Abstract
Polybrominated diphenyl ethers (PBDEs) are a class of flame retardants used in a variety of consumer products. In the past 25 years, PBDEs have become ubiquitous environmental contaminants. They have been detected in soil, air, sediments, birds, marine species, fish, house dust, and human tissues, blood and breast milk. Diet and house dust appear to be the major sources of PBDE exposure in the general population, though occupational exposure can also occur. Levels of PBDEs in human tissues are particularly high in North America, compared to Asian and European countries, and have been increasing in the past 30 years. Concentrations of PBDEs are particularly high in breast milk, resulting in high exposure of infants. In addition, for toddlers, dust has been estimated to account for a large percentage of exposure. PBDEs can also cross the placenta, as they have been detected in fetal blood and liver. Tetra-, penta- and hexa BDEs are most commonly present in human tissues. The current greatest concern for potential adverse effects of PBDEs relates to their developmental neurotoxicity. Pre- or postnatal exposure of mice or rats to various PBDEs has been shown to cause long-lasting changes in spontaneous motor activity, mostly characterized as hyperactivity or decreased habituation, and to disrupt performance in learning and memory tests. While a reduction in circulating thyroid hormone (T4) may contribute to the developmental neurotoxicity of PBDEs, direct effects on the developing brain have also been reported. Among these, PBDEs have been shown to affect signal transduction pathways and to cause oxidative stress. Levels of PBDEs causing developmental neurotoxicity in animals are not much dissimilar from levels found in highly exposed infants and toddlers.
Introduction
Fires are a major health and economic issue, killing yearly more than 3,500 people, injuring more than 18,000, and causing property damage in excess of $10.7 billion in the U.S.A. alone (Karter, 2006). The use of flame retardants corresponded with a drastic drop in fire incidence in the past 30 years. Among chemicals used as flame retardants, there are phosphorus- or nitrogen-containing compounds, and brominated compounds. Some of the latter, e.g. polybrominated biphenyls, were removed from the market following their contamination of animal feed in the 1970s (Dunckel, 1975). Others, such as tetrabromobisphenol A (TBBPA) and hexabromocyclododecane, are still widely used. A third major class of brominated flame retardants is that of polybrominated diphenyl ethers (PBDEs), extensively used in a variety of consumer products such as textiles, carpets, polyurethane foams, electronic cables, television sets and computers. Commercially used PBDEs were marketed as one of three mixtures, known as pentabrominated BDE, octabrominated BDE, and decabrominated BDE. DecaBDE is the most widely used PBDE globally, and is still produced in the USA and in Europe. PentaBDE, whose use was concentrated chiefly in North America, and octaBDE, were banned in the European Union in 2004, and in several states in the USA (e.g. California, Maine, Hawaii) in 2006; they will be banned in the state of Washington from 2008. In the United States, the producer of penta-and octaBDE has voluntarily ceased production in 2004. PentaBDE was added to polyurethane foams used in couches, chairs, automobile seats. OctaBDE was used primarily in plastics used for circuit boards or small appliances, while decaBDE is used in television and computer casings, as well as in textiles.
PBDEs are not fixed in the polymer product through chemical binding, and can thus leak into the environment. PBDEs are chemically similar to the long banned polychlorinated biphenyls (PCBs), and like PCBs, they are persistent organic pollutants, bioaccumulate in the environment and biomagnify up the food chain (Darnerud et al. 2001; Birnbaum and Staskal, 2004; She et al. 2004; Schecter et al. 2005a; Law et al. 2006). There are 209 possible types of PBDE congeners, numbered using the same system as the PCBs. The general structure of PBDEs is shown in Fig. 1, and the chemical names of some of the compounds discussed in this article are shown in Table 1. Widespread contamination of the environment by PBDEs, and their detection in wildlife, and most importantly in human tissues, have raised concerns on possible adverse health effects. As exposure appears to be highest in the young, a major current concern relates to the possible developmental effects of PBDEs (Branchi et al. 2003; Birnbaum and Staskal, 2004; McDonald, 2005). This article intends to discuss aspects of PBDE’s toxicology related to their potential developmental neurotoxicity. Several other reviews on the general toxicology of PBDEs are available (Darnerud et al. 2001; de Wit, 2002; McDonald, 2002; Hardy, 2002; Birnbaum and Staskal, 2004; WDEH, 2006).
Fig. 1.
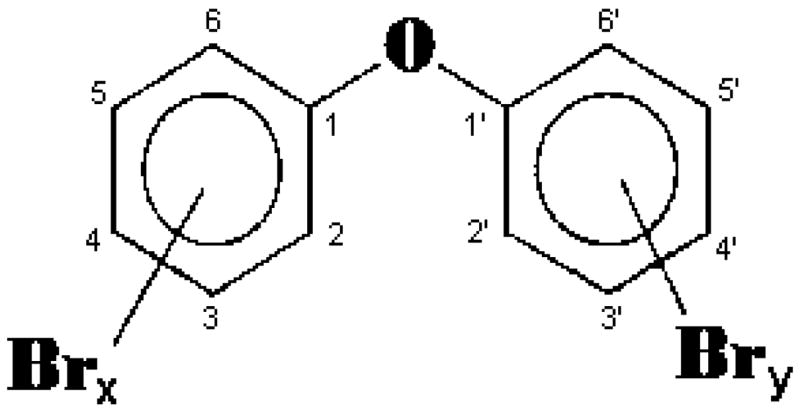
General chemical structure of PBDEs (x + y = 1 to 10).
Table 1.
Major PBDE congeners or mixtures
| BDE-47 | 2,2’,4,4’ - tetrabromodiphenyl ether |
| BDE-99 | 2,2’,4,4’5 - pentabromodiphenyl ether |
| BDE-100 | 2,2’,4,4’,6 - pentabromodiphenyl ether |
| BDE-153 | 2,2’,4,4’,5,5’ - hexabromodiphenyl ether |
| BDE-154 | 2,2’,4,4’,5,6’ – hexabromodiphenyl ether |
| BDE-209 | Decabromodiphenyl ether |
| DE-71a | PentaBDE technical mixture (BDE-99, 44%; BDE-47, 32%; BDE-100, 9%; BDE-153, 4 %) |
| DE-79b | OctaBDE technical mixture (BDE-183, 37%; BDE-197, 22%; BDE-207, 14%; BDE-196, 9%) |
Composition of DE-71 is from Wellington Laboratories (Guelph, ON, Canada). An analysis by La Guardia et al. (2006) similarly indicated the following congeners as the most abundant in DE-71: BDE-99 (49%), BDE-47 (38%), BDE-100 (13%), BDE-153 (5.5%). The European pentaBDE formulation Bromkal 70-5DE had a similar composition (Sjodin et al. 1998).
Composition of DE-79 is from Wellington Laboratories (Guelph, ON, Canada). BDE-183 is a heptaBDE; BDE-197 and BDE-196 are octaBDEs; BDE-207 is a nonaBDE.
PBDEs as environmental pollutants
A number of studies have established the almost ubiquitous presence of PBDEs in the environment, in animals and in humans. PBDEs have been detected in outdoor air, sediments, sludge, soil; in indoor air and house dust; in several food commodities; and in birds, marine species, fish and terrestrial animals (Darnerud et al. 2001; de Wit, 2002; Gill et al 2004; Hites et al. 2004; She et al. 2004; McDonald, 2005; Law et al. 2006; Hazrati and Harrad, 2006; Schecter et al. 2006a; Chen et al. 2007). As in the case with PCBs, they have a biomagnification potential in the food chain (Darnerud et al. 2001). However, in contrast to PCBs and other chlorinated compounds, whose levels in biota have been decreasing in the past three decades, levels of PBDEs have significantly increased (Darnerud et al. 2001). PBDEs have also been detected in human adipose tissue, serum and breast milk (Petreas et al. 2003; Sjodin et al. 2004; Schecter et al. 2005a; Furst, 2006). Five tetra-, penta- and hexa-BDE congeners (BDE-47, −99, −100, −153, −154) predominate in human tissues, usually accounting for 90% of the total body burden (McDonald, 2005). Still widely used decaBDEs (such as BDE-209) are also found in the environment (Law et al. 2006; Chen et al. 2007), where they can be broken down to the lower brominated congeners commonly found in humans (Soderstrom et al. 2004; WDEH, 2006); BDE-209 has been detected in some samples of human milk, and in certain foods (Vieth et al. 2004; Schecter et al. 2005a; Gomara et al. 2006).
