Abstract
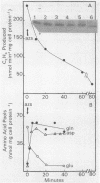
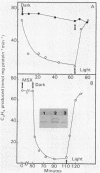
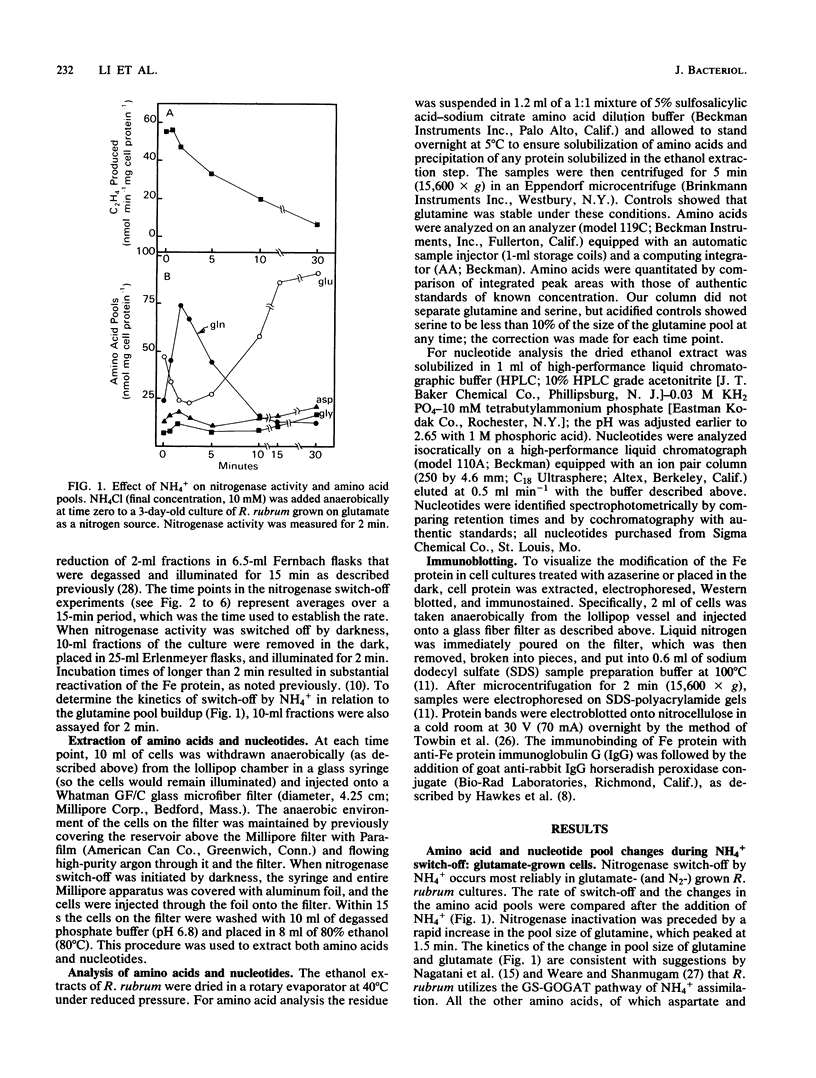
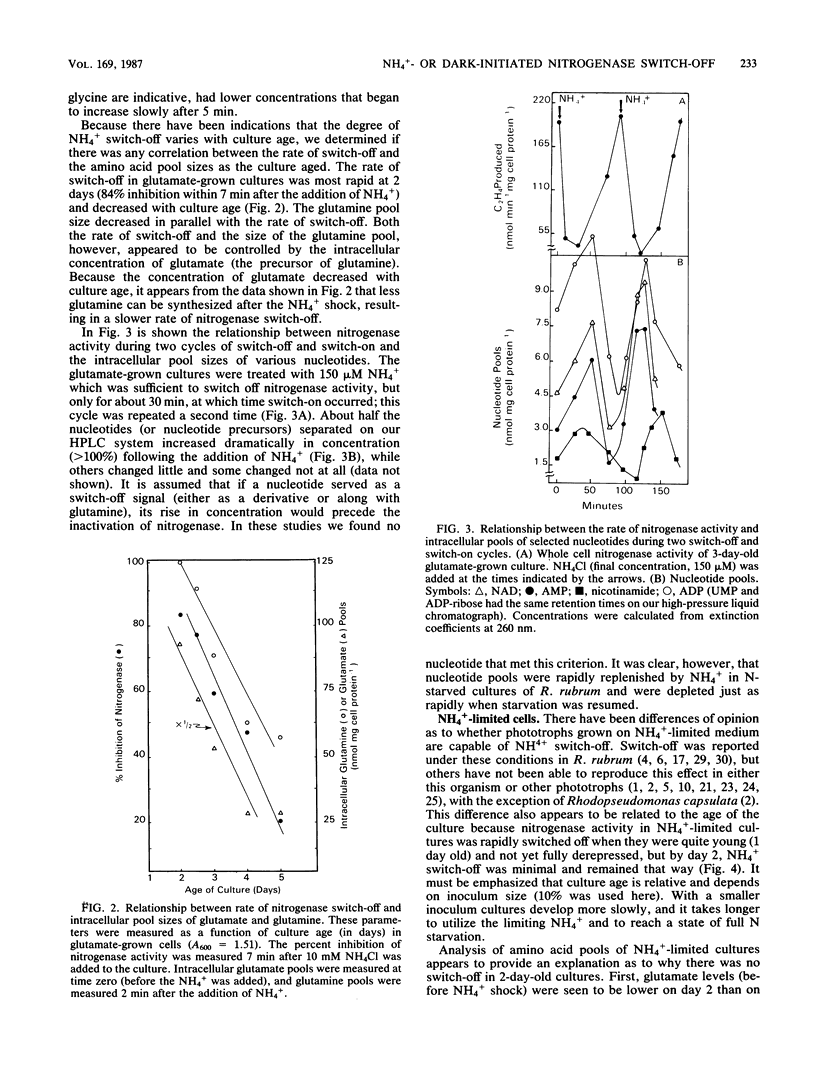
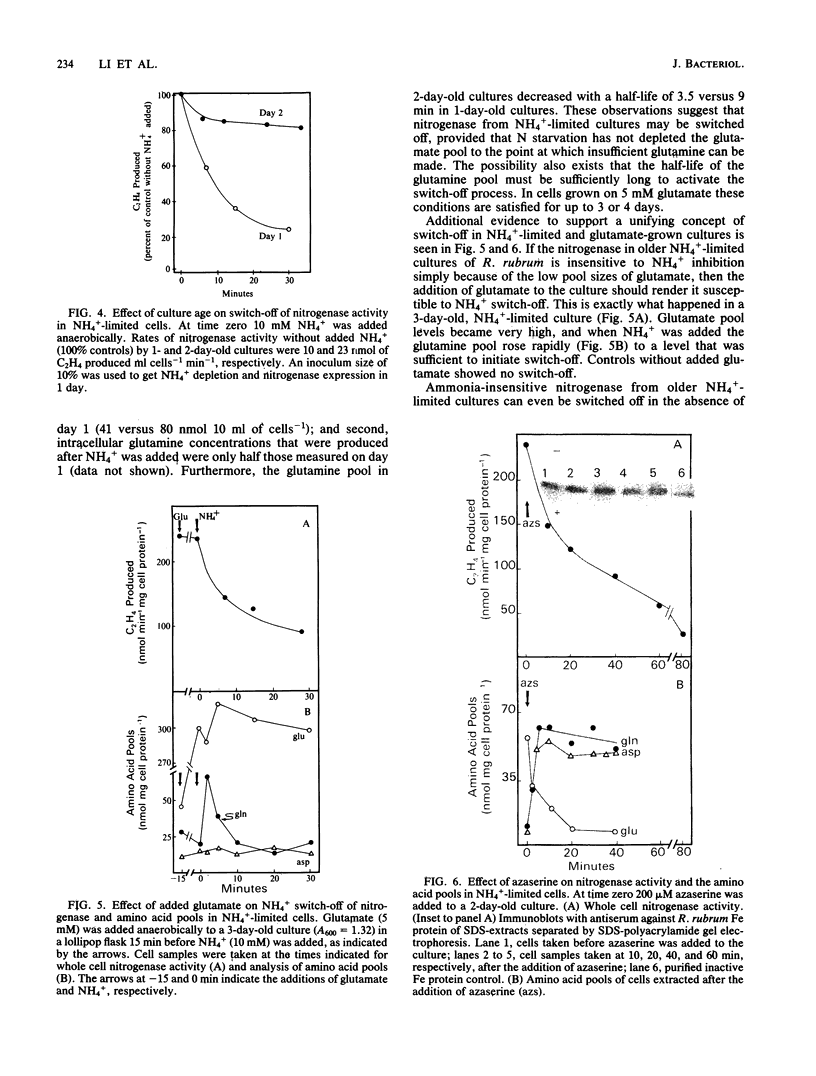
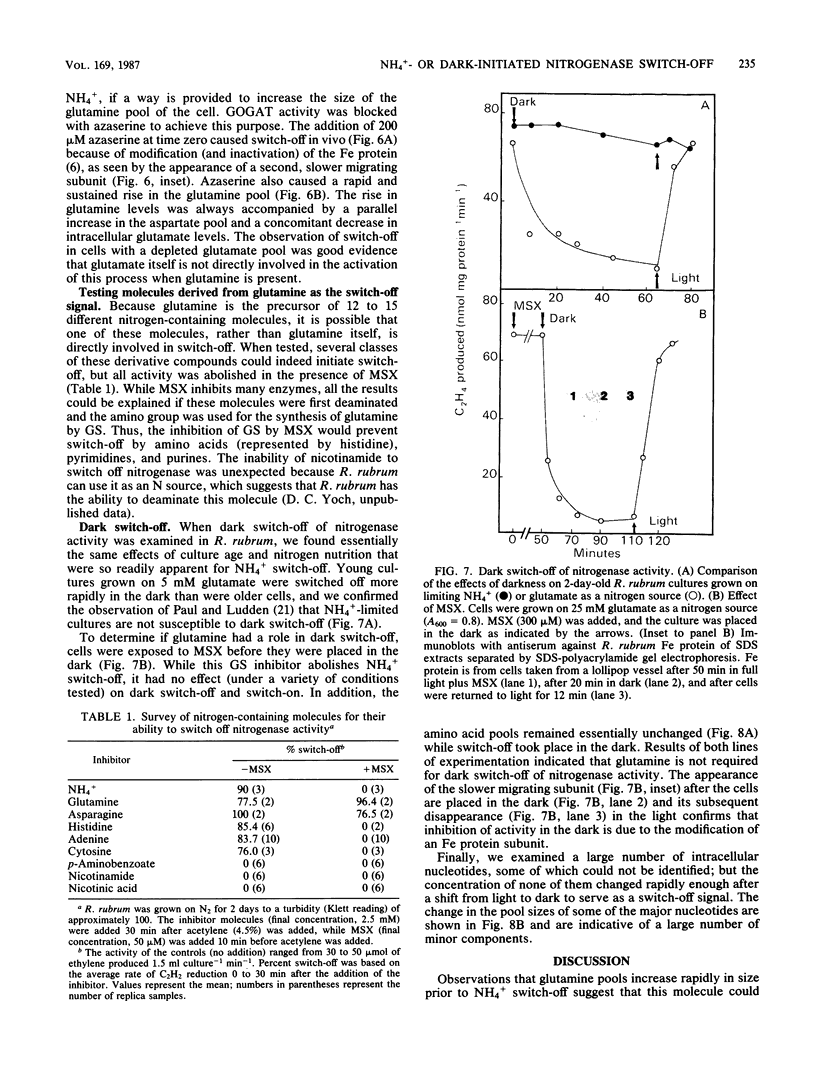
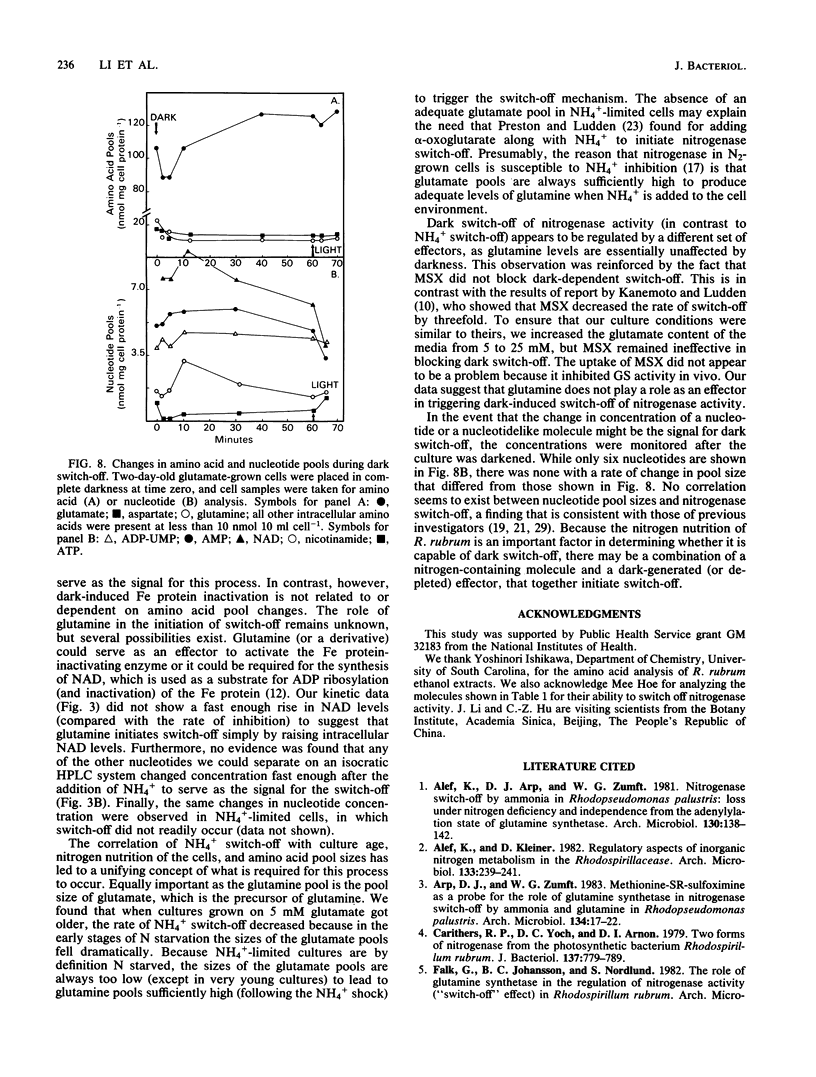
Amino acid and nucleotide pools were measured in nitrogenase-containing Rhodospirillum rubrum cultures during NH4+- or dark-induced inactivation (switch-off) of the Fe protein. A big increase in the glutamine pool size preceded NH4+ switch-off of nitrogenase activity, but the glutamine pool remained unchanged during dark switch-off. Furthermore, methionine sulfoximine had no effect on the rate of dark switch-off, suggesting that glutamine plays no role in this process. In the absence of NH4+ azaserine, an inhibitor of glutamate synthate, raised glutamine pool levels sufficiently to initiate switch-off in vivo. While added NH4+ substantially increased the size of the nucleotide pools in N-limited cells, the kinetics of nucleotide synthesis were all similar and followed (rather than preceded) Fe protein inactivation. Darkness had little effect on nucleotide pool sizes. Glutamate pool sizes were also found to be important in NH4+ switch-off because of the role of this molecule as a glutamine precursor. Much of the diversity reported in the observations on NH4+ switch-off appears to be due to variations