Abstract
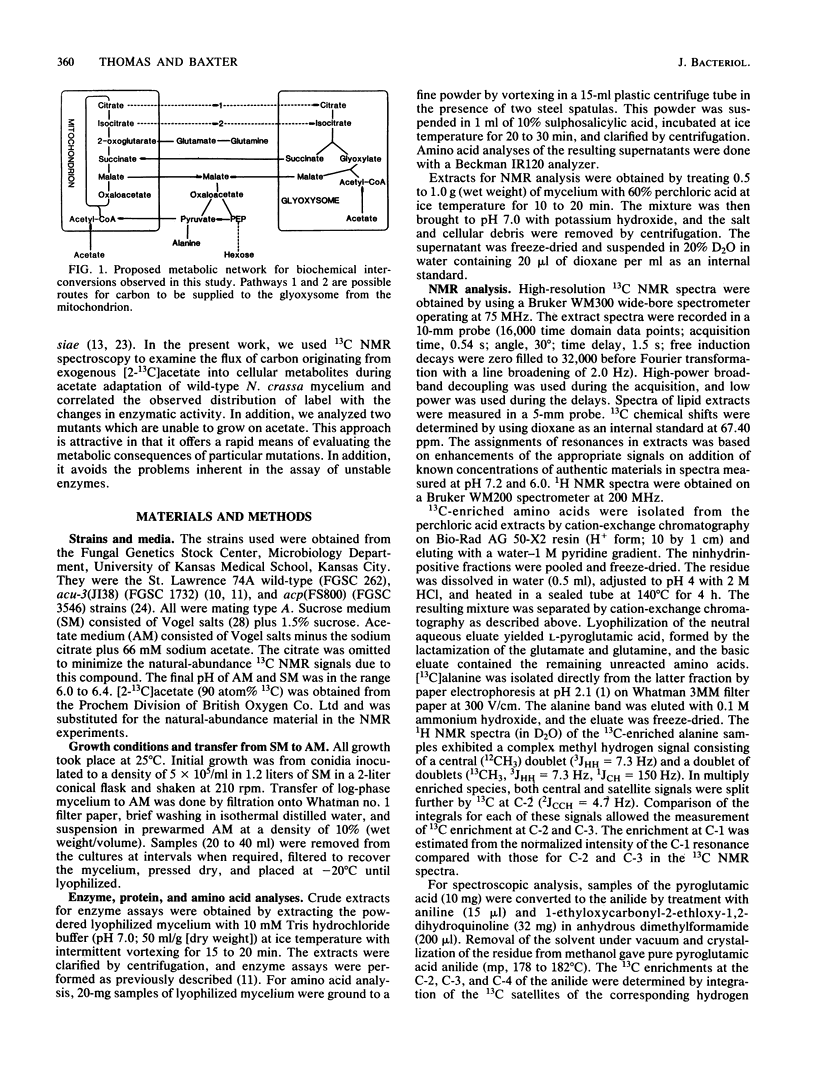
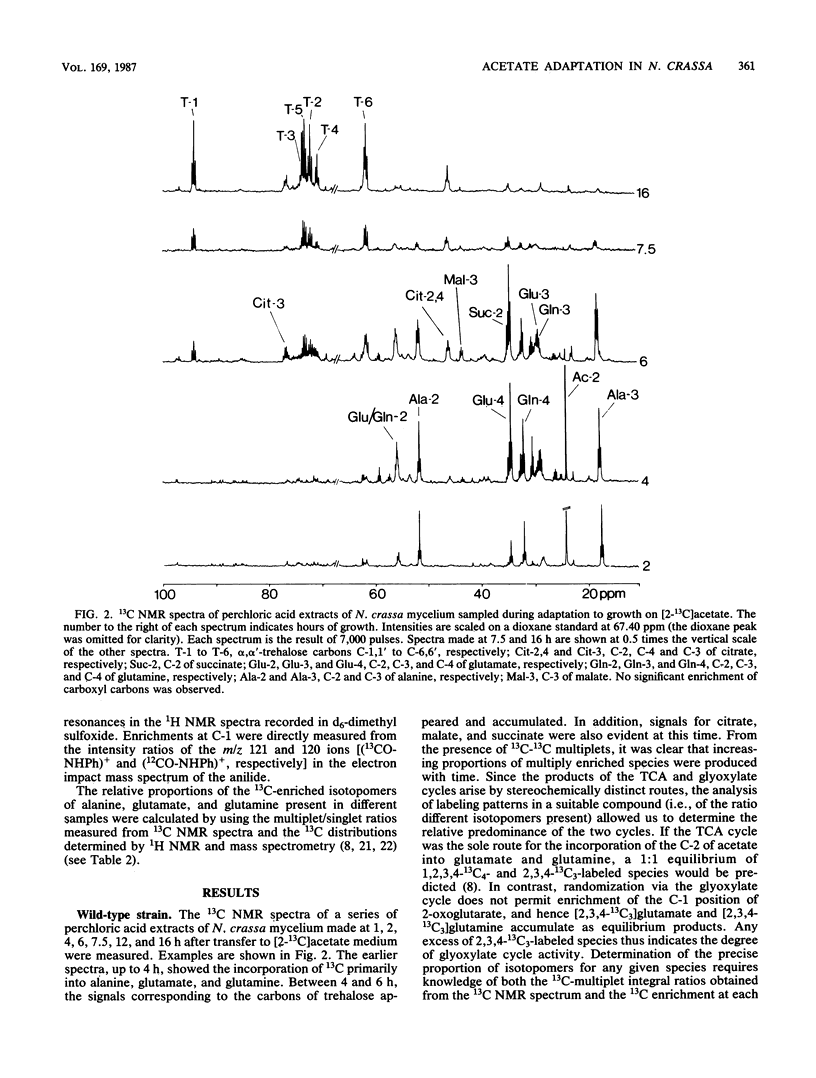
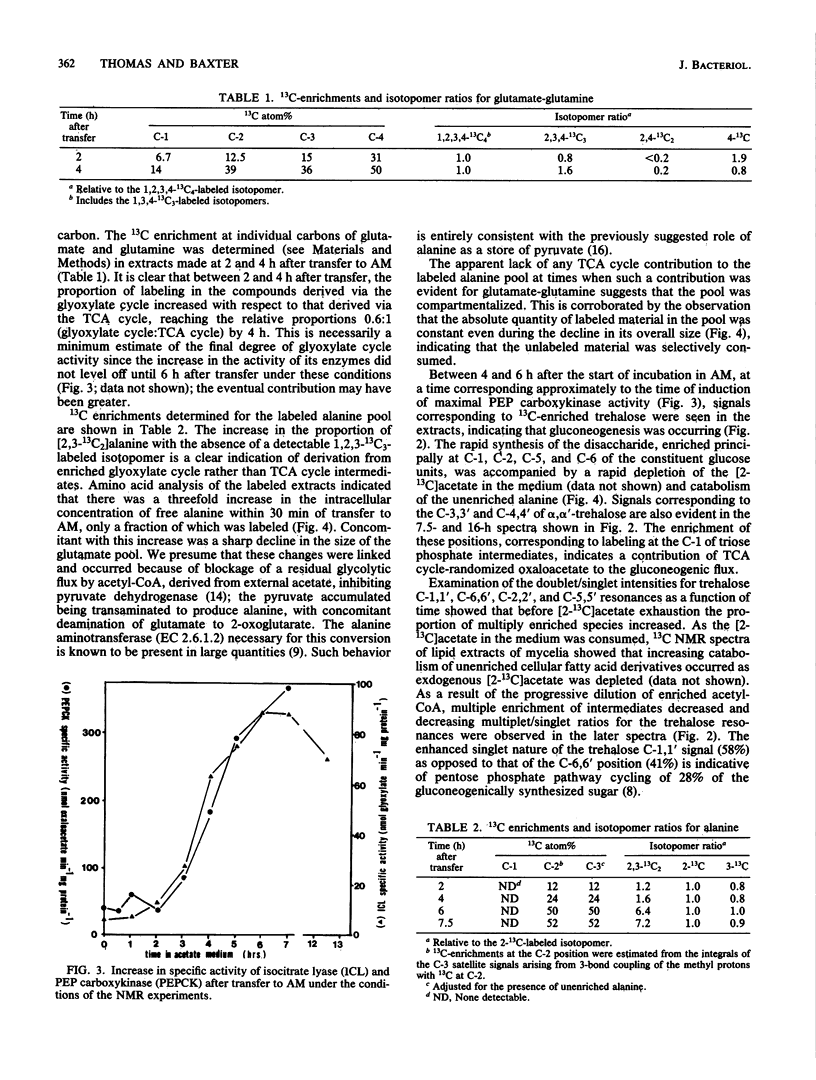
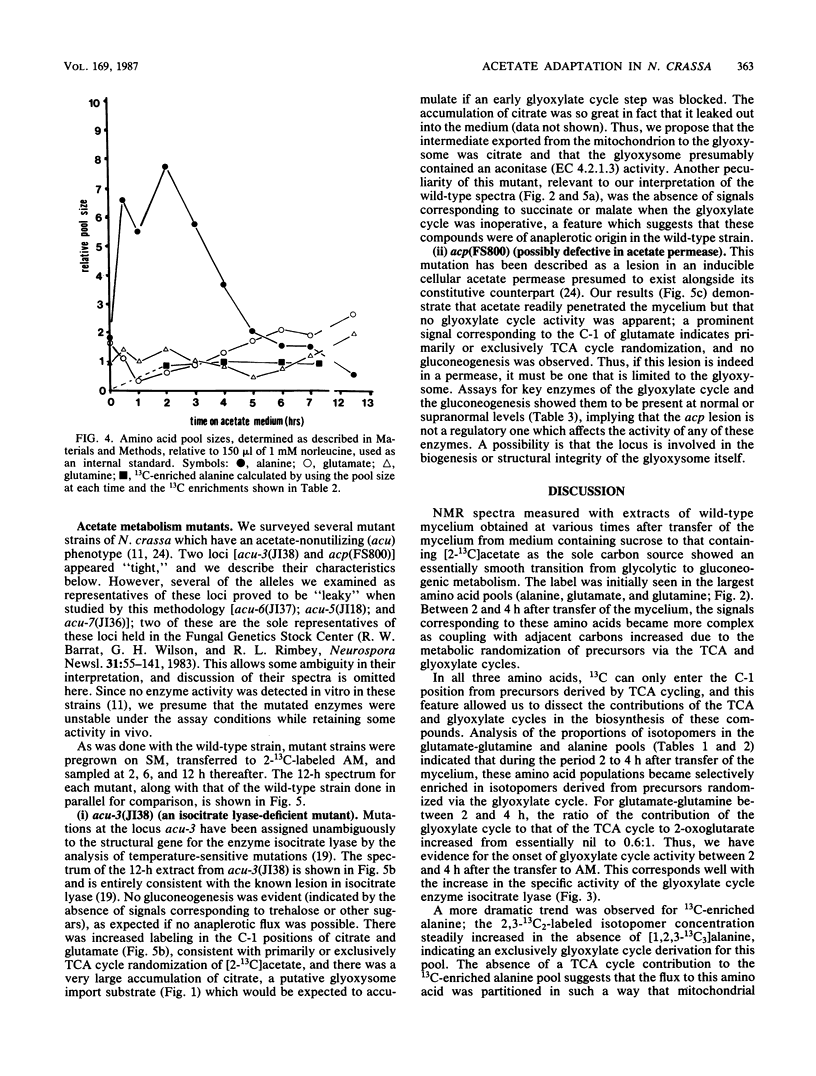
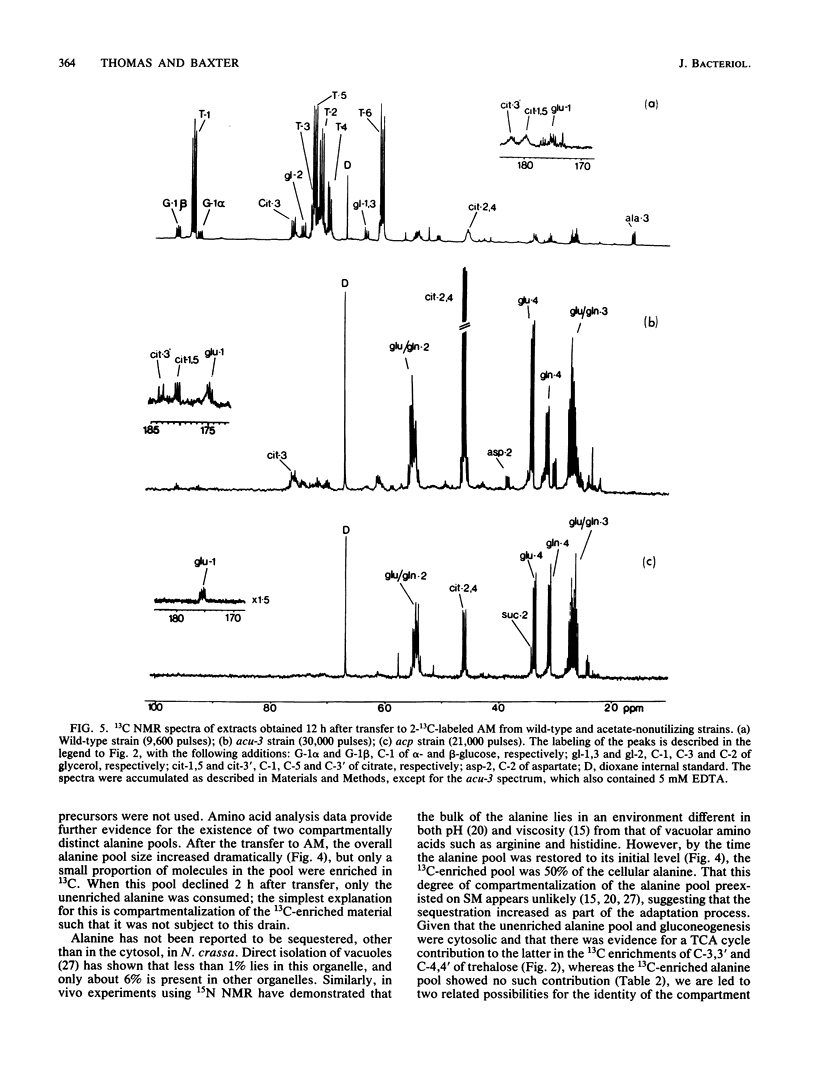
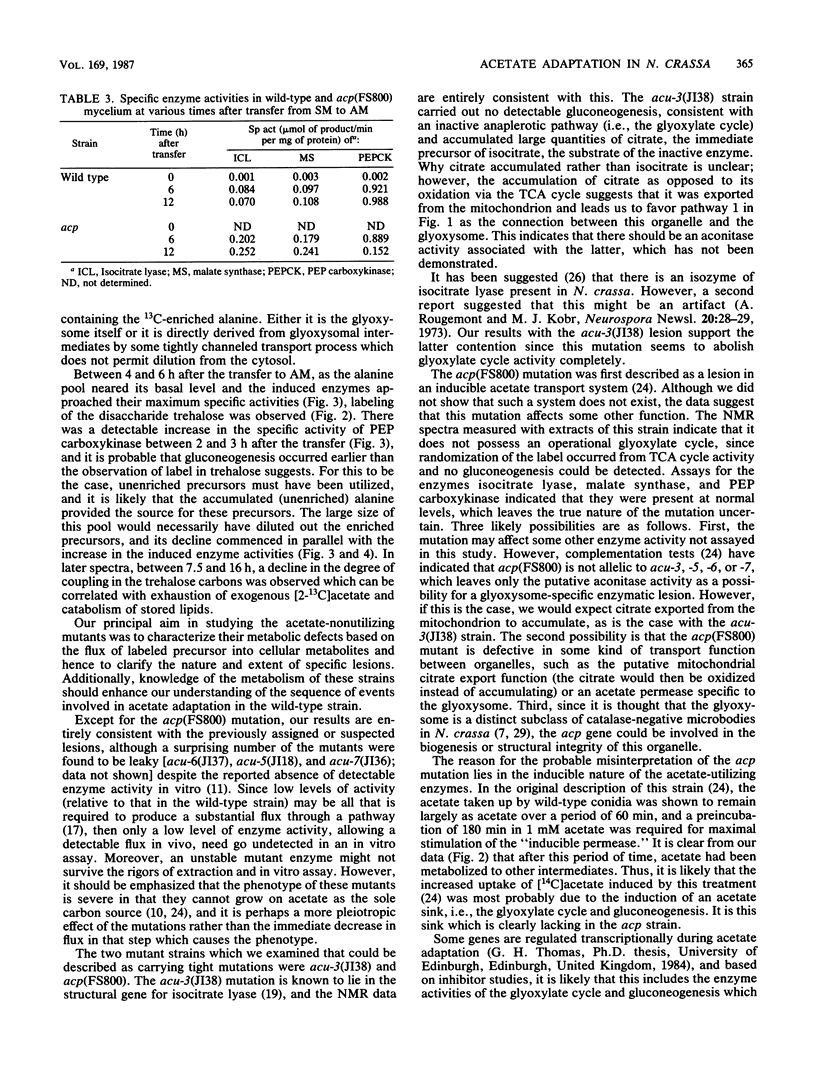
The adaptation of Neurospora crassa mycelium to growth on acetate as the sole carbon source was examined by using 13C nuclear magnetic resonance. Extracts were examined by nuclear magnetic resonance at various times after transfer of the mycelium from medium containing sucrose to medium containing [2-13C]acetate as the sole carbon source. The label was initially seen to enter the alanine, glutamate, and glutamine pools, and after 6 h 13C-enriched trehalose was evident, indicating that gluconeogenesis was occurring. Analysis of the isotopomer ratios in the alanine and glutamate-glutamine pools indicated that substantial glyoxylate cycle activity became evident between 2 and 4 h after transfer. Immediately after transfer of the mycelium to acetate medium, the alanine pool increased to about four times its previous level, only a small fraction of which was enriched with 13C. The