Abstract
Antibodies that inhibit Plasmodium falciparum invasion of erythrocytes are believed to be an important component of immunity against malaria. During blood-stage infection, P. falciparum can use different pathways for erythrocyte invasion by varying the expression and/or utilization of members of 2 invasion ligand families: the erythrocyte-binding antigens (EBAs) and reticulocyte-binding homologs (PfRhs). Invasion pathways can be broadly classified into 2 groups based on the use of sialic acid (SA) on the erythrocyte surface by parasite ligands. We found that inhibitory antibodies are acquired by malaria-exposed Kenyan children and adults against ligands of SA-dependent and SA-independent invasion pathways, and the ability of antibodies to inhibit erythrocyte invasion depended on the pathway used by P. falciparum isolates. Differential inhibition of P. falciparum lines that varied in their use of specific EBA and PfRh proteins pointed to these ligand families as major targets of inhibitory antibodies. Antibodies against recombinant EBA and PfRh proteins were acquired in an age-associated manner, and inhibitory antibodies against EBA175 appeared prominent among some individuals. These findings suggest that variation in invasion phenotype might have evolved as a mechanism that facilitates immune evasion by P. falciparum and that a broad inhibitory response against multiple ligands may be required for effective immunity.
Introduction
Malaria resulting from Plasmodium falciparum infection is a major cause of mortality and morbidity, with up to 3 million deaths and 300–500 million clinical cases each year. The capacity for immune evasion enables P. falciparum to cause repeated and chronic infections, and effective immunity against malaria develops slowly after repeated exposure (1). Antibodies are believed to be an important component of acquired protective immunity, in addition to other factors (2). During blood-stage replication, P. falciparum merozoites invade erythrocytes, and antibodies that inhibit invasion and subsequent replication are believed to be important in mediating both acquired immunity and immunity generated by candidate blood-stage vaccines (3–5). However, the targets of acquired inhibitory antibodies are largely undefined.
Erythrocyte invasion by merozoites involves multiple receptor-ligand interactions, and a number of merozoite proteins have proposed or established roles in invasion and may be targets of inhibitory antibodies (6, 7). Initial attachment is thought to involve antigens on the surface of merozoites, such as merozoite surface protein–1 (MSP1) and other GPI-anchored surface proteins (8, 9), and is followed by apical reorientation of the merozoite involving apical membrane antigen 1 (AMA1) (10). Secondary interactions are then required for activation of invasion processes. These involve 2 invasion ligand families: the erythrocyte-binding antigens (EBAs; EBA175, EBA140/BAEBL, EBA181/JESEBL) and P. falciparum reticulocyte-binding homolog (PfRh) proteins (PfRh1, -2a, -2b, and -4) (11–20). Additional members of these families, EBA165 and PfRh3, occur as pseudogenes (18, 20, 21). The role of these ligands appears conserved to some extent across different Plasmodium species. The EBAs have homology to invasion ligands of P. vivax and P. knowlesi, and the PfRh proteins have homology to ligands of P. vivax and P. yoelii (6).
Merozoites can use different pathways for erythrocyte invasion (22). Changes in the expression and/or use of EBA and PfRh proteins enables the use of alternate invasion pathways (16, 20, 22–27). Variation in invasion phenotypes or pathways has been demonstrated with clinical isolates and laboratory-adapted clones of P. falciparum. Although the mechanisms are not fully understood, differences in invasion phenotypes of P. falciparum variants have been clearly demonstrated based on their sensitivity to cleavage of erythrocyte surface receptors with defined enzymes. Invasion phenotypes can be broadly classified into 2 main groups: (a) sialic acid–dependent (SA-dependent) invasion, demonstrated by poor invasion of neuraminidase-treated erythrocytes (neuraminidase cleaves SA on the erythrocyte surface); and (b) SA-independent invasion, demonstrated by efficient invasion of neuraminidase-treated erythrocytes. SA-dependent (neuraminidase-sensitive) invasion involves the 3 EBAs and PfRh1, with EBA175 probably being the most important (11, 13, 15, 17, 19, 24, 28, 29). These ligands bind to SA on the erythrocyte surface. EBA175 and EBA140 bind to glycophorin A (28–30) and C (13), respectively. EBA181 binds to SA on the erythrocyte surface and to band 4.1 protein (15, 31). PfRh2b and PfRh4 are important in SA-independent invasion, and interactions appear chymotrypsin sensitive (16, 20, 32); however, receptors for binding these ligands are unknown. PfRh2a shares approximately 80% sequence identity with PfRh2b, differing in the C-terminal region of the protein. Presently, there is no evidence that PfRh2a is functional (16). Activation of PfRh4 appears essential for SA-independent invasion. When isolates using an SA-dependent invasion pathway are selected for invasion of neuraminidase-treated erythrocytes, there is a switch to the use of an SA-independent invasion pathway and induction of PfRh4 (20, 32). Disruption of PfRh4 blocks the ability to switch from SA-dependent to SA-independent invasion (20). Similarly, disruption of the EBA175 gene results in switching from SA-dependent to SA-independent invasion, with activation of PfRh4 expression (20). Presently, there is no evidence that other candidate invasion ligands, such as MSP1 and AMA1, are involved in phenotypic variation of invasion pathways.
The biological significance of the use of alternate pathways for erythrocyte invasion and the availability of multiple invasion ligands is not known but may provide a strategy to facilitate parasite invasion in the face of host receptor polymorphisms or immune responses that inhibit ligand binding (16). Although the EBA and PfRh ligand families play important roles in erythrocyte invasion, their significance as targets of inhibitory or protective antibodies has not been established. In order to gain further insights into the mechanisms of acquired immunity and to aid in the prioritization of candidate antigens for vaccine development, we have investigated whether acquired antibodies target specific invasion pathways and whether the use of alternate invasion pathways alters the activity of invasion-inhibitory antibodies, which may contribute to immune evasion. We further examined the acquisition of antibodies against the EBA and PfRh ligand families and evaluated their role as targets of inhibitory antibodies in humans.
Results
Invasion phenotypes and properties of defined P. falciparum lines.
