Abstract
Imaging occupies an important role in the investigation of dementia and neurodegenerative disease. The role of imaging in prion disease used to be one of exclusion of other conditions. Over the past decade, the non‐invasive nature of MRI, the improved range of magnetic resonance sequences and the availability of clinical and neuropathological correlation have led to a more prominent position of MRI and its inclusion in the diagnostic criteria for variant Creutzfeldt–Jakob disease. As experience of imaging in human prion disease increases, patterns of change related to strain and genotype may improve the diagnostic potential of imaging in the future, may reduce the need for more invasive testing and prove useful in future therapeutic trials. This paper reviews the current knowledge of imaging appearances in human prion disease.
Imaging occupies an important role in the investigation of dementia and neurodegenerative disease. Early diagnosis of human prion disease is difficult. At present, there is no diagnostic blood test (except in inherited cases) and the definitive diagnostic tests of brain biopsy, or tonsil biopsy in variant Creutzfeldt–Jakob disease (vCJD), are invasive.
The role of imaging in prion disease used to be one of exclusion of other conditions. Over the past decade, the non‐invasive nature of MRI, the improved range of MRI sequences and the availability of clinical and neuropathological correlation have lead to a more prominent position of MRI and its inclusion in the diagnostic criteria for vCJD (table 1). This paper reviews the current knowledge of imaging appearances in human prion disease.
Table 1 Summary of imaging appearances in Creutzfeldt–Jakob disease.
| Type of CJD | MRI part of diagnostic criteria? | Characteristic appearances | Most useful sequence | Key references |
|---|---|---|---|---|
| Variant | Yes | Pulvinar or hockey stick sign | FLAIR is the most sensitive sequence, DWI sensitivity data limited | World Health Organization,9 Zeidler,10 Collie11 |
| Sporadic | No | Increased cortical and/or basal ganglia signal | DWI is most sensitive clinical diagnostic test. | Tschampa,25 Shiga26 |
| Inherited | No | Cortical/cerebellar atrophy, decreased basal ganglia signal or no change | Little evidence to suggest most useful sequences | Zerr,35, Almer,37 Arata38 |
| Iatrogenic | No | Caudate/putamen hyperintensity; only atrophy seen in dural recipients. Pulvinar sign if donor had vCJD | Little evidence to suggest most useful sequences | Brown,40 Caboclo,41 Oppenheim,42 Wakisaka,43 Kretzschmar,44 Preusser,45 Martinez‐Lage,46 Garcia Santos,47 Rabinstein,48 Llewelyn,49 Peden,50 Wroe51, Collie DA, (personal communication) |
DWI, diffusion weighted imaging; FLAIR, fluid attenuated inversion recovery imaging; vCJD, variant Creutzfeldt–Jakob disease.
Findings in the different forms of human prion disease
Human prion diseases are uniformly fatal, progressive neurodegenerative disorders. These transmissible diseases are associated with an abnormal form of the human prion protein. In addition to abnormal prion protein staining, neuropathological examination is characterised by gliosis, spongiosis and neuronal loss. The most common form of human prion disease is sporadic CJD (sCJD) with an incidence worldwide of 1–2 per million per annum. Inherited prion diseases are a group of autosomal dominantly inherited conditions with high penetrance. Acquired human prion disease includes vCJD, seen mainly in the UK associated with exposure to bovine spongiform encephalopathy prions, iatrogenic CJD, caused by infection from medical or surgical procedures, and Kuru, caused by exposure to human prions via endocannibalism. Kuru is seen only in Papua New Guinea and neuroimaging findings have not been described.
Variant Creutzfeldt–Jakob disease
vCJD was described in 1996,1 and is causally linked to bovine spongiform encephalopathy.2 The transmissible prion agent has a characteristic strain type on western blotting. The characteristic clinical features so far reported in cases of vCJD differ from those of other prion diseases. vCJD predominantly affects a young age group with a median age of presentation of 26 (range 12–74) years,3 occurring in males and females equally.4 There are early psychiatric and behavioural symptoms and in half concurrent sensory symptoms such as limb pain and dysaesthesia occur. As the disease progresses, neurological features become more prominent, particularly ataxia and cognitive impairment. The disease has a median duration of 13 (range 6–39) months.3
Initially the MRI scans of vCJD were reported as being normal.5 The first two case reports of pulvinar change were published in 1996.6,7 The pulvinar sign is now part of the World Health Organization (WHO) diagnostic criteria for vCJD.8 The current WHO definition of the pulvinar sign is bilateral symmetrical pulvinar high signal relative to the signal intensity of other deep grey matter nuclei and cortical grey matter using T2 weighted imaging (T2WI), proton density weighted, fluid attenuated inversion recovery (FLAIR) and axial diffusion weighted imaging (DWI) sequences.9 This, and the “hockey stick” sign of high signal in both pulvinar and dorsomedial thalamic nuclei was described in 2000.10 As yet there is no evidence that the pulvinar sign could be used as a presymptomatic test for vCJD prion infection as scanning has only been carried out in symptomatic patients.