Human exposure and body burden
Levels of PBDEs found in human tissues in North America are particularly alarming, as they are one to two orders of magnitude higher than those reported in Europe and Japan (Petreas et al. 2003; Schecter et al. 2003; Inoue et al. 2006). Sources of PBDE exposure can be found in the occupational setting, in the diet, and in the indoor environment. For example, with regard to occupational exposure, in a group of computer dismantlers, serum levels of PBDEs were 26 ng/g, compared to 3.3 ng/g in a reference group of hospital cleaners (Sjodin et al. 1999). Among foods, fish has the highest content of PBDEs, followed by meat and diary products, with meat estimated to be the major source of PBDEs in the U.S. diet (Schecter et al. 2004; Hites et al. 2004; Jones-Otazo et al. 2005; Schecter et al. 2006a). In a small study, vegetarians (vegans) were found to have somewhat lower plasma levels of PBDEs (range 12.4 – 127 ng/g lipid) than the general U.S. population (4 – 366 ng/g lipid) (Schecter et al. 2006b). Above all, high PBDE levels have been found in human breast milk. Table 2 summarizes reported levels of PBDEs in human milk from different countries. It is readily apparent that levels in North America (U.S. and Canada) are much higher than those reported in Europe, Asia or Australia. Levels of PBDEs in breast milk have been increasing in the past 20–30 years (see Table 3), along with serum levels in the general population (Thomsen et al. 2002; Sjodin et al. 2004). Fig. 2 shows the levels of PBDEs in serum found in the United States in 1973 and in 2003. It is evident that while levels of dioxins, furans and PCBs have declined, those of PBDEs have substantially increased. Given the high levels found in breast milk, it has been estimated that a breastfed infant would be exposed to approximately 306 ng/kg/day of PBDE, as compared to about 1 ng/kg/day for adults (Schecter et al. 2006a; see also Table 4). Although PBDE contamination of food is currently higher in the USA than in other countries, diet alone cannot explain the higher levels of PBDEs in the general U.S. population (Jones-Otazo et al. 2005; Wilford et al. 2005; Schecter et al. 2006a; Fischer et al. 2006; Lorber, 2007). Several studies have indicated that house dust is a major source of exposure to PBDEs (Jones-Otazo et al. 2005; Wilford et al. 2005; Schecter et al. 2005b; Wu et al. 2007). For toddlers in particular, dust has been estimated to account for 80% to 93% of PBDE exposure (Wilford et al. 2005). A toddler would be expected to ingest approximately 100 mg/day of house dust (vs 50 mg for an adult; USEPA, 1997), a difference that is compounded by differences in body weight. Table 5 shows serum levels of PBDES found in a California family, where the toddler had the highest body burden. Indeed, in contrast to PCBs, whose concentration increases with age due to accumulation in adipose tissue, PBDE levels do not appear to vary with age in adults (Thomsen et al. 2002). Rather, the highest serum levels of PBDEs are found in infants and toddlers, as a result of exposure through maternal milk and house dust (Fischer et al. 2006; Table 4). PBDEs can also cross the placenta, and similar concentrations are found in maternal and fetal blood, as illustrated in Table 6. Levels of PBDEs ranging from 4 to 98.5 ng/g lipid have also been found in fetal liver (Schecter et al. 2007). In almost all cases, BDE-47, BDE-99 and BDE-153 were among the PBDEs found in highest amounts.
Table 2.
Concentrations of ΣPBDEs in human milk (ng/g lipid)
| Country | Year | N | Median | Range | Most prominent BDE congeners | Reference |
|---|---|---|---|---|---|---|
| U.S.A. | 2001–04 | 59 | 30.1 | 6.2–418 | BDE-47
BDE-153 |
Schecter et al. 2005a |
| U.S.A. (Boston) | 2004–05 | 46 | 28 | 4–263 | BDE-47
BDE-153 |
Wu et al. 2005 |
| U.S.A. (Texas) | 2002 | 47 | 34.0 | 6.1–419 | BDE-47
BDE-99 |
Schecter et al. 2003 |
| U.S.A. (Texas) | 2004 | 25 | 43.0 | 8.9–246 | ND | Ryan et al. 2006 |
| U.S.A/Canada (Pacific Northwest) | 2003 | 40 | 50.0 | 6–321 | BDE-47
BDE-153 |
She et al. 2007 |
| Canada | 2001–02 | 92 | 22.1 | 0.84–956 | BDE-47
BDE-99 |
Gill et al. 2004 |
| Canada | 2005 | 34 | 20 | 3.7–580 | ND | Ryan et al. 2006 |
| Sweden (Uppsala) | 1996–99 | 93 | 3.15 | 0.91–28.2 | BDE-47
BDE-153 |
Lind et al. 2003 |
| Sweden (Uppsala) | 2000–01 | 31 | 2.90 | 1.20–8.07 | BDE-47
BDE-153 |
Lind et al. 2003 |
| Sweden (Uppsala) | ND | 39 | 3.37 | 1.14–28.2 | BDE-47
BDE-153 |
Atuma et al. 2001 |
| Sweden (Stockholm) | 2000–01 | 15 | 2.14 | 0.56–7.72 | BDE-47
BDE-153 |
Meironyte Guvenius et al. 2003 |
| Norway | ND | 151 | 2.34 | 0.95–21.05 | BDE-47
BDE-153 |
Thomsen et al. 2005 |
| Finland | 1994–98 | 11 | 2.25 | 0.88–5.89 | BDE-47
BDE-99 |
Strandman et al. 2000 |
| Faroe Islands | 1999 | 9 | 5.8 | 4.7–13 | BDE-153
BDE-47 |
Fangstrom et al. 2005 |
| Russia | 2000 | 25 | 0.81 | 0.51–3.62 | BDE-47
BDE-153 |
Polder et al. 2006 |
| Russia | 2003–04 | 10 | 0.96 | 0.46–1.7 | BDE-153
BDE-47 |
Tsydenova et al. 2007 |
| Poland | 2004 | 22 | 2.0 | 0.8–8.4 | BDE-47
BDE-153 |
Jaraczewska et al. 2006 |
| Czech Republic | 2003 | 103 | 0.61* | 0.30–1.43* | BDE-47* | Kazda et al. 2004 |
| Germany | 2001–03 | 93 | 1.78 | ND | BDE-47
BDE-153 |
Vieth et al. 2004 |
| Germany | 2002 | 79 | 3.02 | 0.85–24.6 | BDE-47
BDE-153 |
Furst, 2006 |
| United Kingdom | 2001–03 | 54 | 6.3 | 0.3–69 | BDE-47
BDE-153 |
Kalantzi et al. 2004 |
| France | 2005 | 23 | 2.65 | 1.39–11.63 | BDE-47
BDE-99 |
Antignac et al. 2006 |
| Italy | 1998–01 | 39 | ND | 1.6–4.1 | BDE-47
BDE-99 |
Ingelido et al. 2007 |
| Turkey | 2004 | 37 | ND | 0.0–0.4 | BDE-47
BDE-99 |
Erdogrul et al. 2004 |
| Japan | 1999 | 13 | ~1.3 | 0.56–291 | BDE-47
BDE-28 |
Akutsu et al. 2003 |
| Japan | 2003–04 | 105 | 1.28 | 0.01–23 | BDE-47
BDE-99 |
Eslami et al. 2006 |
| Japan | ND | 12 | ND | 0.67–2.84 | BDE-47
BDE-153 |
Ohta et al. 2002 |
| Japan | ND | 89 | 1.54 | 0.49–4.55 | BDE-47
BDE-153 |
Inoue et al. 2006 |
| South China | ND | 27 | 3.5 | 1.5–17 | BDE-47
BDE-153 |
Bi et al. 2006 |
| Taiwan | 2000–01 | 20 | 3.65 | ND | BDE-47
BDE-153 |
Chao et al. 2007 |
| Indonesia | 2001–03 | 30 | 1.5 | 0.49–13 | BDE-47
BDE-153 |
Sudaryanto et al. 2007 |
| Vietnam | 2000 | 10 | 1.1 | ND | BDE-153
BDE-47 |
Sudaryanto et al. 2005 |
| Cambodia | 2000 | 11 | 1.7 | ND | BDE-47
BDE-99 |
Sudaryanto et al. 2005 |
| Korea | 2004 | 9 | 2.6 | ND | BDE-47
BDE-153 |
Sudaryanto et al. 2005 |
| Australia | 2002–03 | 157 | 11.0 | 6.1–18.7 | BDE-47
BDE-99 |
Toms et al. 2007 |
Values are for BDE-47 only. ND, not determined.
Table 3.