in glutamate pool sizes prior to the NH4+ shock. The nitrogen nutritional background is an important factor in determining whether darkness initiates nitrogenase switch-off; however, no link has yet been established between this and NH4+ (glutamine) switch-off.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., Zumft W. G. L-methionine-SR-sulfoximine as a probe for the role of glutamine synthetase in nitrogenase switch-off by ammonia and glutamine in Rhodopseudomonas palustris. Arch Microbiol. 1983 Jan;134(1):17–22. doi: 10.1007/BF00429400. [DOI] [PubMed] [Google Scholar]
- Carithers R. P., Yoch D. C., Arnon D. I. Two forms of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1979 Feb;137(2):779–789. doi: 10.1128/jb.137.2.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotto J. W., Yoch D. C. Regulation of Rhodospirillum rubrum nitrogenase activity. Properties and interconversion of active and inactive Fe protein. J Biol Chem. 1982 Mar 25;257(6):2868–2873. [PubMed] [Google Scholar]
- Hartmann A., Fu H., Burris R. H. Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol. 1986 Mar;165(3):864–870. doi: 10.1128/jb.165.3.864-870.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Jones B. L., Monty K. J. Glutamine as a feedback inhibitor of the Rhodopseudomonas sphaeroides nitrogenase system. J Bacteriol. 1979 Sep;139(3):1007–1013. doi: 10.1128/jb.139.3.1007-1013.