Abstract
Background
The paraoxonases, PON1–3, play a major protective role both against environmental toxins and as part of the antioxidant defence system. Recently, non‐synonymous coding single nucleotide polymorphisms (SNPs), known to lower serum PON activity, have been associated with sporadic ALS (SALS) in a Polish population. A separate trio based study described a detrimental allele at the PON3 intronic variant INS2+3651 (rs10487132). Association between PON gene cluster variants and SALS requires external validation in an independent dataset.
Aims
To examine the association of the promoter SNPs PON1−162G>A and PON1−108T>C; the non‐synonymous functional SNPs PON1Q192R and L55M and PON2C311S and A148G; and the intronic marker PON3INS2+3651A>G, with SALS in a genetically homogenous population.
Methods
221 Irish patients with SALS and 202 unrelated control subjects were genotyped using KASPar chemistries. Statistical analyses and haplotype estimations were conducted using Haploview and Unphased software. Multiple permutation testing, as implemented in Unphased, was applied to haplotype p values to correct for multiple hypotheses.
Results
Two of the seven SNPs were associated with SALS in the Irish population: PON155M (OR 1.52, p = 0.006) and PON3INS2+3651 G (OR 1.36, p = 0.03). Two locus haplotype analysis showed association only when both of these risk alleles were present (OR 1.7, p = 0.005), suggesting a potential effect modification. Low functioning promoter variants were observed to influence this effect when compared with wild‐type.
Conclusions
These data provide additional evidence that genetic variation across the paroxanase loci may be common susceptibility factors for SALS.
Epidemiological evidence suggests that environmental factors may contribute to the risk of sporadic amyotrophic lateral sclerosis (SALS). Such factors include insecticides, pesticides, arylesterases1,2 and oxidants in cigarette smoke.3 The incidence of SALS is increased in Gulf war veterans who may have been exposed to exogenous neurotoxins.4 These observations have led to the proposition that genetic determinants known to increase susceptibility to exogenous compounds may also increase the risk of SALS.5
The human paraoxonase (PON) gene cluster is located on chromosome 7q21.3. The cluster comprises three genes ordered PON1, PON3 and PON2, with PON1 being the most centromeric.6 The PON1 enzyme acts as the major protective mechanism by which toxic exogenous compounds are hydrolysed in serum.7,8 PON1 has four common functional polymorphisms, two non‐synonymous coding single nucleotide polymorphisms (SNPs) (PON1Q192R and PON1L55M) which alter enzyme activity,9,10 and two promoter SNPs (PON1‐108T>C and PON1‐162G>A) which affect expression levels.11 PON2 and PON3 do not appear to have detoxifying activity, but along with PON1 have important roles in protecting against lipid peroxidation.13,14 Non‐synonymous coding polymorphisms associated with PON2 activity include PON2C311S and PON2A148G.15,16 The risk alleles, associated with lower PON activity, are PON1192R, 55M, −108T and −162G and PON2311C and 148G.9,10,11,12,13,14,15,16
Two recent studies have reported associations between PON gene cluster polymorphisms and increased risk for SALS. The first reported an association of PON1192R and PON2311C with SALS in a Polish population.17 A separate haplotype study of trio pedigrees in the US observed an important intronic marker in PON3 (PON3INS2+3651A>G or rs10487132).18
Reproduction of association in independent populations is of substantial importance to support the hypothesis that PON cluster polymorphisms modify the risk for SALS. Here we test for the association of PON cluster variants with SALS in an Irish population. We selected variants with previous association or an established biological role in PON kinetics, and explored the influence of PON promoter polymorphisms and the PON3 intronic variant.
Methods
Subjects
The study population comprised 221 Irish patients with sporadic ALS (52.9% male; mean age 58.8 (SD 12.8) years) and 202 spousal/population control subjects (50.9% male; mean age 54.9 (14.3) years). All patients fulfilled the El Escorial criteria for clinically definite or probable ALS. Cases with familial ALS, based on a positive family history, were excluded. Control subjects had no history of neurological disease and no family history of ALS. All cases and controls were of Irish Caucasian ethnicity. Informed consent was obtained and the study approved by the Beaumont Hospital Ethics and Medical Research Committee (protocol 49/05).
Marker selection
Comprehensive tagSNP analysis of the PON gene cluster suggests that the majority of the cis effects on serum detoxifying activity are attributable to known non‐synonymous and promoter sequence polymorphisms, which thus capture the functionally important genetic variation across the region.12 Aiming to explore the influence of these functionally relevant PON cluster variants, along with those previously associated with SALS, we selected seven markers for study: the four non‐synonymous polymorphisms known to alter enzyme kinetics (PON1 Q192R and L55M and PON2C311S and A148G), the two promoter polymorphisms known to influence expression levels (PON1−162G>A and −108T>C) and the PON3 intronic marker (PON3INS2+3651A>G) noted from the study in the US.