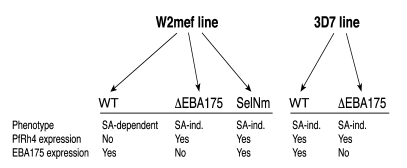
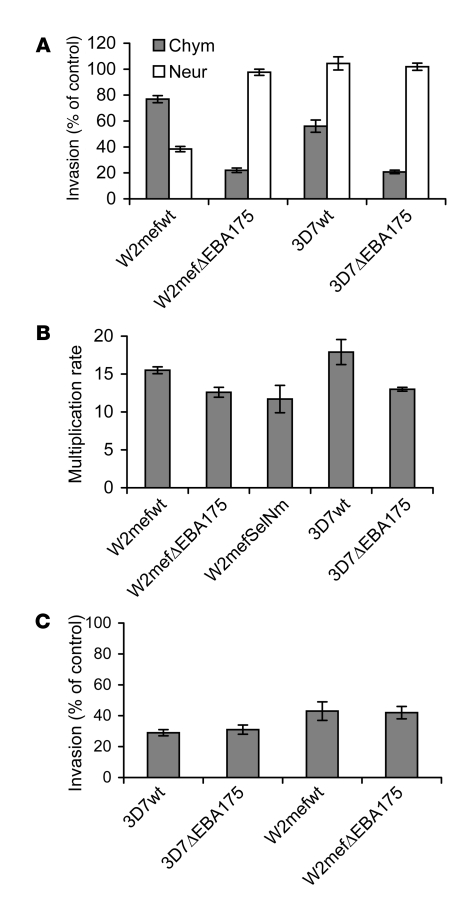
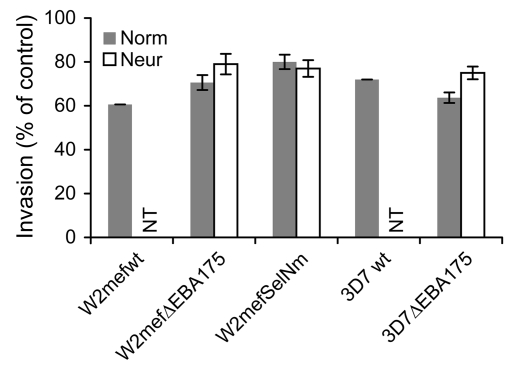
In our studies, we used variants of the clonal parasite lines W2mef and 3D7 using different invasion pathways. Targeted disruption of the gene for EBA175 (33) and selection of W2mef for invasion of neuraminidase-treated erythrocytes (20) generated parasites that used the SA-independent pathway (Figure 1). We characterized the invasion phenotypes of these parasite lines by evaluating their invasion into chymotrypsin- and neuraminidase-treated erythrocytes compared with normal erythrocytes (Figure 2A). Clear differences between the parasite lines in invasion pathway use or phenotype were demonstrated, consistent with previously published findings (33). Invasion of the parental WT W2mef (W2mef-WT) was sensitive to neuraminidase treatment of erythrocytes (SA-dependent invasion) but moderately resistant to chymotrypsin treatment of erythrocytes. In contrast, invasion of W2mef with EBA175 disrupted (W2mefΔEBA175) was resistant to neuraminidase treatment (SA-independent invasion) but sensitive to chymotrypsin. Invasion of 3D7-WT and 3D7 with EBA175 disrupted (3D7ΔEBA175) was resistant to neuraminidase, but the two 3D7 lines differed in their invasion of chymotrypsin-treated erythrocytes.
Figure 1. Overview of the different P. falciparum lines used in invasion inhibition assays.
Invasion phenotypes are grouped as SA dependent or independent (SA-ind.), and differences in the expression of EBA175 and PfRh4 are shown. Other differences in the expression of EBAs and PfRh proteins are not known. SA-dependent invasion involves EBA175, EBA140, EBA181, and PfRh1. SA-independent invasion involves PfRh4 and PfRh2b. ΔEBA175 indicates genetic disruption of EBA175; SelNm indicates that parasites were selected for invasion of neuraminidase-treated erythrocytes; WT indicates WT parental line.
Figure 2. The phenotypes and invasion of the different P. falciparum lines used.
(A) Demonstration of phenotypic differences in erythrocyte invasion by comparing the effect of neuraminidase or chymotrypsin treatment of erythrocytes on the invasion of different P. falciparum lines. Results are expressed as percentage invasion relative to erythrocytes treated with control (buffer); values represent mean ± SEM from 4 different experiments. Chym, chymotrypsin-treated erythrocytes; Neur, neuraminidase-treated erythrocytes. (B) Multiplication rates for different P. falciparum lines, cultured in normal erythrocytes. The multiplication rate represents the fold increase in parasitemia over 2 parasite life cycles for each parasite line. All samples were tested in duplicate; values represent mean ± range from 2 different experiments. (C) Inhibition of invasion of P. falciparum lines by rabbit antibodies against MSP119. All samples were tested in duplicate; values represent mean ± range from 2 different experiments. Results are expressed as the percent of invasion observed with control samples.
Comparison of the invasion rates of the different lines (Figure 2B) revealed a small reduction for the EBA175-knockout parasites compared with parental lines for both W2mef and 3D7. A switch from SA-dependent to SA-independent invasion in the W2mef line, by repeated selection for invasion of neuraminidase-treated erythrocytes (W2mefSelNm), was also associated with a modest reduction in multiplication rate. No substantial differences were found in the proportions of singly or multiply infected erythrocytes (this is referred to elsewhere as the selectivity index; ref. 34) between the different lines (data not shown). This suggests there is no major reduction in the invasion capacity of the transgenic or selected parasites compared with WT. The inhibitory effect of antibodies raised against MSP119 on erythrocyte invasion of the different W2mef and 3D7 lines was similar despite differences in invasion phenotypes (Figure 2C). These observations further indicate that MSP1, which is a potential target of inhibitory antibodies (35), is not involved in phenotypic variation.
The use of alternate erythrocyte invasion pathways alters the efficacy of invasion-inhibitory antibodies.
We examined whether variation in invasion phenotype, or use of alternate erythrocyte invasion pathways, influenced the inhibitory activity of acquired antibodies. Differential inhibition by acquired antibodies of isogenic lines that differ only in invasion phenotype would indicate that alternate pathways may exist as a mechanism that facilitates immune evasion. To examine this question, we compared the inhibitory activity of serum antibodies against W2mef and 3D7 parasite lines with different invasion phenotypes. We tested serum antibodies from a selection of children (n = 60) and adults (n = 20) resident in a malaria-endemic region of coastal Kenya. Total invasion-inhibitory antibodies were common among this population; 68% inhibited W2mef-WT and 62% inhibited 3D7-WT by greater than 25% compared with nonexposed controls. For these studies, differential inhibition was defined as an at least 25% difference in the extent of inhibition between the comparison lines. In all assays, we tested inhibition of W2mef-WT and 3D7-WT using untreated erythrocytes, and inhibition of W2mefΔEBA175, W2mefSelNm, and 3D7ΔEBA175 was tested with untreated and neuraminidase-treated erythrocytes.
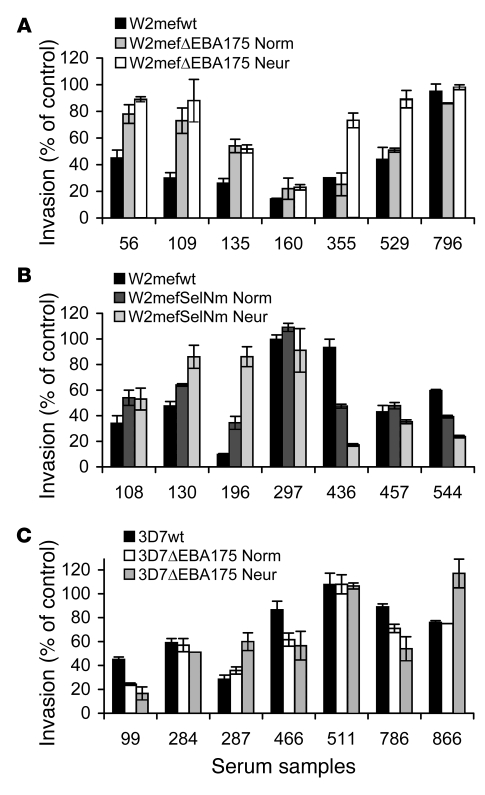
We first compared serum antibody inhibition of W2mef-WT with that of W2mefΔEBA175, the invasion phenotype of which is stable, but different from that of the parental W2mef, and has upregulated expression of PfRh4. W2mef-WT uses SA-dependent invasion mechanisms, whereas invasion of W2mefΔEBA175 is largely SA independent. In comparative inhibition assays, we found that 27% of samples differentially inhibited the 2 lines (e.g., samples 56, 109, and 135 in Figure 3A), indicating that the inhibitory activity of acquired antibodies is influenced by the invasion pathway being used (Figure 3A and Figure 4A). Large differences in inhibitory activity (up to 66%) between the lines were observed for individual samples. Although W2mefΔEBA175 has switched to use a largely SA-independent invasion pathway, it remains possible that other ligands involved in SA-dependent invasion (e.g., EBA140, EBA181, PfRh1) may still be functional to some extent in W2mefΔEBA175, despite the switch in phenotype. To inhibit these interactions, and more clearly compare antibodies against SA-dependent versus SA-independent invasion pathways, we also performed antibody inhibition assays using W2mefΔEBA175 and neuraminidase-treated erythrocytes, in comparison to inhibition of W2mef-WT with normal erythrocytes (Figure 4B). This approach further emphasized differences in antibody activity linked to variation in invasion phenotype; the proportion of samples showing differential inhibition of the 2 lines was 48% compared with 27% when using normal erythrocytes with both lines. The extent of differences in inhibitory activity was strongly increased for some individual samples. This indicates that the inhibitory activity of antibodies against ligands of SA-independent invasion was enhanced once the residual activity of SA-dependent ligands was inhibited by neuraminidase treatment of erythrocytes.