Time-related trend of PBDE levels in human milk (ng/g lipid)
| Country | ΣPBDE (year) | ΣPBDE (year) | Fold increase | Reference |
|---|---|---|---|---|
| Japan | Not detected (1973) | 1.39 (2000) | N.M. | Akutsu et. al. 2003 |
| Sweden | 0.07 (1973) | 4.02 (1997) | 57 | Meironyte et al. 1999 |
| Germany | 1.87 (1992) | 3.75 (2002) | 2 | Furst, 2006 |
| Faroe Islands | 1.9 (1987) | 8.2 (1999) | 4 | Fangstrom et al. 2005 |
| Canada | 3.0 (1992) | 22.0 (2002) | 7 | Ryan et al. 2002 |
N.M. = not meaningful
Fig. 2.
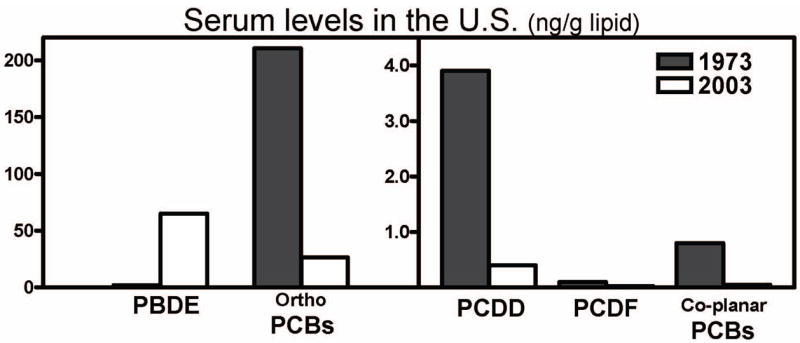
Serum levels (ng/g lipid) of polychlorinated dibenzodioxins (PCDD), polychlorinated dibenzo furans (PCDF), co-planar polychlorinated biphenyls (PCB), mono-ortho substitutes PCBs, and PBDEs in the U.S. population in 1973 and 2003. Data from Schecter et al. (2005).
Table 4.
Estimated exposure of North America population to PBDEs
| Infant | Child (1–5) | Child (6–11) | Adolescent (12–19) | Adult | Reference |
|---|---|---|---|---|---|
| ND | 49.3 | 14.4 | 9.1 | 7.7 | Lorber et al. 2007 |
| ND | ND | ND | ND | 16 | McDonald, 2005 |
| ND | 12–38 | ND | ND | 0.75–4.5 | Wilford et al. 2005 |
| 280 | 20 | ND | ND | 2.2 | Jones-Otazo et al. 2005 |
| 306* | 2.6* | 1.7* | 1.4* | 1.0* | Schecter et al. 2006a |
Data are in ng/kg body weight/day.
Exposure through diet only. ND, not determined.
Table 5.
Serum levels of PBDEs in a family from Northern California in 2004
| Family member | Age | Σ(5)PBDEs* | BDE-47 | BDE-99 | BDE-153 | BDE-209 |
|---|---|---|---|---|---|---|
| Father | 35 | 64 | 32 | 4 | 19 | 23 |
| Mother | 36 | 106 | 60 | 10 | 22 | 14 |
| Female child | 5 | 247 | 137 | 28 | 49 | 143 |
| Male toddler | 1.5 | 418 | 245 | 37 | 75 | 233 |
PBDE levels are expressed as ng/g lipid weight.
Sum of five BDEs (BDE-47, BDE-99, BDE-100, BDE-153, BDE-154). Adapted from Fischer et al. (2006).
Table 6.
PBDE levels in maternal and fetal blood
| Country | Year | N | Maternal blood | Fetal blood | Most prominent BDE congener | Reference |
|---|---|---|---|---|---|---|
| U.S.A. | 1997–99 | 50 | <10–511 (10) | ND | * | Petreas et al. 2003 |
| U.S.A. | 1999–01 | 24 | 5.3–320 (21) | ND | BDE-47 | Bradman et al. 2007 |
| U.S.A. | 2001 | 15 | 15–580 (580) | 14–460 (39) | BDE-47 | Mazdai et al. 2003 |
| Sweden | 2000–01 | 15 | 0.71–8.39 (2.07) | 0.46–4.28 (1.69) | BDE-47 | Meironyte Guvenius et al. 2003 |
| France | 2005 | 26 | 0.21–1.91 (0.69) | 0.14–1.08 (0.41) | ** | Antignac et al. 2006 |
| Japan | ND | 89 | 0.74–21.19 (2.99) | ND | BDE-209 | Inoue et al. 2006 |
| China | ND | 21 | 1.6–17 (4.4) | 1.5–12 (3.9) | BDE-47
BDE-153 (fetus) |
Bi et al. 2006 |
Values of ΣPBDE levels are expressed as ng/g lipid. Results indicate range and median (in parenthesis).
BDE-47 only;
BDE-153 only. ND = not determined.
Toxicity of PBDEs
There is an acceptable body of information on the toxicity of PBDEs, particularly decaBDE, and a comprehensive discussion of PBDE toxicology is outside the goal of this article (see Darnerud et al. 2001; de Wit, 2002; Hardy, 2002; Gill et al. 2004; Birnbaum and Staskal, 2004; McDonald, 2002; 2005; WDEH, 2006). PBDEs have low acute toxicity, with oral LD50s of >5 g/kg. Upon chronic exposure, target organs are the liver, the kidney and the thyroid gland. Different PBDEs appear to have similar toxicological profiles, with decaBDE being less potent than other lower brominated congeners. For instance, in subchronic toxicity studies in rat, NOEL (no-observed-effect-level) values are usually in the g/kg/day range for decaBDE, but less than 10 mg/kg/day for pentaBDE (Darnerud et al. 2001). PBDEs are not genotoxic (Hardy, 2002; Evandri et al. 2003), but an increased incidence of hepatocellular carcinomas and of thyroid adenomas have been observed in rodents upon exposure to BDE-209 (NTP, 1986; Darnerud et al. 2001). Reproductive toxicity has also been reported. Prenatal exposure to BDE-99 has been found to reduce sperm counts in adult rats (Kuriyama et al. 2005), and to alter the ultrastructure of the ovary cells in females (Talsness et al. 2005). Similar findings in the female reproductive system were seen with BDE-47 (Talsness et al. 2004), while BDE-209 (500–1500 mg/kg/day from GD 0 to GD 17) was reported to impair male rat reproductive functions (Hsu et al. 2006). PBDEs can be fetotoxic, but usually at maternally toxic doses, and there is no evidence of teratogenicity. Despite the structural similarities to PCBs, PBDEs do not appear to activate the Ah receptor-AhR nuclear translocator protein-XRE complex, although they can bind to the Ah receptor (Chen and Bunce, 2003; Peters et al. 2006; Hamers et al. 2006). PBDEs may have endocrine disrupting effects, as they have been shown to interact as antagonists or agonists at androgen, progesterone, and estrogen receptors (Meerts et al. 2001; Hamers et al. 2006). For example, most PBDEs have antiandrogenic activity in vitro and in vivo (Stoker et al. 2005); tetra-to hexa-BDEs have potent estrogenic activity in vitro; heptaBDE and 6-OH-BDE-47, a metabolite of BDE-47, have anti-estrogenic activity (Hamers et al. 2006). Hydroxylated BDEs are structurally very similar to thyroid hormones, and have been shown to displace thyroid hormones from the thyroxin plasma transporter transthyretin (TTR) (Meerts et al. 2000). PBDEs have also been reported to decrease levels of total and free T4 in adult animals (Fowles et al. 1994; Hallgren et al. 2001), and following developmental exposure (discussed below). Various PBDEs have been reported to induce mixed-type monoxygenase in vivo. For example, DE-71 was reported to induce CYP1A1 and CYP2B in rats (Zhou et al. 2001), while BDE-47, −99, and −153 upregulate CYP2B and CYP3A, also in rat (Sanders et al. 2005). In a recent study in mice, BDE-47, −99, and −209 were found to induce expression of CYP3A11 and CYP2B10 by activating the pregnane X receptor (PXR) (Pacyniak et al. 2007). Some PBDEs and their metabolites (e.g. OH-BDE-47) have been found to inhibit activity of CYP17, a key enzyme in the synthesis of testosterone, in vitro (Canton et al. 2006). PBDEs have also been shown to induce phase II metabolizing enzymes, such as uridine diphosphoglucuronosyl transferase (UDPGT; Zhou et al. 2002; Skarman et al. 2005) (see section on thyroid hormones for further discussion).