In a study of 36 neuropathologically confirmed vCJD cases compared with 57 controls with suspected CJD, the pulvinar sign had a sensitivity of 78% and specificity of 100%.10 T2WI and proton density sequences on the most recent set of scans available on each patient were used. In a retrospective study of MRI in 86 neuropathologically confirmed cases of vCJD, the most sensitive sequence was FLAIR11 where the pulvinar sign was present in 100% of the 30 FLAIR scans performed. In a further study of 27 vCJD cases confirmed with tonsil biopsy compared with 18 tonsil biopsy negative patients, retrospective blinded analysis of T2WI, DWI, FLAIR and proton density weighted images was performed on all images, solely to assess the presence or absence of the pulvinar sign. A sensitivity of 81% and a specificity of 94% for the pulvinar sign was found.12 High signal in the pulvinar nucleus has been described in sCJD,13,14,15 and in two reports16,17 the WHO criteria were fulfilled9 with pulvinar intensity being higher than other deep grey matter nuclei. There are two reports of an initially positive pulvinar sign disappearing as disease progresses.11,18 Other conditions such as paraneoplastic limbic encephalitis may initially present in a similar way.19
vCJD has the most consistent changes on MRI of any human prion disease type, perhaps representing the fact that only one molecular strain of human prion disease is seen in these patients.2 This consistency is useful as the pulvinar sign can often be identified in scans despite movement artefact. Hyperintensity of other structures in vCJD is seen, which varies between patient and sequence weighting. In particular, dorsomedial thalamic nuclei, periaqueductal grey matter and caudate hyperintensity are seen. Occasionally parieto‐occipital white matter and asymmetric pulvinar hyperintensity is seen, or cerebral and cerebellar atrophy.11
Single photon emission computed tomography (SPECT) has been used in two cases. Areas of cortical hypoperfusion were seen (left temporoparietal in one, widespread reduction in cortical perfusion with cerebellar and basal ganglia reduction in the second). SPECT changes were seen before changes on MRI or on EEG.20 Magnetic resonance spectroscopy may be useful as a measure of disease activity, monitoring of treatment effects21 and a future tool in early diagnosis.22
Sporadic Creutzfeldt–Jakob disease
sCJD has an incidence of 1–2 per million in the worldwide population. The median age of onset is 65 years, its clinical course thereafter being rapid with a median duration of survival of 4 months.23 The clinical phenotype of sCJD is typically one of rapidly progressive dementia and multifocal neurological features, including myoclonus, ataxia, pyramidal and extrapyramidal signs. The host codon 129 genotype and the molecular strain of the transmissible prion agent affect this phenotype.2
Historically, imaging features were not part of the diagnostic criteria for sCJD, which relied more on clinical, neurohistopathological, EEG and CSF features. There is, however, growing support for MRI, and in particular DWI to be included in the diagnostic work up for sCJD24,25,26 (fig 1A–C).
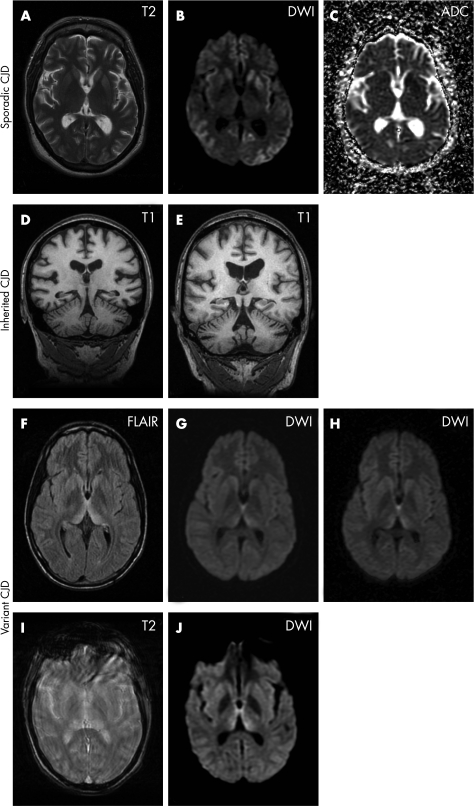
Figure 1 (A–C) A 56‐year‐old female patient with sporadic Creutzfeldt–Jakob disease (CJD). (A) Axial T2 weighted image shows a subtle increase in signal intensity of the left putamen anteriorly. The remainder of the basal ganglia and cortex appear normal. (B) Axial diffusion weighted image (DWI), acquired with b = 1000, shows hyperintense signal in the heads of both caudate nuclei and in both putamina (on the left more than on the right). In addition, there are gyriform areas of hyperintensity in both the insulae and in the frontal temporal and occipital cortices. (C) A map of the apparent diffusion coefficient (ADC) shows reduced signal in the areas which appear hyperintense on the DWI image (B), confirming that the diffusion of water is restricted in these regions. (D–E) Coronal T1 weighted images of a volumetric MRI study in two cases with inherited CJD. (D) A 39‐year‐old female patient with 6‐octapeptide repeat insertion. The MRI shows cerebellar atrophy with widening of the cerebellar fissures and supratentorial atrophy with enlarged lateral ventricles and very prominent Sylvian fissures. (E) A 57‐year‐old male patient with a P102L mutation. There is marked cerebellar atrophy with pronounced enlargement of the horizontal cerebellar fissure and fourth ventricle. There is also supratentorial atrophy, mostly of the central type with ventricular enlargement. (F–H) An 18‐year‐old patient with variant CJD. (F) Axial fluid attenuated inversion recovery image (FLAIR) acquired 7 months after the onset of symptoms demonstrates hyperintensity in the dorsomedial thalamic nuclei and pulvinar bilaterally (“hockey stick sign”). (G) DWI images (b = 1000) acquired at the same time as (F) also show hyperintense signal change. It is, however, less marked than on the FLAIR images, appears more linear and affects predominantly the dorsomedial nuclei. (H) DWI acquired 1 month after (G) demonstrates progression of the signal change in the thalami, which now more obviously involves the pulvinar. At this stage of the disease, both the T2 weighted and FLAIR images were degraded by patient movement. (I–J) A 25‐year‐old patient with variant CJD 7 months after onset of symptoms. (I) The axial T2 weighted image is severely degraded by patient movement and of limited diagnostic value. (J) The axial DWI image (b = 1000). The patient managed to keep still during the acquisition of this sequence which is diagnostic and shows hyperintense signal in the dorsomedial thalamic nuclei and pulvinar, relative to the signal intensity of the putamina.
Traditionally, the recognised features of sCJD on MRI are bilateral high signal in the caudate and putamen.25,27 Recent publications have additionally highlighted the importance of cortical ribbon hyperintensity.26 Shiga and colleagues26 described 26 cases of sCJD (a mix of definite and probable cases); 41.7% showed only cortical lesions, 45.8% showed lesions in both the cortex and basal ganglia, and 12.5% lesions only in the basal ganglia. Sensitivity of DWI was 92.3%, significantly more than T2WI (40%) or FLAIR (50%).