Figure 3. Inhibition of different P. falciparum lines by serum antibodies from malaria-exposed Kenyan children and adults.
Results shown were selected to demonstrate representative examples of the inhibitory activities observed. Values are expressed as percentage invasion relative to nonexposed donors. All samples were tested in duplicate; values represent mean ± range. (A) Inhibition of W2mef-WT compared with W2mefΔEBA175 cultured with normal or neuraminidase-treated erythrocytes. (B) Inhibition of W2mef-WT compared with W2mef-SelNm cultured with normal or neuraminidase-treated erythrocytes. (C) Inhibition of 3D7-WT compared with 3D7ΔEBA175 cultured with normal or neuraminidase-treated erythrocytes. Numbers on the x axis are study codes for individual serum samples. Norm, cultured with normal erythrocytes; Neur, cultured with neuraminidase-treated erythrocytes.
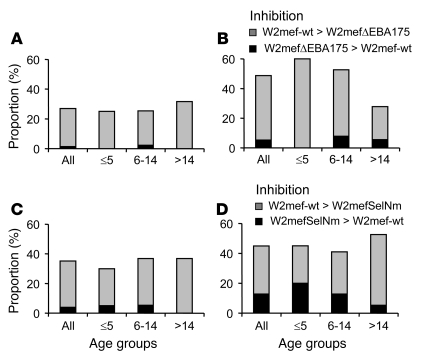
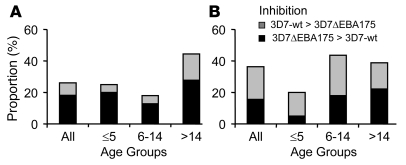
Figure 4. Differential inhibition of W2mef P. falciparum lines by serum antibodies from malaria-exposed Kenyan children and adults.
Results show the proportion of sera (n = 80) that differentially inhibited the 2 parasite lines tested for each comparison shown. Gray bars show the proportion of samples that inhibited the parental WT parasite line more than the W2mefΔEBA175 line or W2mefSelNm line (type A response). Black bars show the proportion of samples that inhibited the W2mefΔEBA175 line or W2mefSelNm line more than the corresponding parental line (type B response). The proportion with differential inhibitory activity is shown for all samples and separately by age groups (≤5, 6–14, and >14 years of age). (A and B) W2mef-WT compared with W2mefΔEBA175 cultured with normal (A) or neuraminidase-treated (B) erythrocytes. (C and D) W2mef-WT compared with W2mefSelNm cultured with normal (C) or neuraminidase-treated (D) erythrocytes. W2mef-WT was cultured with normal erythrocytes in all assays. Differences between the age groups were not statistically significant.
Differential inhibition by samples was also observed with W2mef-WT compared with W2mefSelNm (Figure 3B and Figure 4C). The latter isolate is genetically intact, and its phenotype was generated by selection for invasion of neuraminidase-treated erythrocytes (20). Like W2mefΔEBA175, it uses an alternate SA-independent invasion pathway and has upregulated expression of PfRh4. It still expresses EBA175 (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI32138DS1) but does not depend on this ligand for invasion. We found 35% of samples from children and adults differentially inhibited the 2 lines (e.g., samples 196 and 436 in Figure 3B), confirming that a change in invasion phenotype, or pathway, can substantially alter the efficacy of inhibitory antibodies. As expected, the inhibition of W2mefSelNm and W2mefΔEBA175 by samples was highly correlated (r = 0.61; n = 80; P < 0.001), as these isolates invade via the same pathway and only differ by the presence of EBA175 (20). We also tested antibodies for inhibition of W2mefSelNm invasion into neuraminidase-treated erythrocytes (Figure 4D), compared with W2mef-WT invasion of normal erythrocytes, to more clearly evaluate antibodies against SA-independent versus SA-dependent invasion pathways. This also ensured the phenotype and expression of PfRh4 were maintained in W2mefSelNm and that the parasites did not revert to the parental phenotype during the assay (spontaneous reversion of W2mefSelNm to the parental phenotype in the absence of antibodies took more than 4 weeks [data not shown]). Overall, 45% of samples differentially inhibited the 2 lines. Some samples showed greater differences in the inhibition of W2mef-WT and W2mefSelNm than when normal erythrocytes were used (e.g., samples 196 and 436 in Figure 3B).
Differential antibody inhibition of 3D7 lines with different invasion phenotypes further confirmed that variation in invasion phenotypes influences the activity of inhibitory antibodies (Figure 3C and Figure 5, A and B). The proportion of samples that differentially inhibited parental 3D7 versus 3D7ΔEBA175 was 26% when using normal erythrocytes and 37% when using neuraminidase-treated erythrocytes with 3D7ΔEBA175. These combined results with W2mef and 3D7 lines established that the availability of alternate pathways for erythrocyte invasion is immunologically important, suggesting that it might exist as a mechanism for evasion of acquired inhibitory antibodies in vivo.
Figure 5. Differential inhibition of 3D7 P. falciparum lines by serum antibodies from malaria-exposed Kenyan children and adults.
Results show the proportion of sera (n = 80) that differentially inhibited the 2 parasite lines tested for each comparison shown. Gray bars show the proportion of samples that inhibited parental 3D7 (3D7-WT) more than 3D7ΔEBA175 (type A response). Black bars show the proportion of samples that inhibited 3D7ΔEBA175 more than the parental 3D7 (type B response). The proportion with differential inhibitory activity is shown for all samples and separately by age groups (≤5, 6–14, and >14 years of age). Comparisons are shown with 3D7ΔEBA175 cultured with normal (A) or neuraminidase-treated (B) erythrocytes. 3D7-WT was cultured with normal erythrocytes in all assays. Differences between the age groups were not statistically significant.
The antibody inhibition assays used here have been previously validated and described in detail elsewhere (36). Differences in the inhibitory activity of individual sera against different parasite lines were confirmed by repeat testing. Overall, results from invasion inhibitory assays were highly reproducible. For example, results of repeat testing of 33 samples for inhibition of 3D7-WT and 3D7ΔEBA175 were highly correlated (r = 0.96 for 3D7-WT and r = 0.94 for 3D7ΔEBA175; P < 0.001). Repeat testing of 40 samples for inhibition using different parasite lines also demonstrated a high correlation of results among assays (r = 0.83; P < 0.001). We confirmed that the differential inhibitory activity measured in our assays represented inhibition of invasion and not an effect on parasite intraerythrocytic development. To do this, we tested serum samples in inhibition assays and determined parasitemias at different stages of parasite development, over 1 and 2 parasite life cycles (data not shown). We routinely measured antibody-inhibitory activity over 2 cycles of parasite invasion because this substantially increased the sensitivity of antibody inhibition assays (36) and facilitated the detection of differences in inhibition between parasite lines in this study. Differential inhibition of comparison lines by sera was also observed in single-cycle assays (data not shown).