quantity of 13C-enriched alanine remained almost constant between 2 and 7.5 h after the transfer, whereas the overall alanine pool decreased to its original level. The selective catabolism of the unenriched alanine leads us to suggest that the alanine pool is partitioned into two compartments during adaptation. Two acetate-nonutilizing mutants were also studied by this technique. An acu-3 strain, deficient for isocitrate lyase (EC 4.1.3.1) activity, showed metabolic changes consistent with this lesion. An acp strain, previously thought to be deficient in an inducible acetate permease, took up [2-13C]acetate but showed no evidence of glyoxylate cycle activity despite synthesizing the necessary enzymes; the lesion was therefore reinterpreted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter R. L. Microbiological applications of NMR spectroscopy. Part 2. Microbiol Sci. 1985 Nov;2(11):340–345. [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- Desel H., Zimmermann R., Janes M., Miller F., Neupert W. Biosynthesis of glyoxysomal enzymes in Neurospora crassa. Ann N Y Acad Sci. 1982;386:377–393. doi: 10.1111/j.1749-6632.1982.tb21429.x. [DOI] [PubMed] [Google Scholar]
- Dickinson J. R., Dawes I. W., Boyd A. S., Baxter R. L. 13C NMR studies of acetate metabolism during sporulation of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5847–5851. doi: 10.1073/pnas.80.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINCHAM J. R. S. Transaminases in Neurospora crassa. Nature. 1951 Dec 1;168(4283):957–958. doi: 10.1038/168957b0. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-nonutilizing mutants of Neurospora rassa. II. Biochemical deficiencies and the roles of certain enzymes. J Bacteriol. 1968 Mar;95(3):1063–1068. doi: 10.1128/jb.95.3.1063-1068.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Fincham J. R. Acetate-onutilizing mutants of Neurospora crassa. I. Mutant isolation, complementation studies, and linkage relationships. J Bacteriol. 1968 Mar;95(3):1056–1062. doi: 10.1128/jb.95.3.1056-1062.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. B., Woodward D. O. The concurrent regulation of metabolically related enzymes. The Krebs cycle and glyoxylate shunt enzymes in Neurospora. Eur J Biochem. 1970 Dec;17(2):284–291. doi: 10.1111/j.1432-1033.1970.