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto R. H., Ludden P. W. Effect of ammonia, darkness, and phenazine methosulfate on whole-cell nitrogenase activity and Fe protein modification in Rhodospirillum rubrum. J Bacteriol. 1984 May;158(2):713–720. doi: 10.1128/jb.158.2.713-720.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowery R. G., Saari L. L., Ludden P. W. Reversible regulation of the nitrogenase iron protein from Rhodospirillum rubrum by ADP-ribosylation in vitro. J Bacteriol. 1986 May;166(2):513–518. doi: 10.1128/jb.166.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Activating factor for the iron protein of nitrogenase from Rhodospirillum rubrum. Science. 1976 Oct 22;194(4263):424–426. doi: 10.1126/science.824729. [DOI] [PubMed] [Google Scholar]
- Ludden P. W., Burris R. H. Purification and properties of nitrogenase from Rhodospirillum rubrum, and evidence for phosphate, ribose and an adenine-like unit covalently bound to the iron protein. Biochem J. 1978 Oct 1;175(1):251–259. doi: 10.1042/bj1750251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani H., Shimizu M., Valentine R. C. The mechanism of ammonia assimilation in nitrogen fixing Bacteria. Arch Mikrobiol. 1971;79(2):164–175. doi: 10.1007/BF00424923. [DOI] [PubMed] [Google Scholar]