Antibodies against SA-dependent invasion pathways are common, and inhibitory antibodies are acquired against EBA175.
Having established that antibodies can differentially inhibit alternate invasion pathways, we next aimed to further define the acquisition of antibodies against SA-dependent invasion in the population. Here, we term greater inhibition of SA-dependent invasion than SA-independent invasion as a type A inhibitory response. We define a type B inhibitory antibody response as the opposite; greater inhibition of SA-independent invasion compared with SA-dependent invasion in comparative inhibition assays.
Of those samples that differentially inhibited W2mef-WT versus W2mefΔEBA175 (cultured with normal erythrocytes), 26 of 27 had a type A response, inhibiting the parental W2mef more than W2mefΔEBA175 (P < 0.001; Figure 4). This pattern of inhibition points to inhibitory antibodies targeting EBA175 and other ligands of SA-dependent invasion. Overall, the mean inhibition of W2mef-WT by all samples (39.4%) was significantly greater than that of W2mefΔEBA175 (29.4%; P < 0.01) (Figure 6).
Figure 6. The effect of serum antibodies from Kenyan donors on erythrocyte invasion by different P. falciparum lines.
Results represent the mean from testing 80 Kenyan serum antibody samples; error bars represent 95% confidence intervals. Values are expressed relative to control samples from nonexposed donors. Samples were not tested for inhibition of 3D7-WT or W2mef-WT invasion into neuraminidase-treated erythrocytes; NT, not tested. Norm, invasion into normal erythrocytes; Neur, invasion into neuraminidase-treated erythrocytes.
When W2mefΔEBA175 was cultured with neuraminidase-treated erythrocytes to inhibit any residual SA-dependent interactions, there was an increase in the difference in the mean inhibition of W2mef-WT versus W2mefΔEBA175 by samples (a difference of 18.9% versus 10% using untreated erythrocytes; P < 0.01; Figure 6). Antibodies from 60% of children ≤5 years of age inhibited W2mef-WT to a greater extent than W2mefΔEBA175 (Figure 4B), whereas among adults, 22% showed this pattern of inhibition (P = 0.019). Similar to results from assays using W2mefΔEBA175, 31% of samples inhibited W2mef-WT more than W2mefSelNm (type A response; Figure 4C), whereas only 4% inhibited W2mefSelNm more than W2mef-WT (P < 0.001). Additionally, the mean inhibition of W2mef-WT (39.4%) by all samples was greater than W2mefSelNm (20%; P < 0.01) (Figure 6).
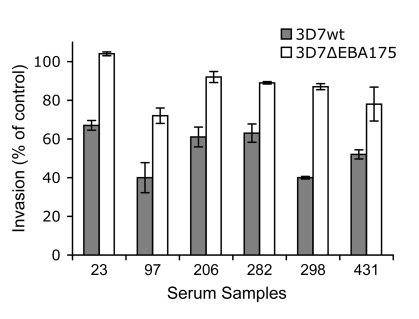
The 3D7 parental line invades erythrocytes through largely SA-independent interactions (Figure 2A), which limits the usefulness of this parasite line for evaluating antibodies against ligands of SA-dependent invasion. However, some samples inhibited the invasion of 3D7-WT into normal erythrocytes more than 3D7ΔEBA175 using neuraminidase-treated erythrocytes (Figure 5B). This indicates the presence of antibodies against the ligands of SA-dependent invasion. Disruption of EBA175 in 3D7, in contrast to W2mef, does not lead to a major switch in invasion phenotype. 3D7ΔEBA175 shows slightly greater resistance to the effect of neuraminidase treatment of erythrocytes compared with 3D7-WT and increased sensitivity to inhibition by chymotrypsin-treatment of erythrocytes, consistent with the loss of function of EBA175 (Figure 2A; ref. 33). Comparing inhibition of 3D7 and 3D7ΔEBA175 is therefore a useful tool to investigate inhibitory antibodies specifically targeting EBA175. Using this approach, we obtained evidence that EBA175 is a target of inhibitory antibodies, as suggested from studies with W2mef lines. Five percent of children and 17% of adults inhibited 3D7-WT more than 3D7ΔEBA175 (Figure 5A), strongly suggesting that at least some individuals in the population have inhibitory antibodies against EBA175. These antibodies were responsible for up to 47% of the total inhibitory activity measured in some individuals (Figure 7), indicating that EBA175 can be an important target.
Figure 7. Differential inhibition of 3D7-WT and 3D7ΔEBA175 by serum antibodies.
A selection of individual samples that inhibit 3D7-WT to a greater extent than 3D7ΔEBA175 is shown. This suggests the presence of inhibitory antibodies against EBA175. Values are expressed as percentage invasion relative to nonexposed donors. All samples were tested in duplicate; values represent mean ± range. Numbers on the x axis refer to study codes of individual serum samples.
Inhibition of SA-independent invasion.
We evaluated the presence of antibodies against ligands of SA-independent invasion by identifying samples that inhibited W2mefΔEBA175 or 3D7ΔEBA175 more than the corresponding parental parasites. Invasion of W2mefΔEBA175 or 3D7ΔEBA175 into neuraminidase-treated erythrocytes is dependent on ligands of the SA-independent invasion pathway. Type B responses, representing inhibitory antibodies against ligands of SA-independent invasion, were present among some samples but were detected significantly less often in our study population than type A responses.
Using the W2mef line, 5% of samples (Figure 4B) showed a type B response and inhibited invasion of W2mefΔEBA175 into neuraminidase-treated erythrocytes more effectively than W2mef-WT. Furthermore, 13% inhibited W2mefSelNm more than W2mef-WT (e.g., sample 436 in Figure 3B). Type B responses were more prevalent with the 3D7 parasite lines than W2mef (P < 0.001). A substantial number of samples inhibited 3D7ΔEBA175 more than 3D7-WT (18% of samples when using normal erythrocytes and 16% when using neuraminidase-treated erythrocytes; Figure 5, A and B). No children ≤5 years inhibited W2mefΔEBA175 more than W2mef-WT (Figure 4, A and B). Type B responses were only seen among samples from older children and adults (P = NS). Only 1 child ≤5 years had antibodies that inhibited invasion of 3D7ΔEBA175 into neuraminidase-treated erythrocytes more than invasion of 3D7-WT.
Acquisition of antibodies against recombinant EBA and PfRh proteins.
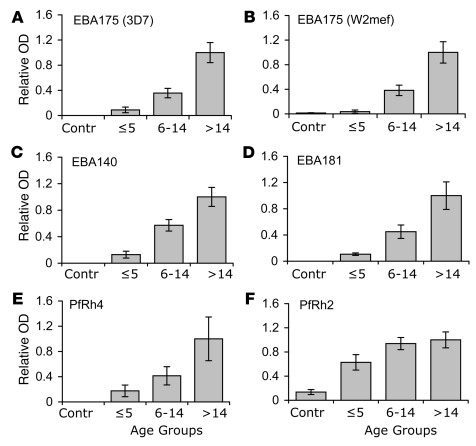
Differential inhibition of parasite lines that vary in their invasion phenotype, but not genotype, suggests that members of the EBA and PfRh proteins are targets of invasion-inhibitory antibodies. We measured antibodies against recombinant EBA and PfRh proteins by ELISA to confirm that these proteins are targets of acquired antibodies. Levels of antibodies against EBA175 (both 3D7 and W2mef alleles), EBA140, EBA181, PfRh2, and PfRh4 were positively associated with increasing age (Figure 8 and Supplemental Figure 3), being significantly higher among older than younger subjects (P < 0.001). There was little or no reactivity of sera from malaria-nonexposed subjects. Antibodies against P. falciparum schizont extract were also significantly correlated with age (data not shown), consistent with increasing exposure to blood-stage malaria. Levels of antibodies against one antigen were generally correlated with levels of antibodies against other antigens examined among the group. The correlations were strongest between the EBAs but were also significant for the PfRh proteins. Because antibody responses were significantly correlated with each other, there was a limited capacity to evaluate the relationship between any antigen-specific response by ELISA and specific inhibitory activity. We found little or no consistent significant association between the presence, or levels, of antibodies to EBA and PfRh proteins measured by ELISA and the extent of invasion inhibition by the same serum samples.