Genotyping
DNA was extracted from peripheral blood. Genotyping was performed by KBiosciences (Herts, UK) using a KASPar PCR system. Quality control criteria were that genotypes formed three distinct clusters, water controls were negative and minor allele frequency was greater than 5%. The number of genotypes callable was 97.6%. All studied polymorphisms were in Hardy–Weinberg equilibrium (HWE) for the case, control and entire study populations.
Statistical analysis
Individual polymorphisms were analysed for association with SALS by the Pearson χ2 test of independence. Estimations of departures from HWE were also calculated by the χ2 test. For single point associations, p<0.05 was considered significant, and the loci found to be associated were entered into two locus haplotypes to investigate potential interactions. Pairwise linkage diseqilibrium (LD) was examined with the program Haploview (version 3.32).19 Haplotype frequencies were estimated using the accelerated expectation–maximisation algorithm as implemented in Haploview (version 3.32) and Unphased (version 3.0.3).20 Haplotypes incorporating one or more risk alleles are compared with the corresponding haplotype with no risk alleles present. The p values for association of haplotypes with SALS were corrected for multiple comparisons by use of a permutation procedure, as implemented by the program Unphased.20 Multiple permutations were performed (up to 1000) until an even distribution curve of the permuted data was obtained. The permutation procedure gives a corrected p value for association so that values <0.05 can be considered significant.
Results
Table 1 shows the allele frequencies and allele tests of association for each of the genotyped polymorphisms. PON155M (SNP2, p = 0.006) was over‐represented in SALS vs controls. A weaker association signal was observed at PON3INS2+3651G (SNP5, p = 0.03). The two positive markers were not in strong LD (r2 = 0.32) (see supplementary fig; the supplementary fig can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental). The other five markers were negative in our population.
Table 1 Association statistics of the genotyped polymorphisms with risk for sporadic amyotrophic lateral sclerosis.
| SNP No | Variant | NCBI ref | Alleles | HWE | Risk allele (functional variant) | RAF (%) (cases; controls) | χ2 | p Value | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PON1Q192R | rs662 | A>G | 0.78 | G (192R) | 28.3; 32.7 | 1.95 | 0.16 | 0.81 (0.6–1.09) |
| 2 | PON1L55M | rs854560 | T>A | 0.64 | A (55M) | 36.6; 27.6 | 7.59 | 0.006* | 1.52 (1.13–2.04) |
| 3 | PON1‐108T>C | rs705379 | T>C | 0.42 | T | 54.3; 54.7 | 0.01 | 0.93 | 0.99 (0.74–1.31) |
| 4 | PON1‐162G>A | rs705381 | G>A | 0.96 | G | 74.9; 73.7 | 0.15 | 0.7 | 1.06 (0.78–1.45) |
| 5 | PON3IVS2+3651A>G | rs10487132 | A>G | 0.86 | G | 41.6; 34.3 | 4.46 | 0.03* | 1.36 (1.02–1.81) |
| 6 | PON2C311S | rs6954345 | C>G | 0.68 | G (311C) | 23.0; 25.4 | 0.62 | 0.43 | 0.88 (0.64–1.21) |
| 7 | PON2A148G | rs12026 | C>G | 0.24 | G (148G) | 23.9; 24.0 | 0.001 | 0.97 | 0.99 (0.71–1.38) |
HWE, Hardy–Weinberg equilibrium p value; NCBI, National Center for Biotechnology Information; RAF, risk allele frequency; SNP, single nucleotide polymorphism.
*Significant p values.
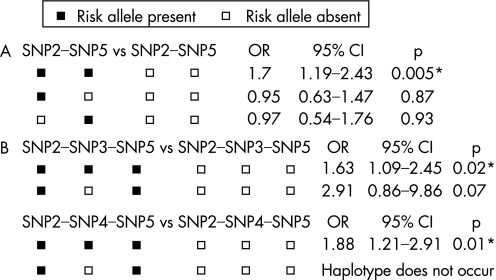
To further explore the influence of the two associated polymorphisms, we next investigated potential interaction between them (fig 1A). The two locus haplotype, comprised of risk alleles at both SNP2 and SNP5, showed association with SALS (p = 0.005) whereas the presence of the risk allele at SNP2 with the wild‐type allele at SNP5 (p = 0.87) and the presence of the risk allele at SNP5 with the wild‐type allele at SNP2 (p = 0.93) did not.