Developmental neurotoxicity of PBDEs
The current greatest concern for potential adverse health effects of PBDEs relates to their developmental neurotoxicity (Branchi et al. 2003; Birnbaum and Staskal, 2004; McDonald, 2005). Such concern is motivated by the following consideration: 1) Animal studies, carried out with different PBDEs, have indicated that pre-and post-natal exposures to PBDEs may cause long-lasting behavioral alterations, particularly in the domains of motor activity and cognitive behavior. Neurochemical changes have also been found following developmental exposure to PBDEs; 2) PBDEs affect thyroid hormone homeostasis, which may result in developmental neurotoxicity; 3) There is indication that young animals may have a reduced ability to excrete PBDEs, and pups have higher tissue (including brain) concentrations than the dams; 4) PBDEs are excreted in milk, and relatively high concentrations are found in North America; 5) Dust has been found to be a major source of exposure; 6) Infants and toddlers have the highest body burden of PBDEs, due to exposure via maternal milk and house dust.
Behavioral studies
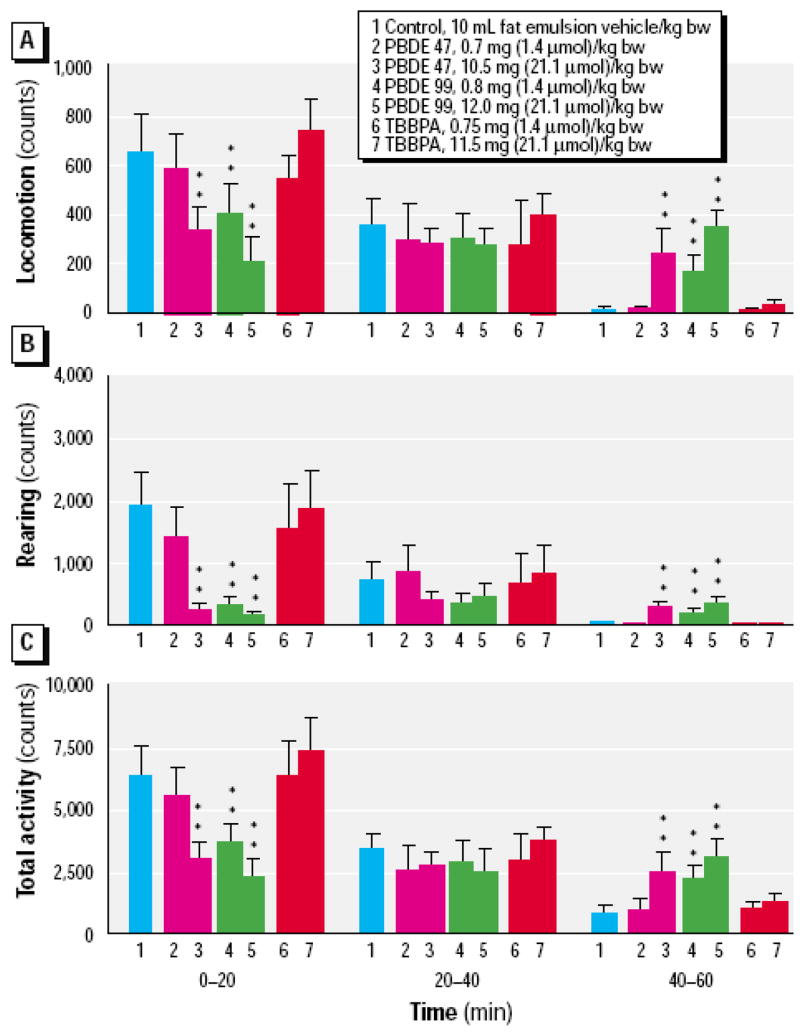
A Swedish group has carried out most studies on developmental neurotoxicity of various PBDEs (see Table 7; Eriksson et al. 2001; 2002; Viberg et al. 2003a; 2003b; 2004a; 2004b; 2006; 2007a). Their experimental protocol involves direct exposure of neonatal mice or rats to PBDEs, as a single oral dose, usually on post-natal day (PND) 10. All PBDEs tested so far (BDE-47, BDE-99, BDE-153, BDE-183, BDE-203, BDE-206, BDE-209) were shown to cause long-lasting changes in spontaneous locomotor behavior. Fig. 3 shows a typical profile of locomotor activity in these experiments. Three variables are measured: horizontal locomotor activity, rearing, and total activity (Eriksson et al. 2001). Mice exposed to PBDEs display significant less activity than controls during the first 20-min. period, but are significantly more active during the third 20-min. period. Control mice display habituation, a decrease in locomotion, rearing and total activity variables in response to the diminishing novelty of the test chamber over 60-min. Thus, the investigators interpret their results (initial hypoactivity and late hyperactivity) as a decrease in habituation caused by PBDEs (Eriksson et al. 2001). An alternative interpretation might be that the decreased activity at the beginning of the session is a possible sign of increased anxiety or arousal, and the elevated activity later in the session is simply the animals finally beginning to explore their environment, as controls did at the beginning of the session. In some cases, the observed behavioral changes appeared to worsen with age (e.g from 2 to 6 month of age; Viberg et al. 2003a; 2003b). Two of the PBDEs tested (BDE-209 and BDE-183, a heptaBDE) did not cause any behavioral change when administered on PND 10; however, they induced the usual changes in locomor activity (decreased habituation) when administered on PND 3 (Viberg et al 2003b; 2006). On the other hand, BDE-99 and BDE-203 (an octaBDE), caused such behavioral alteration when administered on both PND 3 and PND 10 (Eriksson et al. 2001; Viberg et al. 2006). The authors believe that PND 10, at the height of the so-called brain growth spurt, is the most sensitive period for disruption by PBDEs. In case of BDE-209, its ability to induce long-term behavioral changes only when administered on PND 3 has been attributed to its slow accumulation (or that of its metabolites) in the brain (Viberg et al. 2003b). Indeed, levels of [U- 14C]BDE-209 increased from 24.2 nmol/brain on PND 3 to 42.8 nmol/brain on PND 10 (Table 1 in Viberg et al. 2003b). However, similar brain levels (47.8 nmol/brain) were also attained when [U-14C]BDE-209 was given on PND 10 (Table 1 in Viberg et al. 2003b), and they increased even further (to 107.7 nmol/brain) by day 17. This apparent discrepancy may be due to BDE-209 metabolism; if so, metabolites of BDE-209, forming and/or accumulating in the brain over the PND 3–10 time period would be responsible for the observed neurotoxic effects. In case of BDE-99, 3–5% of the administered dose was found in brain (assessed as radioactivity, thus the presence of metabolites cannot be excluded), when exposure occurred on both PND 3 and PND 10 (Eriksson et al. 2002). Brain concentrations of BDE-99 on PND 10 were similar, after administration of the same dose (0.8 mg/kg) on PND 3 or PND 10 (10.7 and 7.2 pmol/g brain, respectively; Eriksson et al 2001; 2002). Thus, persistent similar levels of PBDEs on PND 10 would appear to be associated with long-term behavioral alterations. The susceptibility of this developmental window is also underscored by the fact that BDE-99 and BDE-209 did not cause any behavioral effect when administered on PND 19. In case of BDE-209 levels of brain radioactivity were lower (Viberg et al. 2003b), but for BDE-99 they were comparable to those found after administration on PND 3 or PND 10 (Eriksson et al. 2002). While most of the behavioral studies described so far were carried out in NMRI (Naval Medical Research Institute) mice, similar results were also reported in C57/Bl mice treated with BDE-99 (Viberg et al. 2004a), and in Sprague Dawley rats exposed to BDE-209 (Viberg et al. 2007a). In one study, gender differences in hyperactive behavior were investigated for BDE-99, and none were found (Viberg et al. 2004a).
Table 7.