Figure 8. Age-associated acquisition of antibodies against recombinant EBA and PfRh proteins measured by ELISA (A–F).
Results (n = 150) are grouped by age and show mean ± SEM absorbance expressed relative to the levels for adults (>14 years). P < 0.001 for comparisons between age groups for all antigens. The relative absorbance using sera from nonexposed donors (n = 10) is also shown (Contr).
Discussion
Prior studies have established that P. falciparum uses different erythrocyte invasion pathways, and isolates from clinical infections show a wide diversity in invasion phenotypes and variation in the expression of EBA and PfRh ligands. However, the significance of this phenotypic variation by P. falciparum has been unknown. We evaluated the immunologic significance of these parasite properties by testing acquired antibodies for inhibition of parasite lines with defined invasion phenotypes and ligand expression. Our results demonstrate that the inhibitory activity of acquired antibodies is influenced by variation in invasion phenotypes, suggesting that the use of alternate erythrocyte invasion pathways by P. falciparum may help facilitate immune evasion. We identified inhibitory antibodies specific to ligands of both SA-dependent and SA-independent invasion. Comparing antibody inhibition of W2mef lines with different invasion phenotypes enabled a clear evaluation of the effect of variation in invasion pathway use on the efficacy of inhibitory antibodies. W2mefΔEBA175 uses an alternate SA-independent invasion pathway compared with the parental W2mef-WT (SA-dependent). Many samples that inhibited the parental W2mef lost their inhibitory activity against W2mefΔEBA175, providing evidence that a switch in invasion pathway use may mediate evasion of inhibitory antibodies. We confirmed these results using W2mefSelNm, a line that is genetically intact and uses an SA-independent pathway following selection for invasion into neuraminidase-treated erythrocytes. We also demonstrated that invasion pathway use alters susceptibility to inhibitory antibodies using a genetically different isolate, 3D7. The strategy of varying the use of different members of the EBA and PfRh ligand families therefore appears to enable P. falciparum to evade invasion-inhibitory antibodies targeting specific EBA and PfRh proteins. Effective immunity may depend on the presence of antibodies against a broad range of invasion ligands. Polymorphisms in the EBAs and PfRh proteins occur and may also contribute to immune evasion (37, 38). However, the significance of polymorphisms in evasion of inhibitory antibodies has not been established. Interestingly, a recent study using antibodies against EBA175 raised in rabbits found that polymorphisms in the binding region (F2 domain) of EBA175 did not affect the ability of antibodies to block binding of EBA175 to erythrocytes (39).
Our findings establish that inhibitory antibodies are acquired against both SA-dependent and SA-independent invasion pathways. We have termed these type A and type B inhibitory responses, respectively. In our study population, there was a higher prevalence of type A inhibitory antibody responses, reflecting antibodies against ligands of SA-dependent invasion. W2mef-WT was inhibited to a greater extent and by more samples than W2mef variants using an alternate invasion pathway. This may indicate that antibodies against ligands of SA-dependent invasion (the EBAs and PfRh1) are more prominent than antibodies against ligands of SA-independent invasion or that antibodies to ligands of SA-dependent invasion are acquired before those of SA-independent invasion. Consistent with our findings, studies conducted in Africa suggest that SA-dependent invasion is more common among infections in children (25). This may result in the acquisition of antibodies against SA-dependent invasion more rapidly or to a greater extent than antibodies against SA-independent invasion. Furthermore, studies in the same Kenyan population as investigated here found that expression of PfRh4 was uncommon among isolates (26), which may explain the predominance of type A antibody responses we observed. We established that type B inhibitory responses are also present in the study population, indicating that inhibitory antibodies against ligands of SA-independent invasion are acquired, but are less common. Type B responses were rare among young children in our study, but many children had type A responses. Type B responses were somewhat more prevalent among older children and adults, which may warrant further investigation. Some individuals showed no difference in the inhibition of comparison lines; this occurred because they either lacked inhibitory antibodies or had broadly effective antibodies that inhibited isolates using either invasion pathway.
A switch to the use of an alternate SA-independent pathway might facilitate immune evasion but may also come at the cost of reduced replication (Figure 2B) and could be relevant to parasite virulence. In the absence of immune pressure, an isolate such as W2mef would predominantly use the SA-dependent invasion pathway. The observation of a somewhat reduced replication rate explains prior findings that W2mef selected for invasion of neuraminidase-treated erythrocytes naturally drifts back to the WT invasion phenotype over several weeks (ref. 20 and unpublished observations), as a result of the WT phenotype outgrowing the alternate phenotype.
Data from comparative inhibition assays suggests that the EBA and PfRh ligands are important targets of invasion-inhibitory antibodies (Supplemental Figure 2). Greater inhibition of W2mef-WT compared with W2mefΔEBA175 or W2mefSelNm by samples from exposed donors points to antibodies targeting the EBAs and PfRh1, which define the SA-dependent pathway. EBA175 is likely to be the major target of these antibodies, as it is essential for utilization of the SA-dependent invasion pathway (20, 24). Disruption of EBA175 in W2mef results in a switch to an alternative SA-independent invasion pathway that is PfRh4 dependent (20). This suggests EBA175 is the major determinant of erythrocyte SA-dependent invasion in W2mef-WT parasites. On the other hand, greater inhibition of W2mefΔEBA175 or W2mefSelNm compared with W2mef-WT suggests the presence of antibodies against PfRh4 and PfRh2b. PfRh4 is essential for invasion using the SA-independent pathway and is therefore likely to be a target of inhibitory antibodies. An important role for PfRh2b in invasion was demonstrated with targeted gene deletion with 3D7 (16). However, a role for PfRh2a in invasion has not yet been established. Further studies are needed to understand the molecular interactions of PfRh4 and PfRh2b during invasion. This will facilitate a more precise understanding of the significance of these antigens as targets of inhibitory antibodies. Antibodies raised in rabbits against EBA175, EBA140, PfRh1, and PfRh2b have been shown to inhibit invasion (13, 17, 40), further supporting their role as targets of acquired inhibitory antibodies. Antibodies raised in rabbits against PfRh4 did not inhibit invasion (20); lack of inhibitory activity may have resulted from the use of only small regions of the protein to generate antibodies.
Although antibodies with other specificities could contribute to the total inhibitory activity against parasites used here, differential inhibition of isogenic parasites that vary in invasion phenotype is most likely explained by antibodies against the EBA and PfRh ligands. Presently, there is no evidence that other known or proposed ligands are involved in determining erythrocyte invasion phenotypes, other than the EBA and PfRh proteins. Acquired inhibitory antibodies have also been reported for MSP1 (35, 41) and AMA1 (42), but we found that the ability of these antibodies to inhibit invasion did not change with variation in invasion pathways. In previous studies, targeted disruption of other merozoite proteins, RAP1, MSP5, and MSP8, had no effect on invasion phenotypes (43–45). This suggests that other antibody specificities would not account for the differential inhibition of parasite lines with different invasion phenotypes.