Patterns emerging from work on sCJD subtype analysis include consistent hyperintensity in the caudate and putamen in codon 129 heterozygotes using T2WI. All 26 heterozygotes in the study of Meissner and colleagues,28 90% of 20 MV2 patients in the study of Krasnianski and colleagues,17 and 10 of 11 heterozygotes in Zerr et al's study29 showed this pattern. Methionine homozygotes form 71% of the sCJD population30 but patterns of MRI change are less clear. In a study of MM2 patients, 5 of 8 had no abnormality on any MRI sequence31 but newer imaging techniques such as SPECT may be useful in identifying patterns in these patients.32 In seven VV1 patients (a subtype that mimics vCJD with young onset, prolonged disease course and psychiatric symptoms), all had cortical high signal, atypical of vCJD.33 (Molecular subtypes quoted refer to the Gambetti classification.)
Inherited prion disease
Inherited prion disease comprises approximately 15% of human prion disease, and is caused by point mutations (such as P102L, P105L, E200K) or insertions (such as the 144 base pair octapeptide repeat insertion) in the prion protein gene; the clinicopathological spectrum is also affected by polymorphisms at codon 129 of the prion protein gene.2 Progressive ataxia or cognitive decline with a disease course of several years often occurs but presentation is heterogeneous and depends on the mutation.34 In the early reports of inherited prion disease, imaging was often not performed.
Imaging in inherited prion disease has a role in excluding other diseases (both prion and non‐prion) but changes are non‐specific. Imaging appearances in symptomatic patients fall broadly into four categories: no change,34,35 cortical atrophy, cerebellar atrophy (fig 1D–E) or decreased T2 signal in the basal ganglia.35,36,37 Atrophic changes can be progressive.36,38 Presymptomatic P102L gene carriers have shown early parietal atrophy on MRI,39 and in symptomatic P102L patients with normal MRI examinations, 123I SPECT imaging showed patchy decreases across the whole cerebrum.38 In two series of patients with familial fatal insomnia (D178N mutation), MRI did not show any specific abnormality,35 or at most mild to moderate atrophy.37
Iatrogenic prion disease
Over 260 cases of iatrogenic prion disease have been reported worldwide.40 Iatrogenic prion disease is caused largely by infection from cadaveric human growth hormone, dura mater grafts manufactured and distributed before the mid‐1980s or corneal grafts. Incubation periods range from months to up to 30 years. In peripherally inoculated cases, cerebellar presentation rather than dementia predominates.
The type of donor material and route of inoculation seem to produce different imaging patterns in recipients. In the largest and most recent imaging series, an Anglo–French cohort of 27 human growth hormone recipients (and one dural graft recipient) reported bilateral symmetrical hyperintensity of the caudate head and putamen in 64% of patients, with DWI changes occurring before T2WI changes (Collie DA. MRI of iatrogenic Creutzfeldt–Jakob disease: appearance in an Anglo–French cohort. Presented at the British Society of Neuroradiologists Conference 2003, personal communication). Caboclo et al and Oppenheim et al also reported caudate and putamen hyperintensity in three growth hormone recipients on FLAIR, DWI and T2 imaging,41,42 with additional thalamic hyperintensity on DWI in two cases. Serial scanning showed progressive atrophy with reduction and eventual disappearance of the FLAIR and DWI changes, a similar finding to pulvinar sign disappearance in the late stages of vCJD mentioned previously.
Basal ganglia hyperintensity has been reported in case reports of dura mater recipients,43,44,45 as well as a case of thalamic hyperintensity in end stage disease.43 In 11 cases reported or reviewed by Martinez‐Lage et al and Garcia Santos et al, atrophy was present, which was progressive in some cases.46,47
In the single corneal graft derived case with MRI findings, caudate and putamen hyperintensity was present but FLAIR and DWI also showed cortical hyperintensity.48
Prion infection has been reported in three patients following blood transfusion from asymptomatic donors who later developed vCJD.49,50,51 In two of these cases infection was identified post mortem and one of these was said to be symptomatic with a normal MRI. In a single case diagnosed ante mortem, MRI was normal during the early symptomatic clinical disease course, the pulvinar sign becoming apparent with more advanced clinical disease and significant neurological disability.
Findings on specific imaging modalities
Computerised tomography
CT has been used historically to exclude a focal lesion in the investigation of a patient with dementia. Currently, CT has no place in the routine imaging investigation of human prion disease, having been replaced by MRI.
The early literature on the imaging of human prion disease revealed that CT showed atrophy, with only one report of focal parenchymal abnormality.52 Thirty‐three cases were reported up to 1992 and more recent reports have added no further insights into CT appearances. In the largest single study of 15 patients, 80% had normal scans and 20% had atrophy.53
MRI–T2 weighted imaging
Standard T2WI can be useful in all types of human prion disease. The recognised features of both sCJD (high signal in the cortex, caudate and putamen) and vCJD (the pulvinar sign) on MRI may be demonstrated on T2WI. The basal ganglia, cerebellar and atrophic changes of inherited prion disease and the hyperintensity of the caudate head and putamen in iatrogenic prion disease are also seen in T2WI. Generally, other sequences such as DWI or FLAIR tend to be more sensitive than T2WI.
The mechanism of signal change has been investigated by Chung et al who found that T2 hyperintensity correlated with gliosis and hypointensity with vacuolation in an in vivo hamster scrapie model.54
MRI–fluid attenuated inversion recovery imaging
On FLAIR images the signal from the CSF is suppressed while maintaining a heavy T2 weighting. This results in high lesion contrast in areas close to CSF, and allows better visualisation of lesions in these areas than conventional T2WI. The widespread use of this modality began in the early 1990s, and has been successfully applied to all types of human prion disease.
