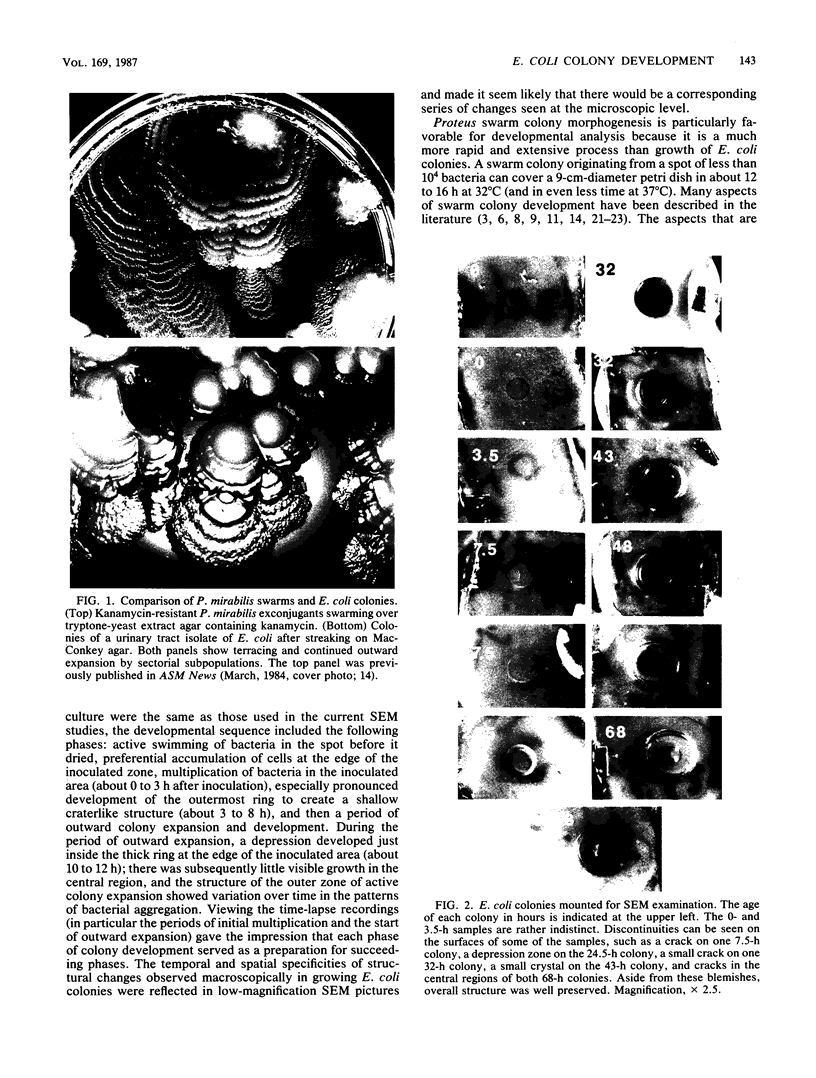
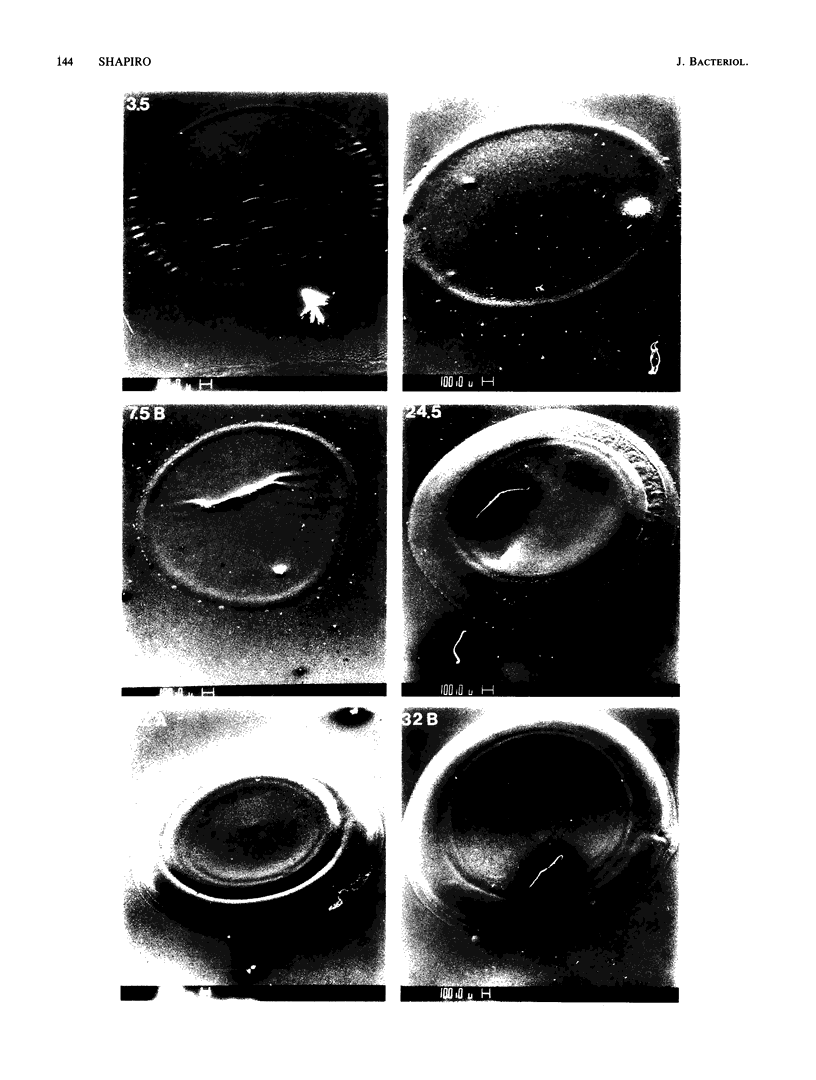
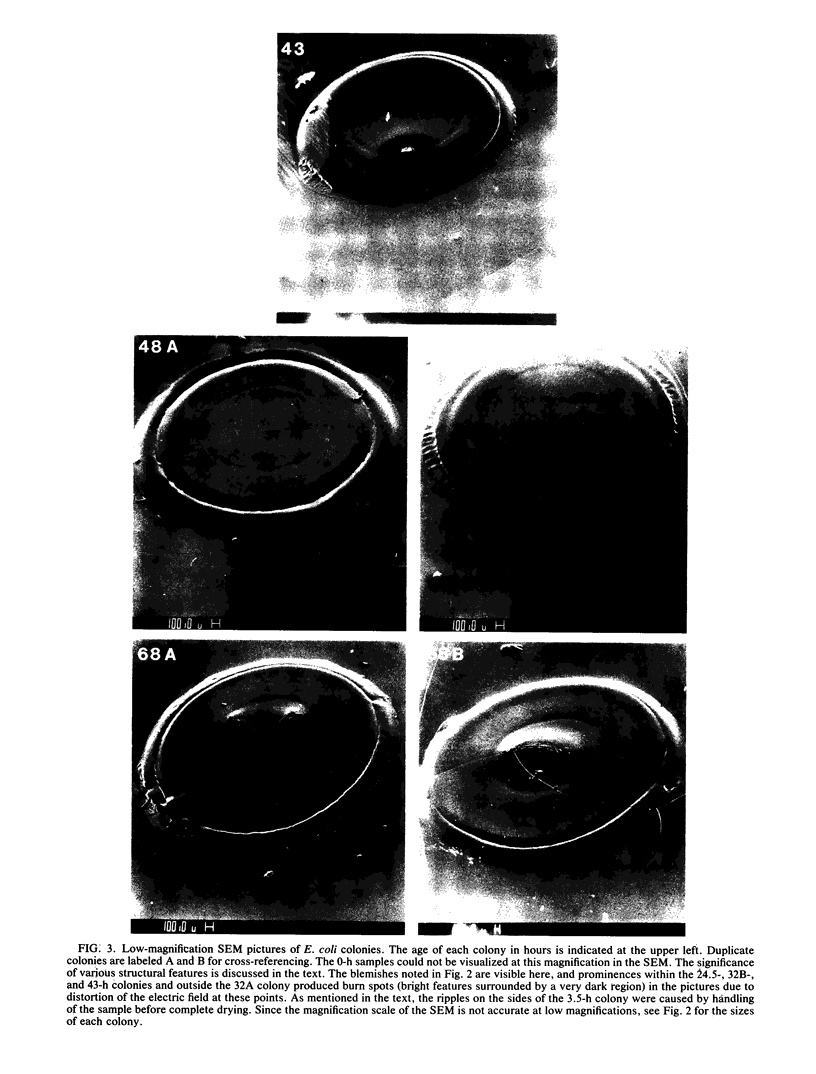
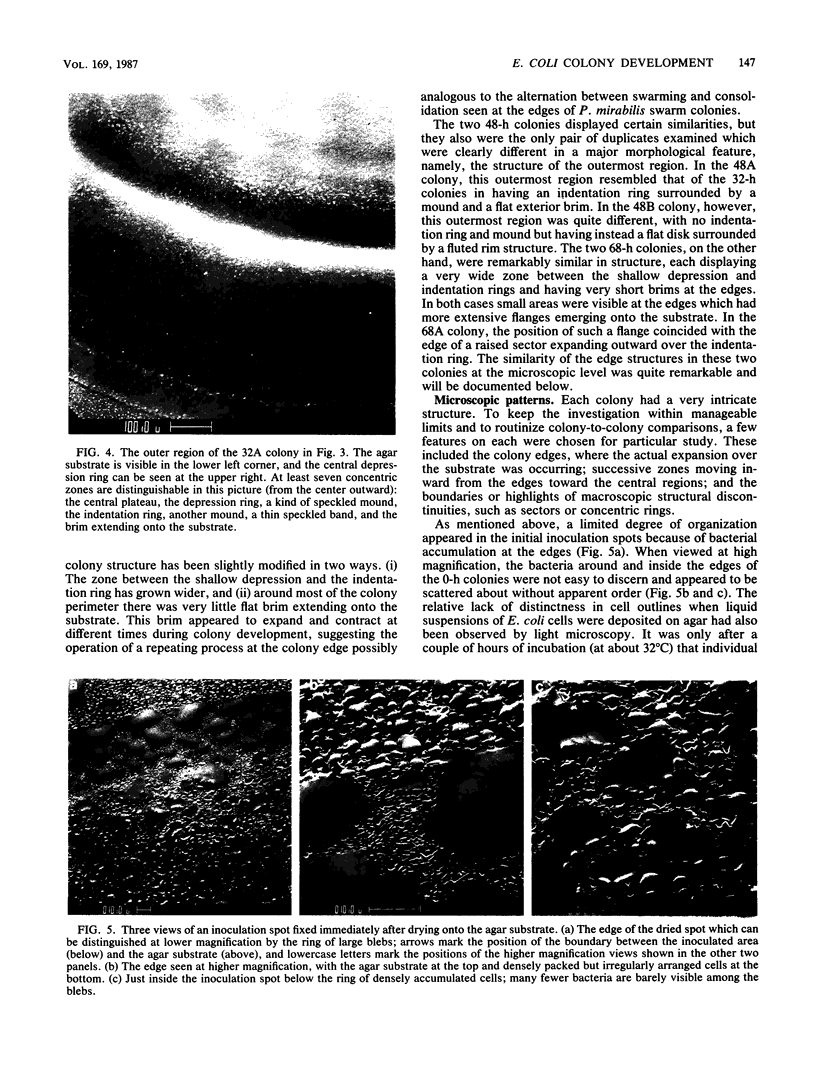
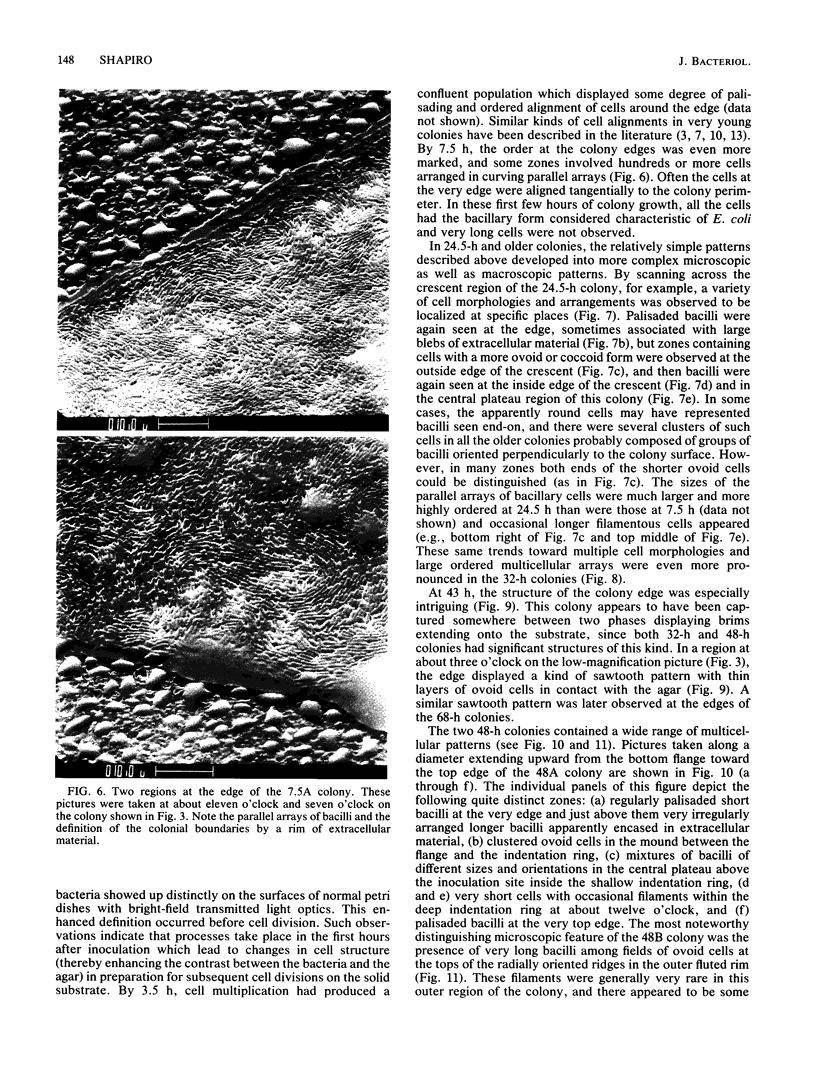
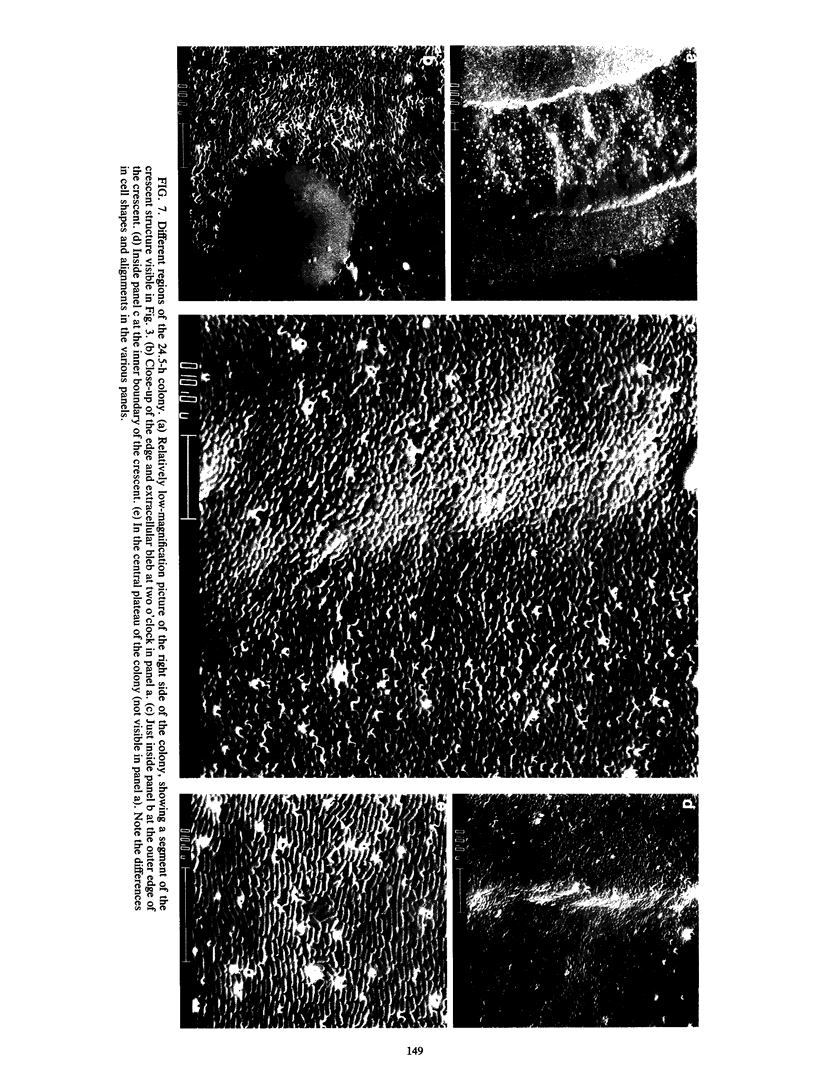
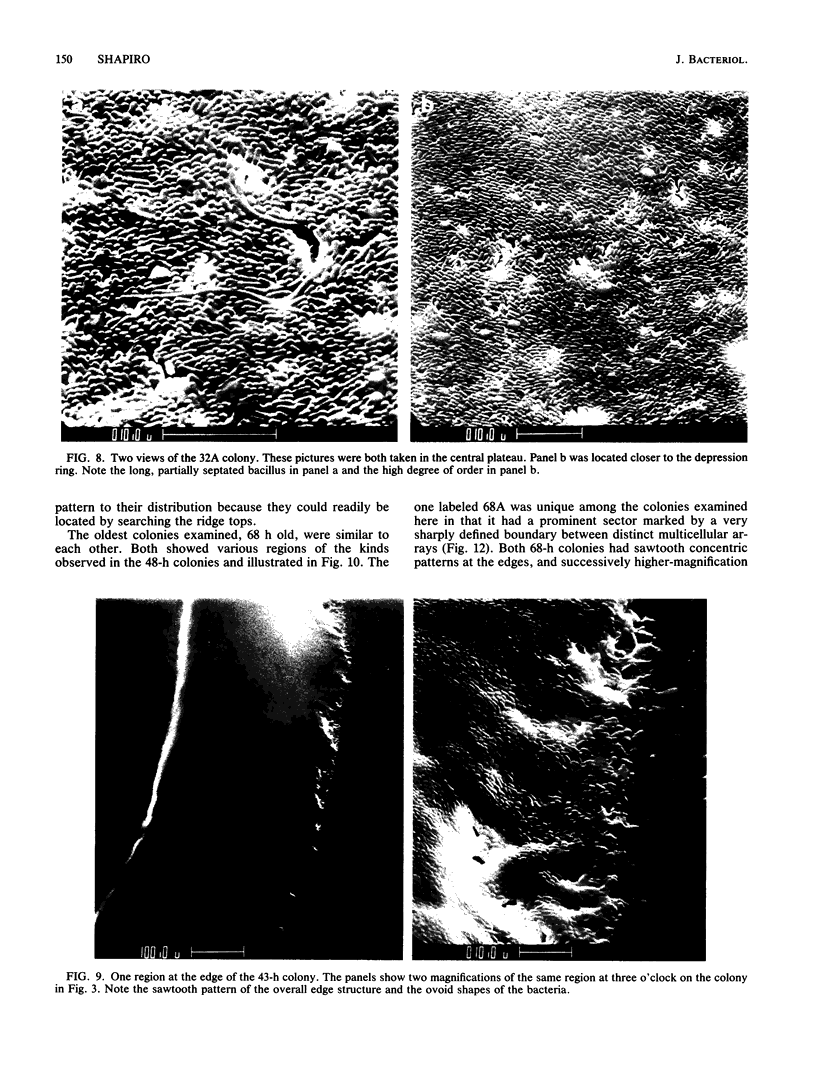
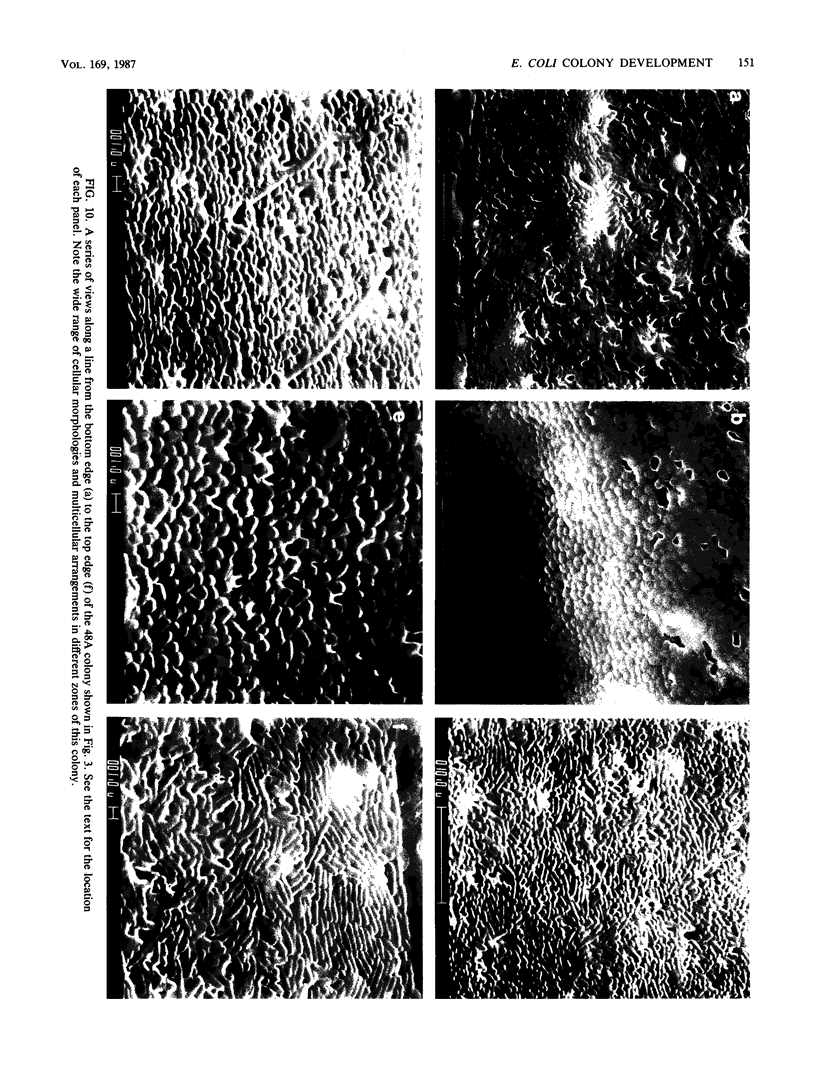
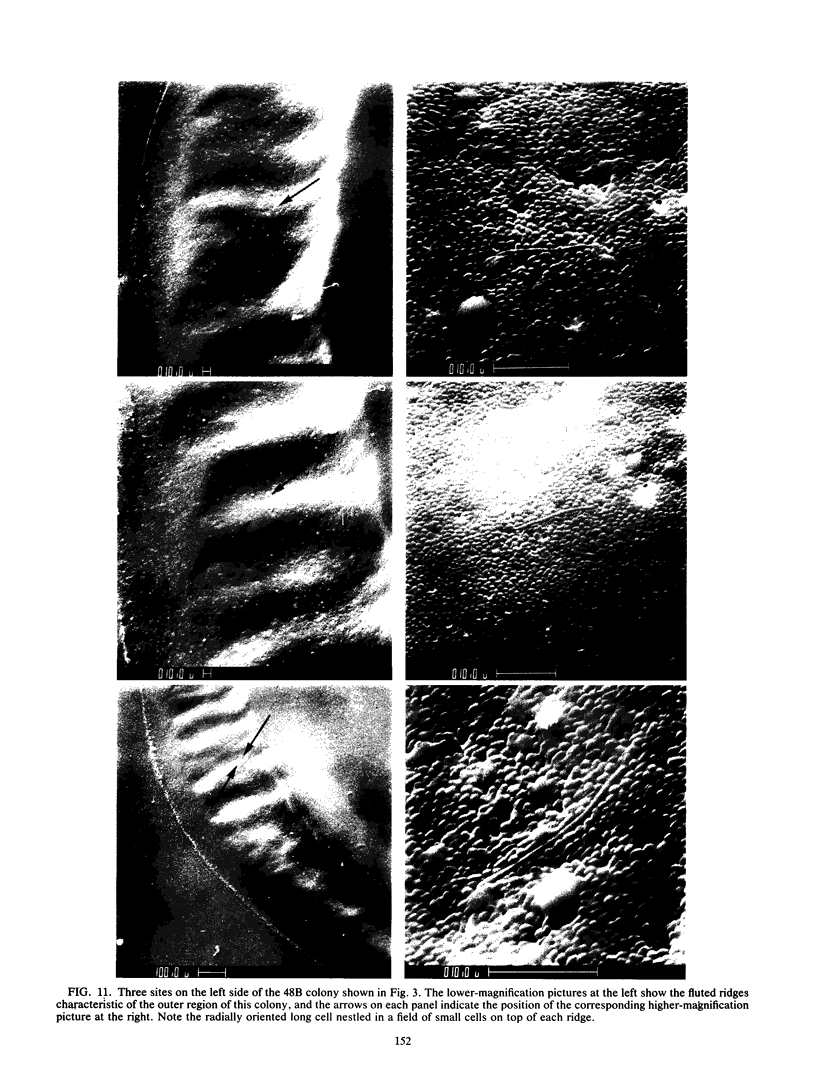
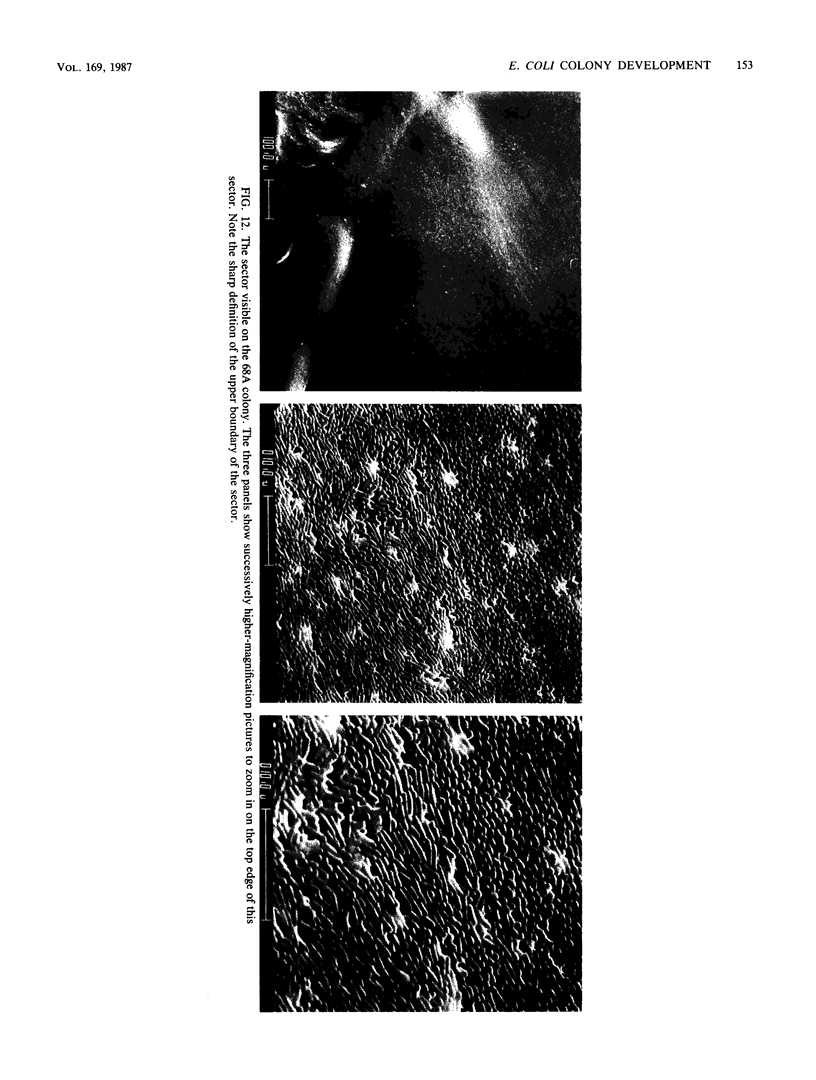
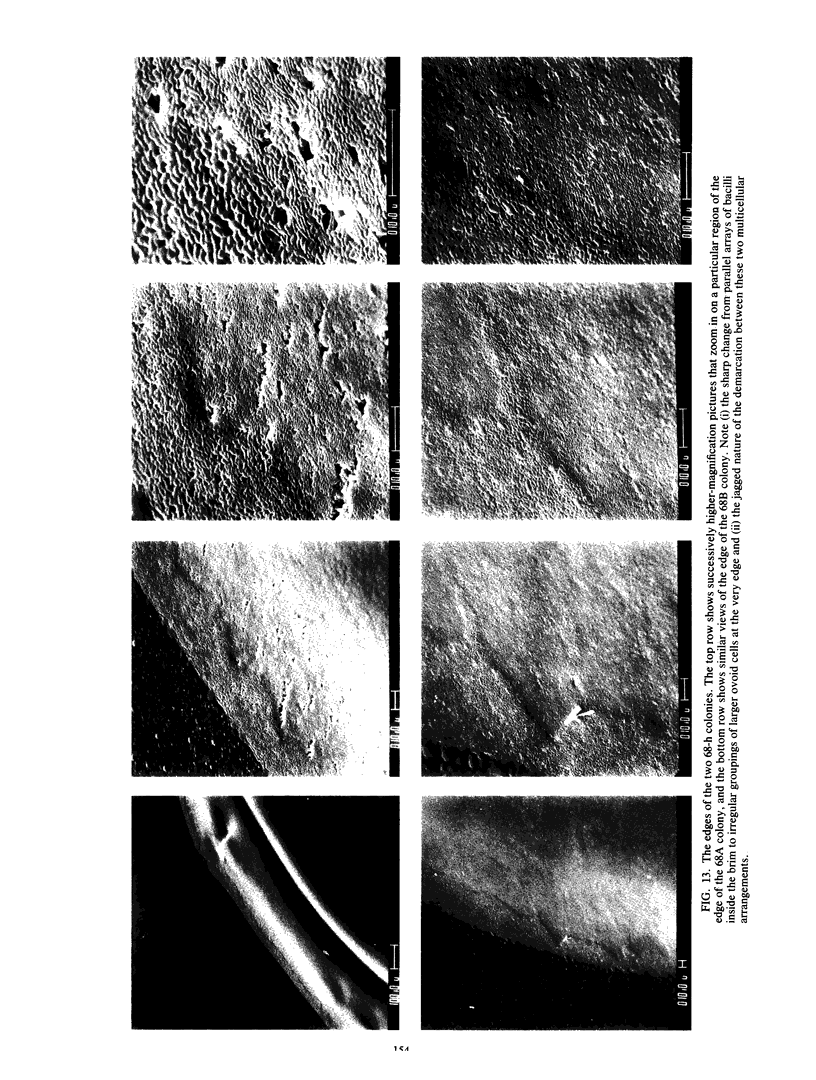