Our findings suggest that comparisons of the inhibitory activity of serum antibodies against 3D7-WT and 3D7ΔEBA175 provide an informative measure of EBA175-specific inhibitory antibodies (Figure 7). Disruption of EBA175 in 3D7 does not result in a switch in invasion phenotype, as observed with W2mef. Therefore any differences in the inhibitory activity of serum antibodies against 3D7-WT compared with 3D7ΔEBA175 may be attributed to antibodies against EBA175. We identified some sera that gave greater inhibition of 3D7-WT than 3D7ΔEBA175, indicating the presence of antibodies against EBA175. Overall, antibodies of this type were not common. However, in some cases, antibodies against EBA175 appeared to constitute a major component of the total invasion-inhibitory activity. Some samples showed less inhibition of 3D7ΔEBA175 when the line was cultured with neuraminidase-treated erythrocytes, suggesting that inhibitory antibodies were present against other ligands of SA-dependent invasion apart from EBA175. Samples that inhibited 3D7ΔEBA175 more than 3D7-WT point to antibodies targeting PfRh2b and/or PfRh4. We anticipate that EBA175 would be a more important target of inhibitory antibodies against isolates that invade predominantly via an SA-dependent pathway, such as W2mef-WT.
Supporting our data from comparative inhibition assays, we confirmed that the EBAs and PfRh proteins are targets of acquired antibodies by evaluating antibodies against recombinant proteins. Antibodies were acquired in an age-dependent manner against EBA175, EBA181, EBA140, PfRh2, and PfRh4, consistent with increasing exposure and the acquisition of immunity in this population (34). To our knowledge, this is the first report in the literature of acquired antibodies against EBA140, EBA181, and the PfRh proteins. Antibodies generated in rabbits against recombinant antigens used here reacted specifically with their corresponding native parasite proteins (13, 16, 19, 20, 24), further validating our assay. This indicates that epitopes expressed by native proteins are represented on the recombinant proteins we used in ELISAs. However, antibodies measured by ELISA cannot be relied on as a direct measure of functional immunity, as we found no clear or consistent association between antibodies by ELISA and differences in inhibitory activity between comparison lines. Consistent with this, prior studies reported no correlation between antibodies against MSP119 by ELISA and MSP119-specific inhibitory antibodies (46). In other studies we have found antibodies against other merozoite antigens measured by ELISA to be poor predictors of inhibitory activity (F. McCallum, K. Persson, D. Wilson, and J. Beeson, unpublished observations). Standard immunoassays, such as ELISA, do not account for epitope specificity or affinity of antibodies that are important for functional activity. The advantage of functional assays is that antigens are expressed by parasites in their native conformation and context, which may be critical for antibody binding and function. Invasion inhibition assays also provide data relevant to mechanisms of immunity in vivo.
Together, our findings establish that the invasion inhibitory activity of naturally acquired antibodies is influenced by phenotypic variation in erythrocyte invasion pathways and point to members of the EBA and PfRh invasion ligand families as important targets of inhibitory antibodies. This suggests that P. falciparum may have evolved a novel mechanism for evasion of antibodies that inhibit host cell invasion, which may be relevant to other organisms. Clonal variation in the expression of merozoite invasion ligands in the rodent malaria P. yoelii (47) suggests that phenotypic variation and immune evasion by merozoites may also occur in other Plasmodium species. However, further studies are needed to determine and quantify the potential significance of this mechanism in immune evasion in natural infections. These findings are important for further understanding of the acquisition of malarial immunity and the capacity of P. falciparum to cause repeated infections and will aid in the prioritization and validation of candidate vaccine antigens. Based on findings here, we propose that a broad inhibitory response against functional epitopes of invasion ligands may be needed to convey substantial protective immunity. Vaccines may therefore need to target multiple invasion ligands in order to be fully effective and counter parasite immune evasion strategies. Further studies are needed to evaluate whether an effective vaccine might include ligands involved in both SA-dependent and SA-independent invasion in order to cover the spectrum of P. falciparum invasion phenotypes.
Methods
Invasion inhibition assays.
Methods for measuring invasion-inhibitory antibodies in serum samples have been described and evaluated in detail elsewhere (36). P. falciparum lines 3D7-WT, 3D7ΔEBA175, W2mef-WT, W2mefΔEBA175, and W2mefSelNm were cultured in vitro as described previously (48). W2mefSelNm (also called Clone4; ref. 20) was generated from W2mef-WT by selection for invasion into neuraminidase-treated erythrocytes. W2mefSelNm was continuously cultured in neuraminidase-treated erythrocytes to maintain the phenotype. Synchronized (by 5% d-sorbitol) parasites were cultured with human 0+ erythrocytes in RPMI-HEPES medium with 50 μg/ml hypoxanthine, 25 mM NaHCO3, 20 μg/ml gentamicin, 5% vol/vol heat-inactivated pooled human Australian sera, and 0.25% Albumax II (Gibco; Invitrogen) in 1% O2, 4% CO2, and 95% N2 at 37°C.
Invasion inhibition assays were started with parasites at the late pigmented trophozoite stage to schizont stage of the parasite’s development. Inhibitory activity was measured over 2 cycles of parasite replication. Starting parasitemia was 0.2%–0.3%, hematocrit 1%, and cells were resuspended in RPMI-HEPES supplemented as described above. Assays were performed in 96-well U-bottom culture plates (25 μl of cell suspension plus 2.5 μl of test sample/well). All samples were tested in duplicate. After 48 hours, 5 μl of fresh culture medium was added. Parasitemia was determined by flow cytometry (FACSCalibur; BD) after 80–90 hours using ethidium bromide (10 μg/ml; Bio-Rad) to label parasitized erythrocytes. Incubation time was influenced by the stage and synchronicity of parasite cultures at commencement of the assay and by the length of the life cycle of the parasite line used. We confirmed the inhibitory effect of treated samples by testing immunoglobulin purified from the same samples (36).
All serum samples tested for inhibitory antibodies were first treated to remove nonspecific inhibitors that may be present and to equilibrate pH (36). Serum samples (100 μl) were dialyzed against PBS (pH 7.3) in 50-kDa-MWCO microdialysis tubes (2051; Chemicon) and subsequently reconcentrated to the original starting volume using centrifugal concentration tubes (100-kDa MWCO; Pall Corp.). Analysis of flow cytometry data was performed using FlowJo software (Tree Star Inc.). Antibodies against MSP119 (raised against GST fusion protein) used in the assays (at 1:10 final dilution) were generated by vaccination of rabbits and were kindly provided by Brendan Crabb (Walter and Eliza Hall Institute of Medical Research) (44). Samples from nonexposed donors were included as negative controls in all assays, and anti-MSP1 and/or anti-AMA1 antibodies acted as a positive control. Samples were tested for inhibition of the different lines in parallel in the same experiments. A difference between the lines of at least 25% in invasion was designated as the cutoff for differential inhibition by samples.
Preadsorption of treated serum samples against erythrocytes did not alter their invasion-inhibitory activity. A selection of sera was also tested for antibodies against surface antigens of uninfected erythrocytes (maintained in culture) by flow cytometry (49); there was very little reactivity against normal erythrocytes, and there was no relationship between antibody binding to erythrocytes and invasion-inhibitory activity.
Enzyme treatment of erythrocytes.
Erythrocytes were first washed with RPMI-HEPES/25 mM NaHCO3, pH 7.4, and subsequently incubated with neuraminidase (0.067 U/ml; 45 minutes; Calbiochem) or chymotrypsin (1 mg/ml; 15 minutes; Worthington Biochemical) at 37°C. Control treatment was RPMI-HEPES only. After incubation, chymotrypsin-treated cells were washed once with RPMI-HEPES containing 20% human serum and twice with normal culture medium (containing 5% serum) to inhibit enzyme activity. The neuraminidase-treated cells were washed with parasite culture medium 3 times. Treated erythrocytes were then used in invasion inhibition assays as described. All results presented are comparisons with control-treated cells.