The FLAIR sequence is more sensitive than either T2WI or DWI for detection of the pulvinar sign in vCJD although DWI sensitivity data are limited (fig 1F–H).11 FLAIR imaging shows a pathological signal intensity increase in the cerebral cortex (termed “cortical ribboning”) and basal ganglia changes more clearly than T2WI.55,56 Increased signal intensity in the cortical ribbon on FLAIR has been colocalised to periodic sharp waves on EEG.57 Increased FLAIR signal corresponded to increased DWI signal intensity in the basal ganglia58 and cortex.58,59,60
The mechanism of signal change in FLAIR images continues to be debated. Astrogliosis was reported to be the cause of high FLAIR signal in one paper61 and another demonstrated a strong correlation between FLAIR signal and accumulation of prion protein (PrPsc) but did not find any correlation between gliosis or spongiform change on FLAIR.58
Work on the quantification of intensity distributions in the basal ganglia using multimodal T1, T2 and FLAIR has differentiated between sCJD and vCJD and has potential for automatic detection of CJD on MRI.62
MRI–diffusion weighted imaging
DWI assesses the mobility of water molecules in tissue and provides physiological information not available on standard MRI. Cellular swelling and other microstructural changes lead to an alteration of the water mobility which becomes apparent on DWI. The spatial resolution of DWI is not as high as that of conventional MRI but it is a rapid imaging technique which represents an advantage in patients with movement disorders. Echoplanar DWI can be acquired within seconds and may be the only sequence yielding diagnostic information in confused and agitated patients where standard MRI sequences are severely degraded by movement artefact (fig 1I, J).63 Although some anatomical detail may be lost, the abnormalities seen in human prion disease can be striking.24 Positive scans show ribbon‐like abnormalities in the cerebral cortex and/or hyperintensity in the caudate and putamen or thalamus. DWI may be the only positive investigation in human prion disease,26,64 may facilitate an early diagnosis as changes have been noted as early as 3 weeks after the onset of symptoms26 and may be used to monitor disease progression.59,65,66,67
DWI abnormalities have been noted in all types of human prion disease. DWI may be the most sensitive imaging modality for sCJD.25,65,68 Some inherited cases show hyperintensity in the basal ganglia and frontoparietal cortices, similar to sporadic cases.69 vCJD shows bilateral hypersignal in the striatum and thalami.70 Caudate and anterior putamen high signal on DWI in iatrogenic CJD is seen before T2 weighted imaging changes (Collie DA, personal communication).
Serial DWI has shown increased intensity and extension of hyperintense signal with disease progression.67,71 The most likely pathophysiological explanation of the DWI changes is altered molecular motion of water and restriction of extracellular space due to spongiform degeneration.68 Other proposed causes include deposition of prion protein,58,72 fibrillary gliosis seen in end stage CJD73 and progressive neuronal death.74 Interestingly, in several studies on serial changes in DWI, initial hyperintensity disappeared in the late stages of disease (Collie DA, personal communication).66,67,74
The apparent diffusion coefficient (ADC) is calculated from two or more images with different degrees of diffusion weighting. It allows quantification of regional water diffusion and is independent of T2 effects. ADC measurements in human prion disease have been performed by several investigators.63,65,68,70,71,75,76 In sCJD most investigators found that ADC values in lesions were lower than in normal brain parenchyma71,72,75 with the exception of one.65 No correlation has been found between reduction in ADC and semiquantitative neuropathological change.71 In a recent paper, thalamic changes in sCJD were only detected by quantitative measurements of ADCs, and were not visible on DWI or T2WI.77 In vCJD, early reports on ADC measurement were conflicting.24,65
High intensity areas on DWI have been correlated with low perfusion on SPECT,72 hypometabolism on positron emission tomography (PET),65,72 clinical findings,72 disease progression,60,71 neuropathology showing spongiform change,64 neuronal loss and gliosis,71 and periodic sharp wave activity on EEG.60,72,76
Magnetic resonance spectroscopy
Proton magnetic resonance spectroscopy (MRS) assesses the spectra of specific brain metabolites such as N‐acetylaspartate (NAA), a decrease in which can serve as a marker of neuronal loss. Decreases in absolute levels or ratios of NAA have been seen in variant,21,22 sporadic,21 iatrogenic74 and inherited CJD.78 The first report of MRS dates from 1991.79 Early reports found little change in NAA in the first stages of disease but at the late stage of CJD when neuronal loss is more obvious neuropathologically, decreases in grey and white matter were seen.79,80 The pattern of metabolite change matches the findings on T2 and FLAIR imaging, with significant reductions in absolute levels or ratios of NAA in the putamen in sporadic patients and in the pulvinar in variant patients.21,22
Positron emission tomography and single photon emission computed tomography
Generally, 2‐(18F)fluorodeoxyglucose (FDG) PET shows widespread hypometabolism in human prion disease, which differentiates it from other dementias such as Alzheimer's disease which show specific patterns on PET.81,82 Hypometabolism on FDG PET has been correlated with histopathological astrocytosis and neuronal death,82,83 and spongiform change.82
In a multitracer PET study83 of 15 patients with probable or definite CJD, changes in regional cerebral blood flow (using oxygen‐15 labelled water), regional cerebral glucose metabolism (FDG) and astrocytosis (using the monoamine oxidase B inhibitor N‐[11C‐methyl]‐L‐deuterodeprenyl) were detected. Hypometabolism was more prominent in the cerebellum and frontal, occipital and parietal lobes than in the thalamus, pons or putamen, which differs from the distribution pattern seen on MRI studies. However, PET seems particularly good at identifying occipital lobe hypometabolism in patients with visual symptoms, including the Heidenhain variant.81,84
Future advances in PET include creating an in vivo probe to label prion plaques, similar to that successfully developed for amyloid plaques in Alzheimer's disease.85 An in vivo method in presymptomatic and symptomatic mice using the ligand methoxy‐X04 has been successful86 as has an in vitro method using fixed sections from patients with sCJD, vCJD and Gerstmann‐Straussler‐Scheinker syndrome (GSS).87
Regions of decreased cerebral blood flow have been demonstrated using SPECT in variant, iatrogenic, familial and sporadic CJD. In the largest series of 19 definite or probable sporadic CJD patients using 99mTc‐HMPAO‐SPECT or 99mTc‐ECD‐SPECT, 89% showed hypoperfusion on SPECT examination, the majority showing widespread reduction in cerebral perfusion, including the occipital lobes, cerebellum or one whole hemisphere.88 As in PET, this pattern of change is different from other types of dementia. SPECT has been reported to be useful in a rare subtype of sCJD with codon 129 methionine homozygosity and a thalamic neuropathological pattern. All four patients showed reduction in cerebral blood flow as well as hypometabolism bilaterally in the thalamus, with preservation in the putamen.32 Regional changes in blood flow (as seen by decreased uptake of tracer, either 99mTc‐HMPAO, 123I‐IMP or Xe‐133 with and without acetazolamide) are seen at earlier stages of disease than MRI changes.20,38,89,90,91 Focal areas of decreased uptake may be seen in the basal ganglia,76 thalamus,32 cerebellum88 and frontotemporal lobes,38,89 or occipital lobes in Heidenhain variant patients,90,91 and inherited patients.38 Sometimes changes are seen in an asymmetric pattern.88,89
At present it remains unclear whether the perfusion changes seen on PET and SPECT could also be investigated with magnetic resonance perfusion imaging. Given the good correlation of SPECT data with magnetic resonance perfusion imaging in neurovascular diseases and tumour imaging, it appears likely that magnetic resonance perfusion imaging might also be useful in human prion disease.