Neurobehavioral effects of developmental PBDE exposure
| PBDE | Species | Treatment | Effect | Reference |
|---|---|---|---|---|
| BDE-47 | NMRI mice | 0.7, 10.5 mg/kg oral in el/po on PND10 | Decreased habituation at 2, 4 mo. (high dose only) | Eriksson et al. 2001 |
| BDE-47 | Wistar rats | 0.14, 0.7 mg/kg, oral, in po, GD6 | Increased locomotor activity on PND35 and 70 (0.7 mg/kg). Decreased thigmotaxis | Kuriyama et al. 2004a |
| BDE-99 | NMRI mice | 0.2, 0.4, 12.0 mg/kg, oral, in el/po, PND10 | Decreased habituation at 4 mo. (high dose only) | Viberg et al. 2004b |
| BDE-99 | NMRI mice | 8 mg/kg, oral, in el/po, PND3 or 10 or 19 | Decreased habituation at 4 mo. Only if treated on PND 3 or 10 | Eriksson et al. 2002 |
| BDE-99 | NMRI mice | 8 mg/kg, oral, in el/po, PND10 | Decreased habituation at 2 mo. Altered response to nicotine at 2 mo. | Viberg et al. 2002 |
| BDE-99 | C57/BI mice | 0.4, 0.8, 4.0, 8.0, 16.0 mg/kg, oral in el/po, PND10 | Decreased habituation at 2 mo at 8.0 and 16.0 mg/kg. No gender differences | Viberg et al. 2004a |
| BDE-99 | Wistar rats | 0.06, 0.3 mg/kg, oral, in po, GD6 | Hyperactivity (0.3 mg/kg) on PND36, and at both doses on PND71. No effect on male sexual behavior | Kuriyama et al. 2005 |
| BDE-99 | Long Evans rats | 1, 10 mg/kg, sc, in olive oil, GD10- GD18 | Increased sweet preference in males on PND120 (10 mg/kg only) | Lilienthal et al. 2006 |
| BDE-99 | Long Evans rats | 1, 10 mg/kg, sc in olive oil, GD10–18 | Decreased sexual behavior in females | Lichtensteiger et al. 2004 |
| BDE-99 | CD-1 Swiss mice | 0.6, 6.0, 30.0 mg/kg, oral, in co, GD6-PND21 | Hyperactivity on PND22 and 60, not on PND120. Decreased thigmotaxis at 2 mo | Branchi et al. 2002 |
| BDE-99 | CD-1 Swiss mice | 18 mg/kg, oral, in co, by self administration, GD6-PND21 | Hyperactivity on PND34, not at PND60, 90, 120 | Branchi et al. 2005 |
| BDE-99 | NMRI mice | 0.8, 12.0 mg/kg, oral, in el/po, PND10 | Decreased habituation at 2 and 4 mo at both doses. Decreased performance in Morris swim maze at 5 mo. | Eriksson et al. 2001 |
| BDE-153 | NMRI mice | 0.45, 0.9, 9.0 mg/kg, oral, in el/po, PND10 | Decreased habituation at 2, 4, 6 mo at the two higher doses. Effect increases with age. Decreased performance in Morris swim maze at 6 mo. (0.9 and 9.0 mg/kg) | Viberg et al. 2003a |
| BDE-183 | NMRI mice | 15.2 mg/kg, oral, el/po, PND3 or 10 | Decreased habituation at 2 mo. (PND3 only) | Viberg et al. 2006 |
| BDE-203 | NMRI mice | 16.8 mg/kg, oral, in el/po, PND3 or 10 | Decreased habituation at 2 mo. Decreased performance in the Morris swim maze (PND10 only) | Viberg et al. 2006 |
| BDE-206 | NMRI mice | 18.5 mg/kg, oral, in el/po PND3 or 10 | Decreased habituation at 2 mo (PND10 only) | Viberg et al. 2006 |
| BDE-209 | NMRI mice | 2.22, 20.1 mg/kg, oral, in el/po, on PND3 or 10 or 19 | Decreased habituation at 2,4, 6 mo. at the high dose, only if exposed on PND3 | Viberg et al. 2003b |
| BDE-209 | Sprague Dawley rats | 6.7, 20.1 mg/kg, oral, in el/po, PND3 | Decreased habituation at 2 mo with both doses. Altered response to nicotine at 2 mo (high dose only) | Viberg et al. 2007a |
| BDE-209 | C57BL6/J mice | 6, 20 mg/kg, oral, in el/po, PND2–15 | Developmental delay in palpebral reflex. Hyperactivity in males (20 mg/kg) at PND70, not at 1 year | Rice et al. 2007 |
| DE-71 | Long Evans rats | 5, 30, 100 mg/kg, oral, in co, GD6- PND21 | No effect on motor activity | MacPhail et al. 2003 (abstract) |
| DE-71 | Rats | 1, 5, 30, 100 mg/kg, oral, in co, GD6-PND21 | No effect on startle reflex. Decreased fear conditioning in adult males | Taylor et al. 2003 (abstract) |
| DE-71 | Long Evans rats | 30 mg/kg, oral, in co, PND6–12 | Impaired learning in a visual discrimination task on PND30–70. No effect on attention. Subsensitivity to scopolamine effect on attention | Dufault et al. 2005 |
| DE-71 | Killifish (Fundulus heteroclitus) | 0.001–100 ug/L in embryos | Hyperactivity (from 0.001 dose level). Decreased predation avoidance (from 0.01 dose level) | Timme-Laragy et al. 2006 |
Abbreviations: co, corn oil; el/po, egg lecithin/peanut oil.
Fig. 3.
Spontaneous behavior in 4 month-old NMRI mice exposed to a single oral dose of BDE-47, BDE-99 and TBBPA (tetrabromo-bis-phenol A) on PND 10. From Eriksson et al. (2001) with permission.
The experimental protocol used by the Swedish investigators has been at times criticized, particularly as the litter is not used as the statistical unit. However, in a study with BDE-99 (reported only in abstract form; Eriksson et al. 2005) the investigators addressed this issue by showing that randomly selected animals from at least three litters, had the same statistical effect and power compared to the use of a litter based study. Issues have also been raised with regard to the experimental design utilized in these studies, which involves administration of a single dose of PBDEs directly to pups. Nevertheless, evidence from a number of other studies is overall supportive of their findings of alterations of locomotor activity following developmental exsposure to PBDEs (Table 7). In a study in rats, a single exposure to low doses of BDE-99 [0.06 or 0.3 mg/kg on gestational day (GD) 6], resulted in hyperactivity on PND 36 (only at the high dose) and on PND 71 (at both doses) (Kuriyama et al. 2005). Two studies by Branchi et al. (2002; 2005) examined the neurobehavioral development of CD-1 Swiss mice following oral developmental exposure of dams to BDE-99 from GD 7 to PND 21. In the first study, 0.6, 6.0 and 30.0 mg/kg/day of BDE-99 did not cause any alteration in the offspring in sensori-motor development, tested by a slightly modified Fox battery (Fox, 1965), with the exception of a delayed maturation of the climbing response in the high dose group (Branchi et al. 2002). Also, no effects on ultrasonic vocalization and homing behavior were found. However, hyperactivity was found in the offspring on PND 34 and PND 60 at all dose levels, but not at a later age (PND 120). Decreased thigmotaxis, an evidence of reduced anxiety-like response, was observed on PND 60 only in the 6.0 mg/kg group (Branchi et al. 2002). In a follow up study, 18 mg/kg/day BDE-99 were administered to mice from GD 6 to PND 21 either by gavage, or by self administration through a syringe (Branchi et al. 2005). Hyperactivity was again observed in the offspring of both experimental groups, but only on PND 34 and not at later time points (PND 60–120). In both studies no gender differences were observed (Branchi et al. 2002; 2005). An increase in locomotor activity and a decrease in thigmotaxis were also found on PND 35 and 70 in offspring of rats exposed in utero (GD 6) to a low single dose of BDE-47 (0.7 mg/kg) (Kuriyama et al. 2004a). An additional study (reported, however, only in abstract form) investigated the developmental effects of DE-71. DE-71, given to rats from GD 6 to PND 21 (5, 30 or 100 mg/kg/day) was found to cause no changes in activity in the offspring when tested as adults (age not specified) (MacPhail et al. 2003). Rice et al. (2007) exposed C57BL6/J mice to BDE-209 postnatally from PND 2–15. They found a delay in the development of the palpebral reflex, and marginal effects on forelimb strength and struggling behavior in males. On PND 70, male rats were hyperactive, but no differences were observed at one year of age (Rice et al. 2007). Finally, motor activity alterations (hypo- or hyperactivity depending on the dose levels and the test) were also reported in the killifish, an estuarine minnow, upon exposure during the embryonic stage (Timme-Laragy et al. 2006).
In addition to changes in locomotor activity, some PBDEs (e.g BDE-99, BDE-153, BDE-203) were also found to cause cognitive impairment, as decreased spatial memory in a swim (Morris) maze was found upon a single postnatal exposure in mice (Eriksson et al. 2001; Viberg et al. 2003a; 2006). In another postnatal exposure study, Long-Evans rats were given the pentaBDE mixture DE-71 by gavage from PND 6 to 12 (Dufault et al. 2005). Starting on PND 30, animals were tested in a visual discrimination learning task, in which they performed significantly worse than controls. In contrast, DE-71 did not alter sustained attention or inhibitory control, though treated animals were sub-sensitive to the effect of the cholinergic muscarinic antagonist scopolamine on attention (Dufault et al. 2005). Finally, in a study (presented, however, only in abstract form) in which DE-71 was given to rats from GD 6 to PND 21 at the doses of 5, 30 and 100 mg/kg, tests of fear conditioning revealed a dose-dependent decrease in cue- but not context-based performance in male offspring tested as adults (Taylor et al. 2003).