Figure 1 Tests of association with sporadic amyotrophic lateral sclerosis (SALS) for (A) two locus haplotypes comprising single nucleotide polymorphisms (SNPs) 2 and 5. (B) Three locus haplotypes with risk alleles present at SNPs 2 and 5, and low functioning versus wild‐type promoter alleles at SNP3 (upper) and at SNP4 (lower). *Significant p value after correction for multiple testing.
Finally, we investigated potential modification by low functioning promoter variants (SNPs 3 and 4) on the associated haplotype. We tested this by constructing three locus haplotypes consisting of low functioning versus wild‐type promoter alleles at one locus, in addition to the risk alleles at SNPs 2 and 5 (fig 1B). The haplotype incorporating the low functioning SNP3 variant showed association (p = 0.02) whereas the haplotype incorporating wild‐type SNP3 did not (p = 0.07). The haplotype incorporating the low functioning SNP4 variant was associated (p = 0.01) while that incorporating wild‐type SNP4 was estimated not to occur in our population.
Discussion
We found a strong association of PON1L55M, and modest association of PON3INS2+3651A>G, with SALS in the Irish population. The presence of two positive markers prompted us to examine their interaction, leading to identification of a two locus haplotype associated with 70% increased risk. These data add support to the previous reports of association between PON gene cluster variants and SALS.
It is unclear why PON1L55M, rather than PON1Q192R reported by Slowik et al,17 is associated in our population. The associated allele from both the Irish and Polish studies leads to an amino acid substitution resulting in the same effect of reduced serum PON1 activity. As the influence of these polymorphisms is substrate dependent,8 it is possible that our population is exposed to a different deleterious agent, for which the PON1L55M variant, rather than PON1Q192R, is critical. An alternative explanation may be that the positive markers from each population are in LD with another, as yet unidentified, key variant which modifies risk.
Association of PON3INS2+3651G with SALS was described by Saeed et al18 in a large American family based trio model, but not replicated in their smaller case control study group. This latter group exhibited different haplostructure with breakdown of strong LD across PON2–3, raising the possibility of a more genetically heterogeneous population. In contrast, we observed strong LD across PON2–3 (supplementary fig; the supplementary fig can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental) similar to that among the American trios.
We also observed a potential effect modification on the associated haplotype conferred by the presence of a low functioning PON1 promoter polymorphism. It is difficult to draw firm conclusions as the rarity of these haplotypes limited power for analysis, and one of the relevant haplotypes for comparison was not present. This rarity within the population may have arisen by selection given the protective role of PON1 from both environmental toxins and premature cardiovascular disease.7,8,21
Taken together, our findings support the suggestion that genetic variation across the paroxanase loci may be common susceptibility factors for SALS. Two biological explanations for this have been proposed.17,18 Exposure to exogenous environmental toxins, in a genetically predisposed host, may precipitate motoneuron degeneration. However, in vitro studies have consistently reported that only PON1 exhibits such detoxifying properties.13,14 Low paraoxonase activity may alternatively predispose to SALS by rendering the antioxidant defence system insufficient to protect against oxidative stress. All three paraoxonase enzymes exhibit antioxidant properties.13,14 We observed associated polymorphisms in both PON1 and PON3, but haplotype comparisons suggested association only when both risk alleles were present. This supports the notion that the PON3 intronic variant could modify PON1 activity through cis effects, but it remains unclear whether the detoxifying or antioxidant activity of PON1 is impaired.
Association of common variations in PONs with SALS and other neurodegenerative diseases22,23 may have important implications. If environmental substrates, such as insecticides and arylesterases, do indeed increase the risk of neurodegeneration, it follows that occupational health strategies to minimise exposure should be prioritised. Additionally, paraoxonases may have therapeutic potential. Further study will be required to assess the full interaction of environmental exposures, enzyme kinetics and genetic predisposition with risk for SALS.
The supplementary figure can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
Copyright © 2007 BMJ Publishing Group Ltd
Supplementary Material
Acknowledgements
We thank the patients, their families and referring neurologists for their participation in this study.
Abbreviations
ALS - amyotrophic lateral sclerosis
HWE - Hardy–Weinberg equilibrium
LD - linkage diseqilibrium
PON - paraoxonase
SALS - sporadic amyotrophic lateral sclerosis
SNP - single nucleotide polymorphism
Footnotes
Funding: This research was supported by a translational research grant from the Charitable Infirmary Charitable Trust
Competing interests: None.
The supplementary figure can be viewed on the J Neurol Neurosurg Psychiatry website at http://www.jnnp.com/supplemental.