Conclusion
The role of imaging in human prion disease continues to evolve as knowledge of different types of disease and their imaging patterns increases. Other than the pulvinar sign in vCJD, it remains to be seen whether MRI can distinguish human infection with other CJD strain types.
Future research will be directed towards finding the earliest point in the disease course where imaging changes are evident. Quantitative techniques such as DWI, diffusion tensor imaging and volumetric imaging may be of use, firstly in presymptomatic patients (either inherited or exposed to infectious prions) to help predict the onset of clinical disease, and secondly in symptomatic patients measuring the response to treatment in therapeutic trials. As current and future MRI sequences evolve, those showing the earliest changes, with the highest sensitivity and specificity, should be prioritised in diagnostic imaging.
Acknowledgements
We thank Ray Young for his assistance in producing the figure.
Abbreviations
ADC - apparent diffusion coefficient
DWI - diffusion weighted imaging
FDG - 2‐(18F)fluorodeoxyglucose
FLAIR - fluid attenuated inversion recovery imaging
MRS - magnetic resonance spectroscopy
NAA - N‐acetylaspartate
PET - positron emission tomography
sCJD - sporadic Creutzfeldt–Jakob disease
SPECT - single photon emission computed tomography
T2WI - T2 weighted imaging
vCJD - variant Creutzfeldt–Jakob disease
Footnotes
Competing interests: None.
References
- 1.Will R G, Ironside J W, Zeidler M.et al A new variant of Creutzfeldt–Jakob disease in the UK. Lancet 1996347921–925. [DOI] [PubMed] [Google Scholar]
- 2.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Ann Rev Neurosci 200124519–550. [DOI] [PubMed] [Google Scholar]
- 3.Spencer M D, Knight R S, Will R G. First hundred cases of variant Creutzfeldt–Jakob disease: retrospective case note review of early psychiatric and neurological features. BMJ 20023241479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry C, Knight R. Clinical features of variant Creutzfeldt–Jakob disease. Rev Med Virol 200212143–150. [DOI] [PubMed] [Google Scholar]
- 5.Zeidler M, Stewart G E, Barraclough C R.et al New variant Creutzfeldt–Jakob disease: neurological features and diagnostic tests. Lancet 1997350903–907. [DOI] [PubMed] [Google Scholar]
- 6.Chazot G, Broussolle E, Lapras C I.et al New variant of Creutzfeldt–Jakob disease in a 26‐year‐old French man. Lancet 19963471181. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizi S J, Scaravilli F, Howard R S.et al Grand Round. Creutzfeldt–Jakob disease in a young woman. Report of a Meeting of Physicians and Scientists, St Thomas' Hospital, London. Lancet 1996347945–948. [PubMed] [Google Scholar]
- 8.Will R G, Zeidler M, Stewart G E.et al Diagnosis of new variant Creutzfeldt–Jakob disease. Ann Neurol 200047575–582. [PubMed] [Google Scholar]
- 9.World Health Organization The Revision of the Surveillance Case Definition for Variant Creutzfeldt‐Jakob Disease: Report of a WHO Consultation, Edinburgh. World Health Organ Tech Rep Ser 2004. (vCJD)
- 10.Zeidler M, Sellar R J, Collie D A.et al The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt–Jakob disease. Lancet 20003551412–1418. [DOI] [PubMed] [Google Scholar]
- 11.Collie D A, Summers D M, Sellar R J.et al Diagnosing variant Creutzfeldt‐Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. Am J Neuroradiol 2003241560–1569. [PMC free article] [PubMed] [Google Scholar]
- 12.Siddique D, Kennedy A, Thomas D.et al Tonsil biopsy in the investigation of suspected variant Creutzfeldt–Jakob disease—A cohort study of 50 patients . J Neurol Sci 2005238(Suppl 1)S52 [Google Scholar]
- 13.Martindale J, Geschwind M D, De Armond S.et al Sporadic Creutzfeldt–Jakob disease mimicking variant Creutzfeldt–Jakob disease. Arch Neurol 200360767–770. [DOI] [PubMed] [Google Scholar]
- 14.Haik S, Brandel J P, Oppenheim C.et al Sporadic CJD clinically mimicking variant CJD with bilateral increased signal in the pulvinar. Neurology 200258148–149. [DOI] [PubMed] [Google Scholar]