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Benoit A. G. NMR analysis of a cell division cycle mutant of Saccharomyces cerevisiae. Biochim Biophys Acta. 1983 Jun 2;762(3):466–470. doi: 10.1016/0167-4889(83)90013-7. [DOI] [PubMed] [Google Scholar]
- Harding R. W., Caroline D. F., Wagner R. P. The pyruvate dehydrogenase complex from the mitochondrial fraction of Neurospora crassa. Arch Biochem Biophys. 1970 Jun;138(2):653–661. doi: 10.1016/0003-9861(70)90393-0. [DOI] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kanamori K., Legerton T. L., Weiss R. L., Roberts J. D. Effect of the nitrogen source on glutamine and alanine biosynthesis in Neurospora crassa. An in vivo 15N nuclear magnetic resonance study. J Biol Chem. 1982 Dec 10;257(23):14168–14172. [PubMed] [Google Scholar]
- Kanamori K., Legerton T. L., Weiss R. L., Roberts J. D. Nitrogen-15 spin-lattice relaxation times of amino acids in Neurospora crassa as a probe of intracellular environment. Biochemistry. 1982 Sep 28;21(20):4916–4920. doi: 10.1021/bi00263a013. [DOI] [PubMed] [Google Scholar]
- Kobr M. J., Vanderhaeghe F., Combépine G. Particulate enzymes of the glyoxylate cycle in Neurospora crassa. Biochem Biophys Res Commun. 1969 Nov 6;37(4):640–645. doi: 10.1016/0006-291x(69)90858-4. [DOI] [PubMed] [Google Scholar]
- Leckie B. J., Fincham J. R. A structural gene for Neurospora crassa isocitrate lyase. J Gen Microbiol. 1971 Jan;65(1):35–43. doi: 10.1099/00221287-65-1-35. [DOI] [PubMed] [Google Scholar]
- Legerton T. L., Kanamori K., Weiss R. L., Roberts J. D. Measurements of cytoplasmic and vacuolar pH in Neurospora using nitrogen-15 nuclear magnetic resonance spectroscopy. Biochemistry. 1983 Feb 15;22(4):899–903. doi: 10.1021/bi00273a029. [DOI] [PubMed] [Google Scholar]
- Navon G., Shulman R. G., Yamane T., Eccleshall T. R., Lam K. B., Baronofsky J. J., Marmur J. Phosphorus-31 nuclear magnetic resonance studies of wild-type and glycolytic pathway mutants of Saccharomyces cerevisiae. Biochemistry. 1979 Oct 16;18(21):4487–4499. doi: 10.1021/bi00588a006. [DOI] [PubMed] [Google Scholar]
- Rao T. K., DeBusk A. G. An inducible acetate transport system in Neurospora crassa conidia. Biochim Biophys Acta. 1977 Nov 1;470(3):475–483. doi: 10.1016/0005-2736(77)90138-9. [DOI] [PubMed] [Google Scholar]
- Sjogren R. E., Romano A. H. Evidence for multiple forms of isocitrate lyase in Neurospora crassa. J Bacteriol. 1967 May;93(5):1638–1643. doi: 10.1128/jb.93.5.1638-1643.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn L. E., Davis R. H. Purification of vacuoles from Neurospora crassa. Mol Cell Biol. 1981 Sep;1(9):797–806. doi: 10.1128/mcb.1.9.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. E., Han C. H., Kollman V. H., London R. E., Matwiyoff N. A. 13C nuclear magnetic resonance studies of the biosynthesis by Microbacterium ammoniaphilum of L-glutamate selectively enriched with carbon-13. J Biol Chem. 1982 Feb 10;257(3):1189–1195. [PubMed] [Google Scholar]
- Wanner G., Theimer R. R. Two types of microbodies in Neurospora crassa. Ann N Y Acad Sci. 1982;386:269–284. doi: 10.1111/j.1749-6632.1982.tb21422.x. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Behar K. L., Shulman R. G. 13C NMR study of transamination during acetate utilization by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):2693–2697. doi: 10.1073/pnas.78.5.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]