Colony growth was initiated by inoculating minimal glucose agar with 1-microliter. spots of a plasmid-free Escherichia coli culture and incubating at 32 degrees C. Inoculations took place over a 3-day period, at the end of which the plates were fixed and dried for scanning electron microscopy. In this way, it was possible to examine the surfaces of colonies ranging in age from 0 to 68 h. Macroscopically, the colonies were organized into different concentric zones, and several morphological features could be seen to develop over this period. These included a shallow depression ring marking the site of inoculation, a deeper indentation ring whose position moved outward as the colony grew, an expanding plateau region between the two rings, a mound outside the indentation ring, and a flat brim extending onto the substrate which was either present or absent at different times. Microscopically, a variety of cell morphologies and cell arrangements were detected. Upon inoculation, the bacteria accumulated at the periphery of the inoculation spot but showed no other kind of order. For the first 7.5 h, all bacteria were rod shaped; at the end of this initial phase, a high degree of alignment was seen in the cells at the colony edge. By 24.5 h, both shorter more ovoid cells and longer filaments had begun to appear, and large multicellular arrays had formed. At later stages of colony development, morphologically distinguishable zones involving cells of different shapes and sizes had formed, and these zones often marked the boundaries of macroscopic features. The edges were particularly interesting and at 68 h displayed very sharp saw-toothed