- 15.Rossetti A O, Glatzel M, Aguzzi A.et al Clinical and radiological mimicry of vCJD in a valine homozygous PrP(Sc) type 1 sCJD patient. J Neurol 2003250491–493. [DOI] [PubMed] [Google Scholar]
- 16.Petzold G C, Westner I, Bohner G.et al False‐positive pulvinar sign on MRI in sporadic Creutzfeldt–Jakob disease. Neurology 2004621235–1236. [DOI] [PubMed] [Google Scholar]
- 17.Krasnianski A, Schulz‐Schaeffer W J, Kallenberg K.et al Clinical findings and diagnostic tests in the MV2 subtype of sporadic CJD. Brain Epub ahead of print 23 May 2006 (doi:10.1093/brain/awl123) [DOI] [PubMed]
- 18.Yamada M, Variant CJD Working Group, Creutzfeldt–Jakob Disease Surveillance Committee, Japan The first Japanese case of variant Creutzfeldt–Jakob disease showing periodic electroencephalogram. Lancet 2006367874. [DOI] [PubMed] [Google Scholar]
- 19.Mihara M, Sugase S, Konaka K.et al The “pulvinar sign” in a case of paraneoplastic limbic encephalitis associated with non‐Hodgkin's lymphoma. J Neurol Neurosurg Psychiatry 200576882–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva R, Patterson J, Hadley D.et al Single photon emission computed tomography in the identification of new variant Creutzfeldt–Jakob disease: case reports. BMJ 1998316593–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandya H G, Coley S C, Wilkinson I D.et al Magnetic resonance spectroscopic abnormalities in sporadic and variant Creutzfeldt‐Jakob disease. Clin Radiol 200358148–153. [DOI] [PubMed] [Google Scholar]
- 22.Cordery R J, Macmanus D, Godbolt A.et al Short TE quantitative proton magnetic resonance spectroscopy in variant Creutzfeldt–Jakob disease. Eur Radiol Epub ahead of print Jan 12 2006 (doi: 10. 1007/s00330‐005‐0090‐4) [DOI] [PubMed]
- 23.Will R G, Matthews W B. A retrospective study of Creutzfeldt–Jakob disease in England and Wales 1970–79 I: Clinical features. J Neurol Neurosurg Psychiatry 198447134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collie D A, Sellar R J, Zeidler M.et al MRI of Creutzfeldt–Jakob disease: Imaging features and recommended MRI protocol. Clin Radiol 200156726–739. [DOI] [PubMed] [Google Scholar]
- 25.Tschampa H J, Kallenberg K, Urbach H.et al MRI in the diagnosis of sporadic Creutzfeldt–Jakob disease: a study on inter‐observer agreement. Brain 20051282026–2033. [DOI] [PubMed] [Google Scholar]
- 26.Shiga Y, Miyazawa K, Sato S.et al Diffusion‐weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt–Jakob disease. Neurology 200463443–449. [DOI] [PubMed] [Google Scholar]
- 27.Schroter A, Zerr I, Henkel K.et al Magnetic resonance imaging in the clinical diagnosis of Creutzfeldt–Jakob disease. Arch Neurol 2000571751–1757. [DOI] [PubMed] [Google Scholar]
- 28.Meissner B, Kortner K, Bartl M.et al Sporadic Creutzfeldt–Jakob disease: magnetic resonance imaging and clinical findings. Neurology 200463450–456. [DOI] [PubMed] [Google Scholar]
- 29.Zerr I, Schulz‐Schaeffer W J, Giese A.et al Current clinical diagnosis in Creutzfeldt–Jakob disease: identification of uncommon variants. Ann Neurol 200048323–329. [PubMed] [Google Scholar]
- 30.Parchi P, Giese A, Capellari S.et al Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 199946224–233. [PubMed] [Google Scholar]
- 31.Krasnianski A, Meissner B, Schulz‐Schaeffer W.et al Clinical features and diagnosis of the MM2 cortical subtype of sporadic Creutzfeldt–Jakob disease. Arch Neurol 200663876–880. [DOI] [PubMed] [Google Scholar]
- 32.Hamaguchi T, Kitamoto T, Sato T.et al Clinical diagnosis of MM2‐type sporadic Creutzfeldt–Jakob disease. Neurology 200564643–648. [DOI] [PubMed] [Google Scholar]
- 33.Meissner B, Westner I M, Kallenberg K.et al Sporadic Creutzfeldt–Jakob disease: clinical and diagnostic characteristics of the rare VV1 type. Neurology 2005651544–1550. [DOI] [PubMed] [Google Scholar]
- 34.Collinge J. Human prion diseases: etiology and clinical features. In: Growden JH, Rossor MN, eds. The Dementias. Boston: Butterworth‐Heinemann, 1998113–150.