Sex hormone-related behavioral effects have also been reported. In a study by Lilienthal et al. (2006), administration of BDE-99 on GD 10–18 at the dose of 10 mg/kg/day, was found to increase sweet preference in male rats on PND 120. Since sweet preference is normally higher in females, the investigators interpreted the finding as a feminization of this sexually dimorphic behavior by BDE-99. Alterations in the levels of circulating sex hormones (estradiol and testosterone) at weaning and in adulthood were also found in this study. In rats treated with BDE-99 under the same experimental protocol, Lichtensteiger et al. (2004) also reported a decrease in sexual behavior in females, while Kuriyama et al. (2005) did not find any alteration in sexual behavior in male rats prenatally exposed to a single low dose of BDE-99.
In summary, available behavioral studies indicate that all PBDEs tested in rodents under different exposure protocols cause alterations in neurobehavioral development. Mostly, changes are seen in the motor domain, with hyperactivity apparently being the most prominent effect, and in the cognitive domain, with reduced learning and memory. Still unclear is whether gender differences exist, as males appear to be more affected in some studies but not in others, and whether the hyperactivity is permanent (or even worsening with age; e.g. Viberg et al. 2003a), or only transient (Branchi et al. 2002; 2005; Rice et al. 2007).
Effects on thyroid hormones
Thyroid hormones are known to play a relevant role in brain development (Chan and Rovet, 2003; LaFranchi et al. 2005), and hypothyroidism has been associated with a large number of neuroanatomical and behavioral effects (Schalock et al. 1977; Haddow et al. 1999; Zoeller and Crofton, 2005). Several chemicals can disrupt thyroid function leading to a decrease in thyroxine (T4). Among these are propylthiouracyl (PTU), which inhibits thyroid peroxidase (Zoeller and Crofton, 2005), perchlorate, which inhibits iodine uptake into the thyroid (Wolff, 1998), and various environmental contaminants (e.g. PCBs, polyaromatic hydrocarbons), that may act by inducing T4 metabolism (Zoeller et al. 2007).
Various studies have found that PBDEs can perturb the thyroid system both in adulthood and during development. Fowles et al. (1994) first reported that adult mice exposed for two weeks to DE-71 had decreased levels of circulating T4. Exposure of adult mice and rats to BDE-47 or to the pentaBDE mixture Bromkal 70-5DE, decreased serum total and free T4 levels, without altering thyrotropin (TSH) levels (Hallgren et al. 2001; Hallgren and Darnerud, 2002). Exposure of two month-old C57BL/6 mice to BDE-47 for 4 days was found to cause a significant decrease of total T4 levels (Richardson et al. 2006). With regard to developmental exposures (see Table 8), Zhou et al. (2001) reported that treatment of weanling female rats with DE-71 or DE-79, but not DE-83R (98% decaBDE), caused a reduction of serum T4 levels, without altering those of triiodotyronine (T3). In a subsequent paper, Zhou et al. (2002) found that exposure of rats to DE- 71 from GD 6 to PND 21 caused a significant decrease of serum T4 in the dam on GD 20 and PND 22, and in the fetuses and pups on GD 20, PND 4 and PND 14, with a recovery by PND 36. A similar treatment with DE-71 in rats (GD 6-PND 18) was found to decrease serum T4 levels in dams on PND 19, and in pups on PND 18, with a full recovery by PND 31 (Ellis-Hutchings et al. 2006). There were no changes in T3 and TTR levels, but TSH levels in dams were increased. Upon exposure of mice to BDE-99 or Bromkal 70-5DE from GD 4 to PND 18 no changes in serum T4 were found in dams on GD 17 and PND 20 (Skarman et al. 2005). In contrast, Bromkal 70-5DE, but not BDE-99, decreased serum T4 concentration in pups on PND 11 and 18, but not on PND 37. Branchi et al. (2005) found a non significant decrease of serum T4 on PND 22, following developmental exposure to BDE-99 in mice. Postnatal exposure of rats to BDE-209 was reported to decrease the serum levels of T4 in male animals on PND 22 (Rice et al. 2007). Exposure to BDE-47 on GD 6 was found to decrease T4 levels in dams on PND1, but not on PND 22 (Kuriyama et al. 2004b), and to decrease T4 levels in pups on PND 14, but not on PND1 or PND 22 (Talsness et al. 2004). American kestrels (Falco sparverius) given a pentaBDE mixture in ovum , also displayed lower serum T4 levels at 36 days of age (Fernie et al. 2005). Also, ranch minks (Mustela vison) fed a diet containing 5 or 10 ppm DE-71 for 11 weeks from weaning, had lower levels of T4, as well as of T3 (Martin et al. 2004).
Table 8.
Effect of developmental exposure to PBDEs on plasma T4 levels
| PBDE | Species | Treatment | Effect | Reference |
|---|---|---|---|---|
| BDE-47 | Wistar rats | 0.14, 0.7 mg/kg, oral, in po, GD6 | Decrease in females on PND14, not on PND1, 22. Decrease in dams on PND1, not on PND22 | Talsness et al. 2004; Kuriyama et al. 2004b |
| BDE-99 | CD-1 Swiss mice | 18 mg/kg, oral, in co, GD6- PND21, by self administration | N.S. decrease on PND22 | Branchi et al. 2005 |
| BDE-99 | NMRI mice | 80 umol/kg, oral, in co, every 3 days, GD4- PND17 | No effect | Skarman et al. 2005 |
| BDE-209 | C57BL6/J mice | 6, 20 mg/kg, oral, in el/po, PND2–15 | Decrease in males on PND21 (20 mg/kg) | Rice et al. 2007 |
| DE-71 | Long Evans rat | 0.3, 1, 3, 10, 30, 100, 300 mg/kg, oral in co, PND28–31 (female only) | Decrease on PND32. No effects on T3 | Zhou et al. 2001 |
| DE-71 | Long Evans rats | 1, 10, 30 mg/kg, oral, in co, GD6- PND21 | Decrease on GD20 (fetus), PND4, 22 (10,30 mg/kg) Recovery by PND36 | Zhou et al. 2002 |
| DE-71 | Sprague Dawley rats | 18 mg/kg, oral in co, GD6- PND18 | Decrease on PND18. Recovery by PND31 | Ellis-Hutchings et al. 2006 |
| DE-71 | Rats | 1, 5, 30, 100 mg/kg, oral, in co, GD6- PND21 | Decrease on PND5, 14 (5–100 mg/kg) | Taylor et al. 2003 (abstract) |
| DE-71 | Ranch mink (Mustela vison) | 1, 5, 10 ppm in feed for 11 wks from weaning | Decrease at 5, 10 ppm. Decrease of T3 | Martin et al. 2004 |
| Bromkal- 705DE | NMRI mice | 80 umol/kg, oral, in co, every 3 days GD4-PND17 | Decrease on PND11. Recovery by PND18 | Skarman et al. 2005 |
| DE-79 | Long Evans rats | 0.3, 1, 3, 10, 30, 60, 100 mg/kg, oral in co, PND28–31 (female only) | Decrease on PND32. No effect on T3 | Zhou et al. 2001 |
| DE-83R | Long Evans rats | 0.3, 1, 3, 10, 30, 60, 100 mg/kg, oral in co, PND28–31 (female only) | No effect | Zhou et al. 2001 |
Abbreviations: co, corn oil; el/po, egg lecithin/peanut oil; N.S., not significant.
DE-83R is a >98% decaPBDE mixture.
Though these studies are quite consistent in showing a decrease in T4 levels following exposure to PBDEs, less is known on possible underlying mechanisms. Current hypotheses relate to an enhanced metabolism and excretion of T4 as a result of exposure to PBDEs, or to an interaction of PBDEs with the thyroid hormone transport system. Zhou et al. (2002) found that the decrease in T4 was associated with induction of UDPGT, a key phase II metabolizing enzyme involved in conjugation of T4. Such increased metabolism results in enhanced excretion and hence in reduced circulating levels of T4 (Barter and Klaassen, 1992). Induction of UDPGT was also found by Skarman et al. (2005) in mice. However, induction of UDPGT alone cannot explain the reduced T4 levels induced by PBDEs. Indeed, in the Zhou et al. (2002) study, a decrease in T4 levels was seen at a lower dose level (10 mg/kg/day) than that necessary to cause UDPGT induction (30 mg/kg/day). Hallgren et al. (2001) reported a similar finding in adult rats; additionally, in the same study, T4 levels were also decreased in mice, where no induction of UDPGT was present. Similarly, in adult mice, the decrease in serum T4 caused by BDE-47 was not paralled by an increase of hepatic UDPGT activity, though its hepatic mRNA levels were increased (Richardson et al. 2006). Also, changes in serum T4 levels following gestational exposure to BDE-47 did not parallel changes in hepatic UDPGT activity (Kuriyama et al. 2004b).