boundaries between concentrically organized groups of bacteria. There were some transient irregularities in the concentric organizations of growing colonies, and one colony had entered upon a distinct developmental pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisset K. A., Douglas C. W. A continuous study of morphological phase in the swarm of Proteus. J Med Microbiol. 1976 May;9(2):229–231. doi: 10.1099/00222615-9-2-229. [DOI] [PubMed] [Google Scholar]
- Douglas C. W., Bisset K. A. Development of concentric zones in the Proteus swarm colony. J Med Microbiol. 1976 Nov;9(4):497–500. doi: 10.1099/00222615-9-4-497. [DOI] [PubMed] [Google Scholar]
- HOENIGER J. F. CELLULAR CHANGES ACCOMPANYING THE SWARMING OF PROTEUS MIRABILIS. I. OBSERVATIONS OF LIVING CULTURES. Can J Microbiol. 1964 Feb;10:1–9. doi: 10.1139/m64-001. [DOI] [PubMed] [Google Scholar]
- HOFFMAN H., FRANK M. E. Form and internal structure of cellular aggregations in early Escherichia coli microcultures. J Gen Microbiol. 1961 Jul;25:353–364. doi: 10.1099/00221287-25-3-353. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A. Scanning electron microscope study of Pseudomonas putida colonies. J Bacteriol. 1985 Dec;164(3):1171–1181. doi: 10.1128/jb.164.3.1171-1181.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. The use of Mudlac transposons as tools for vital staining to visualize clonal and non-clonal patterns of organization in bacterial growth on agar surfaces. J Gen Microbiol. 1984 May;130(5):1169–1181. doi: 10.1099/00221287-130-5-1169. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Sturdza S. A. Expansionserscheinungen der Proteuskulturen. I. Die Schwärmexpansion. Zentralbl Bakteriol Orig A. 1975 Dec;233(4):505–530. [PubMed] [Google Scholar]
- Sturdza S. Expansionserscheinungen der Proteuskulturen II. Die Verschiebungsexpansion. Zentralbl Bakteriol Orig A. 1977 Aug;238(4):444–474. [PubMed] [Google Scholar]
- Williams F. D., Schwarzhoff R. H. Nature of the swarming phenomenon in Proteus. Annu Rev Microbiol. 1978;32:101–122. doi: 10.1146/annurev.mi.32.100178.000533. [DOI] [PubMed] [Google Scholar]