Antibodies against recombinant proteins by ELISA.
We coated 96-well plates (MaxiSorp; Nunc) with recombinant GST fusion proteins at 0.5 μg/ml in PBS overnight at 4°C. Plates were washed and blocked with 10% skim milk powder (Diploma) in PBS-Tween 0.05% for 2 hours. After washing, serum samples (100 μl/well in duplicate), at 1:500 dilution in PBS Tween 0.05% plus 5% skim milk, were incubated for 2 hours. Plates were washed and incubated for 1 hour with HRP-conjugated anti-human IgG at 1:5,000 (Chemicon) in PBS-Tween 0.05% plus 5% milk. After washing, color was developed by adding O-Phenylenediamine (Sigma-Aldrich; stopped with concentrated sulfuric acid) or azino-bis(3-ethylbenthiazoline-6-sulfonic acid) liquid substrate system (Sigma-Aldrich; stopped with 1% SDS) and absorbance read by spectrophotometry. All washes were performed with PBS containing 0.05% Tween-20, and all incubations were at room temperature. For each serum, the absorbance from wells containing GST only was deducted from the absorbance from EBA or PfRh GST fusion proteins. Positive and negative controls were included on all plates to enable standardization. Recombinant proteins used were EBA140 (aa 746–1,045) (13), EBA175 W2mef and 3D7 alleles (aa 761–1,271) (50), EBA181 (aa 755–1,339) (15), PfRh4 (aa 1,160–1,370) (20), and PfRh2 (aa 2,027–2,533) (16). Schizonts were separated on a 60% Percoll gradient, washed 3 times in serum-free RPMI-1640, pelleted by centrifugation, and resuspended. The cells were lysed through freeze-thawing, and the supernatant was preserved. Antibody reactivity of a sample was considered positive if the OD was greater than mean + 3 SDs of the nonexposed controls.
Study population and serum samples.
Serum samples (50 adults and 100 children age ≤14 years) were randomly selected from a community-based cross-sectional survey of children and adults resident in the Kilifi District, Kenya, in 1998, a year that was preceded with a relatively high incidence of malaria in the region (50). The area is endemic for P. falciparum. Samples were also obtained from nonexposed adult residents in Melbourne, Victoria, Australia (n = 20), and Oxford, UK (n = 20). Ethical approval was obtained from the Ethics Committee of the Kenya Medical Research Institute, Nairobi, Kenya, and from the Walter and Eliza Hall Institute Ethics Committee, Melbourne, Victoria, Australia. All samples were obtained after receipt of written informed consent. All serum samples were tested for antibodies by ELISA. A subset of 80 of these samples was randomly selected (26% children age ≤5 years; 49% children 6–14 years; 25% adults >14 years) for use in invasion inhibition assays. The same 80 samples were used in all comparative inhibition assays.
Statistics.
Statistical analyses were performed with SPSS and STATA software. The χ2 test or Fischer’s exact test was used for comparisons of proportions. For comparisons of continuous variables, Mann-Whitney U test or Kruskal-Wallis tests were used for nonparametric data, and 2-tailed t tests or ANOVA were used for normally distributed data, as appropriate. Associations between antibodies against recombinant antigens by ELISA and invasion-inhibitory antibodies were examined by 2 approaches. We tested for correlations between ELISA OD values and total invasion inhibition by samples, or the extent of differential inhibition of 2 comparison parasite lines; and we compared the mean or median inhibition by samples grouped as high or low responders according to reactivity by ELISA. For all analyses, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank J. Chesson, C. Mugyenyi, M. Mosobo, T. Mwangi, and B. Lowe for assistance with samples; J. Thompson and J. Richards for EBA/Rh proteins; and B. Crabb and S. Elliott for helpful discussions and reviewing the manuscript. We are grateful to the participants in the study. Erythrocytes were provided by The Red Cross Blood Bank, Melbourne. This manuscript is published with the permission of the director, Kenya Medical Research Institute, and was supported by the National Health and Medical Research Council, Australia (to J. Beeson, A.F. Cowman, and F.J. McCallum); the Wellcome Trust, UK (to K. Marsh and J. Beeson); Teggerstiftelsen, Maud and Birger Gustavssons Stiftelse, Wenner-Gren Fellowship, Sweden (to K.E.M. Persson); The Miller Fellowship (to J. Beeson); and an International Howard Hughes Medical Institute Fellowship (to A.F. Cowman).
Footnotes
Nonstandard abbreviations used: AMA1, apical membrane antigen 1, EBA, erythrocyte-binding antigen; MSP1, merozoite surface protein–1; PfRh, P. falciparum reticulocyte-binding homolog; SA, sialic acid.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:342–351 (2008). doi:10.1172/JCI32138
References
- 1.Marsh K., Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 2.Yazdani S.S., Mukherjee V.S., Chauhan V.S., Chitnis C.E. Immune responses to asexual blood-stages of malaria parasites. Curr. Mol. Med. 2006;6:187–203. doi: 10.2174/156652406776055212. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S., Butcher G.A., Crandall R.B. Action of malarial antibody in vitro. Nature. 1969;223:368–371. doi: 10.1038/223368a0. [DOI] [PubMed] [Google Scholar]
- 4.Brown G.V., Anders R.F., Mitchell G.F., Heywood P.F. Target antigens of purified human immunoglobulins which inhibit growth of Plasmodium falciparum in vitro. . Nature. 1982;297:591–593. doi: 10.1038/297591a0. [DOI] [PubMed] [Google Scholar]
- 5.Wipasa J., Elliott S., Xu H., Good M.F. Immunity to asexual blood stage malaria and vaccine approaches. Immunol. Cell. Biol. 2002;80:401–414. doi: 10.1046/j.1440-1711.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller L.H., Baruch D.I., Marsh K., Doumbo O.K. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 7.Cowman A.F., Crabb B.S. Invasion of red blood cells by malaria parasites. Cell. 2006;124:755–766. doi: 10.1016/j.cell.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Goel V.K., et al. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5164–5169. doi: 10.1073/pnas.0834959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilson P.R., et al. Identification and stoichiometry of glycosylphosphatidylinositol-anchored membrane proteins of the human malaria parasite Plasmodium falciparum. . Mol. Cell. Proteomics. 2006;5:1286–1299. doi: 10.1074/mcp.M600035-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell G.H., Thomas A.W., Margos G., Dluzewski A.R., Bannister L.H. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merzoites to host red blood cells. Infect. Immun. 2004;72:154–158. doi: 10.1128/IAI.72.1.154-158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camus D., Hadley T.J. A Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. . Science. 1985;230:553–556. doi: 10.1126/science.3901257. [DOI] [PubMed] [Google Scholar]
- 12.Mayer D.C., Kaneko O., Hudson-Taylor D.E., Reid M.E., Miller L.H. Characterization of a Plasmodium falciparum erythrocyte binding protein paralogous to EBA175. . Proc. Natl. Acad. Sci. U. S. A. 2001;98:5222–5227. doi: 10.1073/pnas.081075398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maier A.G., et al. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. . Nat. Med. 2003;9:87–92. doi: 10.1038/nm807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson J.K., Triglia T., Reed M.B., Cowman A.F. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. . Mol. Microbiol. 2001;41:47–58. doi: 10.1046/j.1365-2958.2001.02484.x. [DOI] [PubMed] [Google Scholar]
- 15.Gilberger T.W., et al. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. . J. Biol. Chem. 2003;278:14480–14486. doi: 10.1074/jbc.M211446200. [DOI] [PubMed] [Google Scholar]