An alternative/complementary hypothesis is that PBDEs may interfere with thyroid hormone transport. Meerts et al. (2000) reported that several PBDEs could interact with TTR, one of the thyroid hormone-binding proteins in plasma, thereby displacing T4. However, such interaction only occurred in the presence of phenobarbital-treated microsomes (enriched in CYP 1A, 2B and 4A3), implicating one or more PBDE metabolites. Some hydroxylated PBDEs [e.g. 2, 6-dibromo -4- (2,4,6-tribromo-phenoxy) phenol], synthesized for their structural resemblance to thyroid hormones, were most potent in displacing T4 from TTR. A high potency of 6-OH-BDE-47 (a metabolite of BDE-47; Darnerud et al. 2001) in displacing T4 from TTR was confirmed by Hamers et al. (2006). While the exact role that this mechanism may play in the regulation of serum concentrations of T4 is unknown, displacement of T4 from TTR may lead to increased glucuronidation and a consequent lower level of T4. Of interest is also that BDE-47 increases hepatic mRNA levels of TTR in adult mice (Richardson et al. 2006).
Concern has been expressed that PBDEs may contribute to thyroid and neurodevelopmental health effects in North America (Muir, 2005). Independent of the underlying mechanisms, the effect of PBDEs on thyroid hormone homeostasis may be of interest and significance. Behavioral studies in hypothyroidism (induced by developmental exposure to PTU from GD 3 to PND 20) have evidenced decreases in learning and habituation to maze tests, changes in anxiety-like behavior, and increases in locomotor activity in rats (Negishi et al. 2005). As indicated in a previous section, some of these effects are seen following developmental exposure to PBDEs. However, the PTU treatment caused T4 level to fall below the limit of detection in offspring at PND 21 (Negishi et al. 2005), while decreases of T4 following developmental exposure to PBDEs are less pronounced (10–60%). Nevertheless, in rats, a 60% decrease in T4 levels has been associated with a 15-dB hearing loss (Crofton, 2004), and a good correlation between ototoxicity and decrease of T4 has been established for PCBs (Crofton and Zoeller, 2005). Investigating potential ototoxicity of developmental exposure to PBDE would thus be of interest. It should also be pointed out that decrements in neurological development in children of mothers with 25% decrease in T4 have been reported (Haddow et al. 1999), suggesting indeed that effects of PBDEs on thyroid hormones may contribute to their developmental neurotoxicity.
Biochemical studies following in vivo developmental exposure
There are only few studies that have investigated biochemical/molecular changes occurring in the central nervous system of animals following developmental exposure to PBDEs (Table 9). The rationale for some of these studies is not always clear, though some experiments appear to have been designed on the basis of previous findings with PCBs. Viberg and collegues found a decreased number of cholinergic nicotinic receptors in hippocampus of mice following postnatal exposure (single dose at PND 10) to BDE-99 and BDE-153 (Viberg et al. 2003a; 2004b). In both cases the decrease was in the range of 20–30%, and was observed only at the high dose level of PBDE. Strangely, however, levels of [3H]-alpha-bungarotoxin binding in hippocampi of control animals differed by 3.5-fold in the two experiments (9.83 vs. 35.16 pmol/mg protein), though the only appreciable difference was the age of the animals (4 vs. 6 months). It is also unclear how these nicotinic receptor changes would correlate with the observed altered response to nicotine, which caused hyperactivity in 2 month-old control mice, but hypoactivity in mice exposed to BDE-99 on PND-10 (Viberg et al. 2002). A similar altered response to nicotine was also observed in 2 month-old rats exposed on PND 3 to BDE-209 (Viberg et al. 2007a). Similar changes in hippocampal nicotinic receptors and in response to nicotine had been previously observed in mice exposed to a PCB (Eriksson and Fredriksson, 1996). In contrast to these findings, Bull et al. (2007) did not find any changes in nicotinic receptors in cerebral cortex of ranch mink (Mustela vison) following chronic developmental exposure to DE-71; muscarinic receptors, acetylcholinesterase activity, and acetylcholine levels were also unaffected (Bull et al. 2007).
Table 9.
Neurochemical effects of developmental exposure to PBDEs
| PBDE | Species | Treatment | Effect | Reference |
|---|---|---|---|---|
| BDE-47 | C57BI/6 mice | 6.8 mg/kg, oral in el/po, on PND10 | Decrease GluR subunits NR2B, GluR1, CaMKII | Dingemans et al. 2007 |
| BDE-99 | NMRI mice | 0.2,0.4,12.0 mg/kg oral, in el/po, on PND10 | Decreased NR in hippocampus at 4 mo., only at 12 mg/kg | Viberg et al. 2004b |
| BDE-99 | NMRI mice | 12 mg/kg, oral in el/po, on PND10 | On PND12 changes in a number of proteins in hippocampus, striatum (proteomics analysis) | Alm et al. 2006 |
| BDE-99 | Wistar rats | 30 mg/kg, sc, in olive oil, GD2–9 | Increase in cGMP, NMDA-stimulated cGMP, calmodulin , soluble GC alpha subunit. No change in nNOS | Llansola et al. 2007 |
| BDE-153 | NMRI mice | 0.45, 0.9, 9.0 mg/kg, oral, in el/po, on PND10 | Decreased NR in hippocampus on PND180 (9.0 mg/kg only) | Viberg et al. 2003a |
| BDE-209 | NMRI mice | 20.1 mg/kg, oral, in el/po, on PND3 | Decreased BDNF, Gap-43, CaMKII at PND10, not at PND4 | Viberg et al. 2007b (abstract) |
| DE-71 | Ranch mink | 0.01, 0.05, 0.25 mg/kg, in feed, GD0-PND42 (weaning) | No effects on MR, NR, AChE, ACh levels (PND 42, 190) | Bull et al. 2007 |
Abbreviations: el/po, egg lecithin/peanut oil; ACh, acetylcholine; AChE, acetylcholinesterase; MR, muscarinic receptors; NR, nicotinic receptors; GC, guanylate cyclase; nNOS, neuronal nitric oxide synthase.
In vivo exposure of mice to BDE-47 (6.8 mg/kg on PND 10) was found to reduce hippocampal long term potentiation and post-tetanic potentiation, and to decrease key postsynaptic proteins involved in glutamate receptor signaling [e.g. glutamate receptor subunits NR2B and GluR1, and autophosphorylated-active alpha Ca2+/calmodulin-dependent protein kinase II (CaMKII)] at PND 17–19 (Dingemans et al. 2007). A decrease of CaMKII, in addition to decreases in the levels of brain derived neurotrophic factor (BDNF) and of Gap-43 (neuromodulin) were also found on PND 10 in mice exposed to BDE-209 on PND 3 (Viberg et al. 2007b). A proteomic analysis of hippocampus and striatum of mice 24 h after the administration of BDE-99 (12 mg/kg on PND 10) revealed changes in the levels of a number of proteins (Alm et al. 2006). Among these, Gap-43, which was increased in the striatum, and stathmin, which was decreased. Both are substrates of protein kinase C (PKC), which has been shown to be affected by PBDEs in vitro (Madia et al. 2004; Kodavanti et al. 2005; Kodavanti and Ward, 2005). Various enolases, as well as alpha synuclein were also increased in the hippocampus (Alm et al. 2006). In a study in rats, prenatal exposure to BDE-99 was shown to increase the activity of the glutamate -nitric oxide-cGMP pathway, as assessed by microdialysis (Llansola et al. 2007). This effect was possibly secondary to an increase in calmodulin and soluble guanylate cyclase, and would lead to increased production of nitric oxide in brain, which in turn may increase nitrosylation of proteins thereby altering their functions (Llansola et al. 2007).
Overall, the few and scattered studies available provide only limited information on possible mechanisms underlying developmental neurotoxic effects of PBDEs. More work is certainly needed in this area, particularly keeping in consideration the results of the behavioral studies (to investigate possible neurochemical substrates of behavioral changes), and of the in vitro studies (to validate the findings in vivo upon developmental exposure).