References
- 1.McGuire V, Longstreth W T, Jr, Nelson L M.et al Occupational exposures and amyotrophic lateral sclerosis. A population‐based case‐control study. Am J Epidemiol 19971451076–1088. [DOI] [PubMed] [Google Scholar]
- 2.Chancellor A M, Slattery J M, Fraser H.et al Risk factors for motor neuron disease: a case‐control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry 1993561200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson L M, McGuire V, Longstreth W T., Jret al Population‐based case‐control study of amyotrophic lateral sclerosis in western Washington State. I. Cigarette smoking and alcohol consumption. Am J Epidemiol 2000151156–163. [DOI] [PubMed] [Google Scholar]
- 4.Horner R D, Kamins K G, Feussner J R.et al Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology 200361742–749. [DOI] [PubMed] [Google Scholar]
- 5.Haley R W, Billecke S, La Du B N. Association of low PON1 type Q (type A) arylesterase activity with neurologic symptom complexes in Gulf War veterans. Toxicol Appl Pharmacol 1999157227–233. [DOI] [PubMed] [Google Scholar]
- 6.Primo‐Parmo S L, Sorenson R C, Teiber J.et al The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene family. Genomics 199633498–507. [DOI] [PubMed] [Google Scholar]
- 7.Li W F, Furlong C E, Costa L G. Paraoxonase protects against chlorpyrifos toxicity in mice. Toxicol Lett 199576219–226. [DOI] [PubMed] [Google Scholar]
- 8.Davies H G, Richter R J, Keifer M.et al The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet 199614334–336. [DOI] [PubMed] [Google Scholar]
- 9.Adkins S, Gan K N, Mody M.et al Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet 199352598–608. [PMC free article] [PubMed] [Google Scholar]
- 10.Mackness B, Mackness M I, Arrol S.et al Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non‐insulin dependent diabetes mellitus. Atherosclerosis 1998139341–349. [DOI] [PubMed] [Google Scholar]
- 11.Brophy V H, Jampsa R L, Clendenning J B.et al Effects of 5′ regulatory‐region polymorphisms on paraoxonase‐gene (PON1) expression. Am J Hum Genet 2001681428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson C S, Heagerty P J, Hatsukami T S.et al TagSNP analyses of the PON gene cluster: effects on PON1 activity, LDL oxidative susceptibility, and vascular disease. J Lipid Res 2006471014–1024. [DOI] [PubMed] [Google Scholar]
- 13.Ng C J, Wadleigh D J, Gangopadhyay A.et al Paraoxonase‐2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell‐mediated oxidative modification of low density lipoprotein. J Biol Chem 200127644444–44449. [DOI] [PubMed] [Google Scholar]
- 14.Reddy S T, Wadleigh D J, Grijalva V.et al Human paraoxonase‐3 is an HDL‐associated enzyme with biological activity similar to paraoxonase‐1 protein but is not regulated by oxidized lipids. Arterioscler Thromb Vasc Biol 200121542–547. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki H, Scherer S W, Xi T.et al Human PON2 gene at 7q21. 3: cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene 1998213149–157. [DOI] [PubMed] [Google Scholar]
- 16.Martinelli N, Girelli D, Olivieri O.et al Interaction between smoking and PON2 Ser311Cys polymorphism as a determinant of the risk of myocardial infarction. Eur J Clin Invest 20043414–20. [DOI] [PubMed] [Google Scholar]
- 17.Slowik A, Tomik B, Wolkow P P.et al Paraoxonase gene polymorphisms and sporadic ALS. Neurology 200667766–770. [DOI] [PubMed] [Google Scholar]
- 18.Saeed M, Siddique N, Hung W Y.et al Paraoxonase cluster polymorphisms are associated with sporadic ALS. Neurology 200667771–776. [DOI] [PubMed] [Google Scholar]
- 19.Barrett J C, Fry B, Maller J.et al Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 200521263–265. [DOI] [PubMed] [Google Scholar]
- 20.Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 200325115–121. [DOI] [PubMed] [Google Scholar]
- 21.Baum L, Ng H K, Woo K S.et al Paraoxonase 1 gene Q192R polymorphism affects stroke and myocardial infarction risk. Clin Biochem 200639191–195. [DOI] [PubMed] [Google Scholar]
- 22.Erlich P M, Lunetta K L, Cupples L A.et al Polymorphisms in the PON gene cluster are associated with Alzheimer disease. Hum Mol Genet 20061577–85. [DOI] [PubMed] [Google Scholar]
- 23.Zintzaras E, Hadjigeorgiou G M. Association of paraoxonase 1 gene polymorphisms with risk of Parkinson's disease: a meta‐analysis. J Hum Genet 200449474–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.