- 16.Duraisingh M.T., et al. Phenotypic variation of Plasmodium falciparum merozoite proteins directs receptor targeting for invasion of human erythrocytes. EMBO J. 2003;22:1047–1057. doi: 10.1093/emboj/cdg096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rayner J.C., Vargas-Serrato E., Huber C.S., Galinski M.R., Barnwell J.W. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. . J. Exp. Med. 2001;194:1571–1581. doi: 10.1084/jem.194.11.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triglia T., Thompson J.K., Cowman A.F. An EBA175 homologue which is transcribed but not translated in erythrocytic stages of Plasmodium falciparum. . Mol. Biochem. Parasitol. 2001;116:55–63. doi: 10.1016/s0166-6851(01)00303-6. [DOI] [PubMed] [Google Scholar]
- 19.Triglia T., Duraisingh M.T., Good R.T., Cowman A.F. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol. Microbiol. 2005;55:162–174. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 20.Stubbs J., et al. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. . Science. 2005;309:1384–1387. doi: 10.1126/science.1115257. [DOI] [PubMed] [Google Scholar]
- 21.Taylor H.M., Grainger M., Holder A.A. Variation in the expression of a Plasmodium falciparum protein family implicated in erythrocyte invasion. . Infect. Immun. 2002;70:5779–5789. doi: 10.1128/IAI.70.10.5779-5789.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dolan S.A., Miller L.H., Wellems T.E. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. . J. Clin. Invest. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoyeh J.N., Pillai C.R., Chitnis C.E. Plasmodium falciparum field isolates commonly use erythrocyte invasion pathways that are independent of sialic acid residues of glycophorin A. . Infect. Immun. 1999;67:5784–5791. doi: 10.1128/iai.67.11.5784-5791.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed M., et al. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. . Proc. Natl. Acad. Sci. U. S. A. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum J., Pinder M., Conway D.J. Erythrocyte invasion phenotypes of Plasmodium falciparum in the Gambia. . Infect. Immun. 2003;71:1856–1863. doi: 10.1128/IAI.71.4.1856-1863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nery S., et al. Expression of Plasmodium falciparum genes involved in erythrocyte invasion varies among isolates cultured directly from patients. . Mol. Biochem. Parasitol. 2006;149:208–215. doi: 10.1016/j.molbiopara.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bei A.K., et al. Variant merozoite protein expression is associated with erythrocyte invasion phenotypes in Plasmodium falciparum isolates from Tanzania. . Mol. Biochem. Parasitol. . 2007;153:66–71. doi: 10.1016/j.molbiopara.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Orlandi P.A., Klotz F.W., Haynes J.D. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(alpha 2-3)Gal- sequences of glycophorin A. . J. Cell. Biol. 1992;116:901–909. doi: 10.1083/jcb.116.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim B.K.L., Chitnis C.E., Wasniovska K., Hadley T.J., Miller L.H. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. . Science. 1994;264:1941–1944. doi: 10.1126/science.8009226. [DOI] [PubMed] [Google Scholar]
- 30.Sim B.K.L. Plasmodium falciparum: further characterization of a functionally active region of the merozoite invasion ligand EBA-175. Exp. Parasitol. 1994;78:259–268. doi: 10.1006/expr.1994.1027. [DOI] [PubMed] [Google Scholar]
- 31.Lanzilotti R., Coetzer T.L. The 10kDa domain of human erythrocyte protein 4.1 binds the Plasmodium falciparum EBA-181 protein. . Malar. J. 2006;5:100. doi: 10.1186/1475-2875-5-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaur D., et al. Upregulation of expression of the reticulocyte homology gene 4 in the Plasmodium falciparum clone Dd2 is associated with a switch in the erythrocyte invasion pathway. . Mol. Biochem. Parasitol. 2006;145:205–215. doi: 10.1016/j.molbiopara.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Duraisingh M.T., Maier A.G., Triglia T., Cowman A.F. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. . Proc. Natl. Acad. Sci. U. S. A. 2003;100:4796–4801. doi: 10.1073/pnas.0730883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson J.A., Silamut K., Chotivanich K., Pukrittayakamee S., White N.J. Red cell selectivity in malaria: a study of multiple-infected erythrocytes. Trans. R. Soc. Trop. Med. Hyg. 1999;93:165–168. doi: 10.1016/s0035-9203(99)90295-x. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell R.A., et al. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. . 2001;193:1403–1412. doi: 10.1084/jem.193.12.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson K.E.M., Chee T.L., Marsh K., Beeson J.G.2006The development and optimization of high throughput methods to measure Plasmodium falciparum growth inhibitory antibodie s . J. Clin. Microbiol. 441665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baum J., Thomas A.W., Conway D.J. Evidence for diversifying selection on erythrocyte-binding antigens of Plasmodium falciparum and P. vivax. Genetics. 2003;163:1327–1336. doi: 10.1093/genetics/163.4.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rayner J.C., et al. Dramatic difference in diversity between Plasmodium falciparum and Plasmodium vivax reticulocyte binding-like genes. Am J. Trop. Med. Hyg. 2005;72:666–674. [PubMed] [Google Scholar]
- 39.Mamillapalli A., et al. Sequence polymorphisms in the receptor-binding domain of Plasmodium falciparum EBA-175: implications for malaria vaccine development. Mol. Biochem. Parasitol. 2006;146:120–123. doi: 10.1016/j.molbiopara.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Sim B.K., et al. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. . J. Cell Biol. 1990;111:1877–1884. doi: 10.1083/jcb.111.5.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan A.F., Burghaus P., Druilhe P., Holder A.A., Riley E.M. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. . Parasite Immunol. 1999;21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 42.Hodder A.N., Crewther P.E., Anders R.F. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. . 2001;69:3286–3294. doi: 10.1128/IAI.69.5.3286-3294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldi D.L., Andrews K.T., Waller R.F., Drabb B.S., Cowman A.F. RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum. . EMBO J. 2000;19:2435–2443. doi: 10.1093/emboj/19.11.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drew D.R., O’Donnell R.A., Smith B.J., Crabb B.S. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent Plasmodium surface proteins MSP-1 and MSP-8. J. Biol. Chem. 2004;279:20147–20153. doi: 10.1074/jbc.M401114200. [DOI] [PubMed] [Google Scholar]
- 45.Sanders P.R., et al. A set of glycosylphospatidyl inositol-anchored membrane proteins of Plasmodium falciparum is refractory to genetic deletion. . Infect. Immun. 2006;74:4330–4338. doi: 10.1128/IAI.00054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.John C.C., et al. Evidence that invasion-inhibitory antibodies specific for the 19-kDa fragment of merozoite surface protein-1 (MSP-1 19) can play a protective role against blood-stage Plasmodium falciparum infection in individuals in a malaria endemic area of Africa. . J. Immunol. 2004;173:666–672. doi: 10.4049/jimmunol.173.1.666. [DOI] [PubMed] [Google Scholar]
- 47.Preiser P.R., Jarra W., Capiod T., Snounou G. A rhoptry-protein-associated mechanism of clonal phenotypic variation in rodent malaria. Nature. 1999;398:562–563. doi: 10.1038/19309. [DOI] [PubMed] [Google Scholar]
- 48.Beeson J.G., et al. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. . J. Infect. Dis. 1999;180:464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beeson J.G., et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. . J. Infect. Dis. 2004;189:540–551. doi: 10.1086/381186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mwangi T.W., Ross A., Snow R.W., Marsh K.2005Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J. Infect. Dis. 1911932 – 1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.