- Neilson A. H., Nordlund S. Regulation of nitrogenase synthesis in intact cells of Rhodospirillum rubrum: inactivation of nitrogen fixation by ammonia, L-glutamine and L-asparagine. J Gen Microbiol. 1975 Nov;91(1):53–62. doi: 10.1099/00221287-91-1-53. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U., Baltscheffsky H. Properties of the nitrogenase system from a photosynthetic bacterium, Rhodospirillum rubrum. Biochim Biophys Acta. 1978 Nov 9;504(2):248–254. doi: 10.1016/0005-2728(78)90173-1. [DOI] [PubMed] [Google Scholar]
- Nordlund S., Eriksson U. Nitrogenase from Rhodospirillum rubrum. Relation between 'switch-off' effect and the membrane component. Hydrogen production and acetylene reduction with different nitrogenase component ratios. Biochim Biophys Acta. 1979 Sep 11;547(3):429–437. doi: 10.1016/0005-2728(79)90023-9. [DOI] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Paul T. D., Ludden P. W. Adenine nucleotide levels in Rhodospirillum rubrum during switch-off of whole-cell nitrogenase activity. Biochem J. 1984 Dec 15;224(3):961–969. doi: 10.1042/bj2240961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope M. R., Murrell S. A., Ludden P. W. Covalent modification of the iron protein of nitrogenase from Rhodospirillum rubrum by adenosine diphosphoribosylation of a specific arginine residue. Proc Natl Acad Sci U S A. 1985 May;82(10):3173–3177. doi: 10.1073/pnas.82.10.