- 35.Zerr I, Giese A, Windl O.et al Phenotypic variability in fatal familial insomnia (D178N‐129M) genotype. Neurology 1998511398–1405. [DOI] [PubMed] [Google Scholar]
- 36.Wimberger D, Uranitsch K, Schindler E.et al Gerstmann–Sträussler–Scheinker syndrome: MR findings. J Comput Assist Tomogr 199317326–327. [DOI] [PubMed] [Google Scholar]
- 37.Almer G, Hainfellner H A, Jellinger K.et al Fatal familial insomnia: a new Austrian family. Brain 19991225–16. [DOI] [PubMed] [Google Scholar]
- 38.Arata H, Takashima H, Hirano R.et al Early clinical signs and imaging findings in Gerstmann–Straussler–Scheinker syndrome (Pro102Leu). Neurology 2006661672–1678. [DOI] [PubMed] [Google Scholar]
- 39.Fox N C, Freeborough P A, Mekkaoui K F.et al Cerebral and cerebellar atrophy on serial magnetic resonance imaging in an initially symptom free subject at risk of familial prion disease. BMJ 1997315856–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown P, Preece M, Brandel J P.et al Iatrogenic Creutzfeldt–Jakob disease at the millennium. Neurology 2000551075–1081. [DOI] [PubMed] [Google Scholar]
- 41.Caboclo L O, Huang N, Lepski G A.et al Iatrogenic Creutzfeldt–Jakob disease following human growth hormone therapy: case report. Arq Neuropsiquiatr 200260(2‐B)458–461. [DOI] [PubMed] [Google Scholar]
- 42.Oppenheim C, Zuber M, Galanaud D.et al Spectroscopy and serial diffusion MR findings in hGH‐Creutzfeldt–Jakob disease. J Neurol Neurosurg Psychiatry 2004751066–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakisaka Y, Santa N, Doh‐ura K.et al Increased asymmetric pulvinar magnetic resonance imaging signals in Creutzfeldt–Jakob disease with florid plaques following a cadaveric dura mater graft. Neuropathology 20062682–88. [DOI] [PubMed] [Google Scholar]
- 44.Kretzschmar H A, Sethi S, Foldvari Z.et al Iatrogenic Creutzfeldt–Jakob disease with florid plaques. Brain Pathol 200313245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preusser M, Strobel T, Gelpi E.et al Alzheimer‐type neuropathology in a 28 year old patient with iatrogenic Creutzfeldt–Jakob disease after dural grafting. J Neurol Neurosurg Psychiatry 200677413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martinez‐Lage J F, Poza M, Sola J.et al Accidental transmission of Creutzfeldt–Jakob disease by dural cadaveric grafts. J Neurol Neurosurg Psychiatry 1994571091–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia Santos J M, Lopez Corbalan J A, Martinez‐Lage J F.et al CT and MRI in iatrogenic and sporadic Creutzfeldt–Jakob disease: as far as imaging perceives. Neuroradiology 199638226–231. [DOI] [PubMed] [Google Scholar]
- 48.Rabinstein A A, Whiteman M L, Shebert R T. Abnormal diffusion‐weighted magnetic resonance imaging in Creutzfeldt–Jakob disease following corneal transplantations. Arch Neurol 200259637–639. [DOI] [PubMed] [Google Scholar]
- 49.Llewelyn C A, Hewitt P E, Knight R S G.et al Possible transmission of variant Creutzfeldt–Jakob disease by blood transfusion. Lancet 2004363417–421. [DOI] [PubMed] [Google Scholar]
- 50.Peden A H, Head M W, Ritchie D L.et al Preclinical vCJD after blood transfusion in a PRNP codon 129 heterozygous patient. Lancet 2004364527–529. [DOI] [PubMed] [Google Scholar]
- 51.Wroe S J, Pal S, Siddique D.et al Clinical presentation and pre‐mortem diagnosis of blood transfusion associated variant CJD. Lancet 20063682061–2067. [DOI] [PubMed] [Google Scholar]
- 52.Falcone S, Quencer R M, Bowen B.et al Creutzfeldt–Jakob disease: focal symmetrical cortical involvement demonstrated by MR imaging. Am J Neuroradiol 199213403–406. [PMC free article] [PubMed] [Google Scholar]
- 53.Galvez S, Cartier L. Computed tomography findings in 15 cases of Creutzfeldt–Jakob disease with histological verification. J Neurol Neurosurg Psychiatry 1984471244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung Y L, Williams A, Ritchie D.et al Conflicting MRI signals from gliosis and neuronal vacuolation in prion diseases. Neuroreport 1999103471–3477. [DOI] [PubMed] [Google Scholar]
- 55.Vrancken A F J E, Frijns C J M, Ramos L M P. FLAIR MRI in sporadic Creutzfeldt–Jakob disease. Neurology 200055147–148. [DOI] [PubMed] [Google Scholar]
- 56.Schwaninger M, Winter R, Hacke W.et al Magnetic resonance imaging in Creutzfeldt–Jakob disease: Evidence focal involvement of the cortex. J Neurol Neurosurg Psychiatry 199763408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cambier D M, Kantarci K, Worrell G A.et al Lateralized and focal clinical, EEG, and FLAIR MRI abnormalities in Creutzfeldt–Jakob disease. Clin Neurophysiol 20031141724–1728. [DOI] [PubMed] [Google Scholar]
- 58.Haik S, Dormont D, Faucheux B A.et al Prion protein deposits match magnetic resonance imaging signal abnormalities in Creutzfeldt–Jakob disease. Ann Neurol 200251797–799. [DOI] [PubMed] [Google Scholar]
- 59.Young G S, Geschwind M D, Fischbein N J.et al Diffusion‐weighted and fluid‐attenuated inversion recovery imaging in Creutzfeldt–Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol 2005261551–1562. [PMC free article] [PubMed] [Google Scholar]
- 60.Mao‐Draayer Y, Braff S P, Nagle K J.et al Emerging patterns of diffusion‐weighted MR imaging in Creutzfeldt–Jakob disease: case report and review of the literature. Am J Neuroradiol 200223550–556. [PMC free article] [PubMed] [Google Scholar]
- 61.Urbach H. Creutzfeldt–Jakob disease: analysis of the MRI signal. Neuroreport 200011L5. [DOI] [PubMed] [Google Scholar]
- 62.Linguraru M G, Ayache N, Bardinet E.et al Differentiation of sCJD and vCJD forms by automated analysis of basal ganglia intensity distribution in multisequence MRI of the brain – definition and evaluation of new MRI‐based ratios. IEEE Trans Med Imaging 2006251052–1067. [DOI] [PubMed] [Google Scholar]