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. G., Ludden P. W. Change in subunit composition of the iron protein of nitrogenase from Rhodospirillum rubrum during activation and inactivation of iron protein. Biochem J. 1982 Sep 1;205(3):489–494. doi: 10.1042/bj2050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet W. J., Burris R. H. Inhibition of nitrogenase activity by NH+4 in Rhodospirillum rubrum. J Bacteriol. 1981 Feb;145(2):824–831. doi: 10.1128/jb.145.2.824-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weare N. M., Shanmugam K. T. Photoproduction of ammonium ion from N2 in Rhodospirillum rubrum. Arch Microbiol. 1976 Nov 2;110(23):207–213. doi: 10.1007/BF00690229. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Cantu M. Changes in the regulatory form of Rhodospirillum rubrum nitrogenase as influenced by nutritional and environmental factors. J Bacteriol. 1980 Jun;142(3):899–907. doi: 10.1128/jb.142.3.899-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Gotto J. W. Effect of light intensity and inhibitors of nitrogen assimilation on NH4+ inhibition of nitrogenase activity in Rhodospirillum rubrum and Anabaena sp. J Bacteriol. 1982 Aug;151(2):800–806. doi: 10.1128/jb.151.2.800-806.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C. Manganese, an essential trace element for N2 fixation by Rhodospirillum rubrum and Rhodopseudomonas capsulata: role in nitrogenase regulation. J Bacteriol. 1979 Dec;140(3):987–995. doi: 10.1128/jb.140.3.987-995.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]