- 63.Waldman A D, Jarman P, Merry R T. Rapid echoplanar diffusion imaging in a case of variant Creutzfeldt–Jakob disease; where speed is of the essence. Neuroradiology 200345528–531. [DOI] [PubMed] [Google Scholar]
- 64.Mittal S, Farmer P, Kalina P.et al Correlation of diffusion‐weighted magnetic resonance imaging with neuropathology in Creutzfeldt–Jakob disease. Arch Neurol 200259128–134. [DOI] [PubMed] [Google Scholar]
- 65.Demaerel P, Heiner L, Robberecht W.et al Diffusion‐weighted MRI in sporadic Creutzfeldt–Jakob disease. Neurology 199952205–208. [DOI] [PubMed] [Google Scholar]
- 66.Matoba M, Tonami H, Miyaji H.et al Creutzfeldt–Jakob disease: Serial changes on diffusion‐weighted MRI. J Comput Assist Tomogr 200125274–277. [DOI] [PubMed] [Google Scholar]
- 67.Ukisu R, Kushihashi T, Kitanosono T.et al Serial diffusion‐weighted MRI of Creutzfeldt–Jakob disease. Am J Roentgenol 2005184560–566. [DOI] [PubMed] [Google Scholar]
- 68.Bahn M M, Parchi P. Abnormal diffusion‐weighted magnetic resonance images in Creutzfeldt–Jakob disease. Arch Neurol 199956577–583. [DOI] [PubMed] [Google Scholar]
- 69.Nitrini R, Mendonça R A, Huang N.et al Diffusion‐weighted MRI in two cases of familial Creutzfeldt–Jakob disease. J Neurol Sci 2001184163–167. [DOI] [PubMed] [Google Scholar]
- 70.Oppenheim C, Brandel J P, Hauw J J.et al MRI and the second French case of vCJD. Lancet 2000356253–254. [DOI] [PubMed] [Google Scholar]
- 71.Russmann H, Vingerhoets F, Miklossy J.et al Sporadic Creutzfeldt–Jakob disease. A comparison of pathological findings and diffusion weighted imaging. J Neurol 2005252338–342. [DOI] [PubMed] [Google Scholar]
- 72.Na D L, Suh C K, Choi S H.et al Diffusion‐weighted magnetic resonance imaging in probable Creutzfeldt–Jakob disease—A clinical–anatomic correlation. Arch Neurol 199956951–957. [DOI] [PubMed] [Google Scholar]
- 73.Masters C L, Richardson E P., Jr Subacute spongiform encephalopathy (Creutzfeldt–Jakob disease). The nature and progression of spongiform change. Brain 1978101333–344. [DOI] [PubMed] [Google Scholar]
- 74.Oppenheim C, Zuber M, Galanaud D.et al Spectroscopy and serial diffusion MR findings in hGH–Creutzfeldt–Jakob disease. J Neurol Neurosurg Psychiatry 2004751066–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bahn M M, Kido D K, Lin W L.et al Brain magnetic resonance diffusion abnormalities in Creutzfeldt–Jakob disease. Arch Neurol 1997541411–1415. [DOI] [PubMed] [Google Scholar]
- 76.Lim C C, Tan K, Verma K K.et al Combined diffusion‐weighted and spectroscopic MR imaging in Creutzfeldt–Jakob disease. Magn Reson Imaging 200422625–629. [DOI] [PubMed] [Google Scholar]
- 77.Tschampa H J, Murtz P, Flacke S.et al Thalamic involvement in sporadic Creutzfeldt–Jakob disease: a diffusion‐weighted MR imaging study. Am J Neuroradiol 200324908–915. [PMC free article] [PubMed] [Google Scholar]
- 78.Konaka K, Kaido M, Okuda Y.et al Proton magnetic resonance spectroscopy of a patient with Gerstmann–Straussler–Scheinker disease. Neuroradiology 200042662–665. [DOI] [PubMed] [Google Scholar]
- 79.Bruhn H, Weber T, Thorwirth V.et al In‐vivo monitoring of neuronal loss in Creutzfeldt–Jakob disease by proton magnetic resonance spectroscopy. Lancet 19913371610–1611. [DOI] [PubMed] [Google Scholar]
- 80.Graham G D, Petroff O A, Blamire A M.et al Proton magnetic resonance spectroscopy in Creutzfeldt–Jakob disease. Neurology 1993432065–2068. [DOI] [PubMed] [Google Scholar]
- 81.Henkel K, Zerr I, Hertel A.et al Positron emission tomography with [(18)F]FDG in the diagnosis of Creutzfeldt–Jakob disease (CJD). J Neurol 2002249699–705. [DOI] [PubMed] [Google Scholar]
- 82.Goldman S, Laird A, Flament‐Durand J.et al Positron emission tomography and histopathology in Creutzfeldt–Jakob disease. Neurology 1993431828–1830. [DOI] [PubMed] [Google Scholar]
- 83.Engler H, Lundberg P O, Ekbom K.et al Multitracer study with positron emission tomography in Creutzfeldt–Jakob disease. Eur J Nucl Med Mol Imaging 200330187. [DOI] [PubMed] [Google Scholar]
- 84.Tsuji Y, Kanamori H, Murakami G.et al Heidenhain variant of Creutzfeldt–Jakob disease: diffusion‐weighted MRI and PET characteristics. J Neuroimaging 20041463–66. [PubMed] [Google Scholar]
- 85.Agdeppa E D, Kepe V, Liu J.et al Binding characteristics of radiofluorinated 6‐dialkylamino‐2‐naphthylethylidene derivatives as positron emission tomography imaging probes for beta‐amyloid plaques in Alzheimer's disease. J Neurosci 200121RC189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadowski M, Pankiewicz J, Scholtzova H.et al Targeting prion amyloid deposits in vivo. J Neuropathol Exp Neurol 200463775–784. [DOI] [PubMed] [Google Scholar]
- 87.Bresjanac M, Smid L M, Vovko T D.et al Molecular‐imaging probe 2‐(1‐[6‐[(2‐fluoroethyl)(methyl) amino]‐2 naphthyl]ethylidene) malononitrile labels prion plaques in vitro. J Neurosci 2003238029–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henkel K, Meller J, Zerr I.et al Single photon emission computed tomography (SPECT) in 19 patients with Creutzfeldt–Jakob disease. J Neurol 2004246103, P490 [Google Scholar]
- 89.Matsuda M, Tabata K, Hattori T.et al Brain SPECT with 123I‐IMP for the early diagnosis of Creutzfeldt–Jakob disease. J Neurol Sci 20011835–12. [DOI] [PubMed] [Google Scholar]
- 90.Jibiki I, Fukushima T, Kobayashi K.et al Utility of 123I‐IMP SPECT brain scans for the early detection of site‐specific abnormalities in Creutzfeldt–Jakob disease (Heidenhain type): A case study. Neuropsychobiology 199429117–119. [DOI] [PubMed] [Google Scholar]
- 91.Mathews D, Unwin D H. Quantitative cerebral blood flow imaging in a patient with the Heidenhain variant of Creutzfeldt–Jakob disease. Clin Nucl Med 200126770–773. [DOI] [PubMed] [Google Scholar]