Biochemical studies in vitro
A number of studies (see Table 10) have examined potential effects of PBDEs in vitro, mostly in neuronal or astroglial cell preparations. As for the in vivo studies, many of the end-points studied in vitro were chosen as they had been shown to be affected by PCBs. Most effects are seen at micromolar concentrations of PBDEs. However, a study by Mundy et al. (2004) has indicated that, at least for BDE-47, concentrations present in the medium underestimate tissue concentrations by up to two orders of magnitude. For example, incubation of rat cortical neurons with 1 uM BDE-47 for 60 min, resulted in an intracellular concentration of 100 uM. As all PBDEs are highly lipophilic compounds, this observation may extend to other congeners as well. This study also noted that if serum was present in the incubation medium, accumulation of BDE-47 in cells was lower by 5-fold, suggesting that PBDEs may bind to serum proteins.
Table 10.
In vitro effects of PBDEs in neuronal or astroglial cells
| PBDE | Cell type | Concentration | Effect | Reference |
|---|---|---|---|---|
| BDE-47 | CGN rat | 10–50 uM | Increased PBDU binding (10 uM). Increased LDH release (100 uM) | Kodavanti et al. 2005 |
| BDE-47 | PC12 cells | 2, 20 uM | Increased Ca++ and increased vesicular release of catecholamines (both at 20 uM) | Dingemans et al. 2007 |
| BDE-47 | SON tissue punches from LE and SD rats | 5, 15 uM | Decreased vasopressin release at both concentrations | Coburn et al. 2007 |
| BDE-47 | SH-SY5Y (human neuroblastoma) | 4–16 uM | Increased levels of ROS and lipid peroxidation. Increased apoptotic cell death | Zhang et al. 2007 |
| BDE-77 | CGN rat | 10–50 uM | Increased PBDU binding at 30 uM | Kodavanti et al. 2005 |
| BDE-77 | SON tissue punches from LE and SD rats | 15 uM | Decreased vasopressin release | Coburn et al. 2007 |
| BDE-99 | CGN Wistar rat | 0.1, 0.3, 1.0 uM | Increased cGMP, NMDA- stimulated cGMP, calmodulin, soluble GC | Llansola et al. 2007 |
| BDE-99 | 1321N1 cells (human astrocytoma) | 1–100 uM | Decreased cell viability (MTT, IC50=47 uM). Increased translocation of PKC,(100 uM). Increased apoptosis and p53 (50, 100 uM). No increase in LDH release. | Madia et al. 2004 |
| BDE-99 | PC12 cells, rat astrocytes | ?uM | Increased Ca++ | Smolnikar et al. 2001 (abstract) |
| BDE-99 | CGN rat | 10–50 uM | Increased PDBU binding (10 uM) | Kodavanti et al. 2005 |
| BDE-100 | CGN rat | 10–50 uM | Increased PBDU binding (10 uM) | Kodavanti et al. 2005 |
| BDE-153 | CGN rat | 10–50 uM | Increased PBDU binding (10 uM) | Kodavanti et al. 2005 |
| DE-71 | Rat brain synaptosomes | 2–20 uM | Inhibition of vesicular dopamine uptake (5 uM). No effect on synaptosomal uptake of dopamine, GABA, glutamate | Mariussen and Fonnum, 2003 |
| DE-71 | CGN rat | 2–100 uM | Increased arachidonic acid release (10 ug/ml) | Kodavanti and Derr-Yellin, 2002 |
| DE-71 | CGN rat | 6–60 uM | Increased PBDU binding. No LDH release up to 50 ug/ml | Kodavanti and Ward, 2005 |
| DE-71 | Microsomes and mitochondria from rat brain areas | 6–60 uM | Decreased microsomal and mitochondrial Ca++ uptake (>3 ug/ml). | Kodavanti and Ward, 2005 |
| DE-71 | CGN rat | 2–20 uM | Increased apoptotic cell death. Prevented by Vit. E but no increase in ROS. No increase in Ca++ influx | Reistad et al. 2006 |
| DE-71 | SON tissue punches from LE and SD rats | 10, 30 uM | Decreased vasopressin release (30 uM) | Coburn et al. 2007 |
| DE-71 | Rat cortical neurons | 1–30 uM | Increased MAPK phosphorylation | Mundy et al. 2002 |
| DE-71 | CGN mouse | 1–50 uM | Increased levels of ROS. Increased apoptotic cell death | Costa et al. 2007 |
| DE-79 | CGN rat | 2–100 uM | No effect on arachidonic acid release | Kodavanti and Derr-Yellin, 2002 |
| DE-79 | CGN rat | 6–60 uM | No effect on PBDU binding, microsomial and mitochondrial Ca++ uptake | Kodavanti and Ward, 2005 |
| DE-79 | Rat brain synaptosomes | 2–20 uM | No effect on neurotransmitter uptake | Mariussen and Fonnum, 2003 |
| DE-83R | Rat brain synaptosomes | 2–20 uM | No effect on neurotransmitter uptake | Mariussen and Fonnum, 2003 |
Abbreviations: CGN, cerebellar granule neurons; SON, supraoptic nerve; LE, SD rats, Long-Evans, Sprague Dawley rats ; GC, guanylate cyclase.
Studies in rat cerebellar granule neurons have shown that PBDEs can interfere with signal transduction pathways. DE-71 caused stimulation of arachidonic acid release by activating the phospholipase A2 pathway (Kodavanti and Derr-Yellin, 2002). On a molar basis, DE-71 was as potent as the PCB mixture Aroclor 1254, and the minimal effective concentration of DE-71 was about 15 uM. In contrast, the octaBDE mixture DE-79 was inactive in this regard. DE-71 also increased the binding of a phorbol ester (3H-PDBu), an index of PKC translocation (Kodavanti and Ward, 2005), but with less potency than Aroclor 1254. Again, DE-79 was ineffective. A subsequent examination of individual PBDE congeners showed that several PBDEs (BDE-47, BDE-99, BDE-77, BDE-100, BDE-153) could increase 3H-PDBu binding at concentrations of 10 uM and higher (Kodavanti et al. 2005). The potency of PBDE congeners appeared to correlate with their accumulation in neurons, with BDE-47 being the most potent (IC50 = 34 uM). Activation of various PKCs (alpha, epsilon, zeta) by BDE-99 was also observed in a human astrocytoma cell line (1321N1; Madia et al. 2004). In microsomal and mitochondrial preparations from rat brain (frontal cortex, hippocampus, cerebellum) DE-71 was found to inhibit uptake of 45Ca++ (Kodavanti and Ward, 2005). Effective concentrations were as low as 5 uM, while DE-79 was devoid of inhibitory activity. Micromolar concentrations of BDE-99 were reported to increase Ca++ concentrations in rat astrocytes, PC-12 cells and human macrophages [Wiegand et al. 2001; Smolnikar et al. 2001 (abstract)]. Similarly, BDE-47 (20 uM) was reported to increase [Ca++] in PC-12 cells (Dingemans et al. 2007). In contrast, DE-71 (30 uM) did not cause any Ca++ elevation in cerebellar granule cells (Reistad et al. 2006), and octaBDE (10–40 uM) did not alter Ca++ levels in mouse thymocytes (Sandal et al. 2004).
A number of studies have also examined the effects of PBDEs on cell viability. In rat cerebellar granule cells, DE-71 did not cause any release of lactate dehydrogenase (LDH) at up to 60 uM for 240 min (Kodavanti and Ward, 2005), while following the same incubation period, BDE-47 caused a small but significant increase of LDH release at 50 uM (Kodavanti et al. 2005). Similarly, in human astrocytoma cells, no cytotoxicity (assessed by measuring LDH release or Trypan blue exclusion) was found with up to 100 uM BDE-99 for 24 h (Madia et al. 2004). However, the mitochondrial activity that cleaves MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was significantly decreased by BDE-99, with an IC50 of 46.5 uM (Madia et al. 2004). Further experiments in this glial cell line indicated that a PKC inhibitor (GF 109023X) or down-regulation of classical and novel PKCs by pre-incubation with a phorbol ester, did not affect the cytotoxicity of BDE-99 observed in the MTT assay (Madia et al. 2004). The membrane permeable calcium chelator BAPTA-AM also failed to affect BDE-99 cytotoxicity, suggesting that the observed effects on PKC and calcium homeostasis may not be involved in BDE-99 toxicity in astrocytoma cells.
In apparent contrast with the results of Kodavanti and Ward (2005), in rat cerebellar granule cells DE-71, but not DE-79, caused cytotoxicity (measured by the Trypan blue exclusion assay) at relatively low concentrations (IC50 = 7 uM) (Reistad et al. 2006). Cytotoxicity was partially reduced by an antagonist of the N-methyl D-aspartate (NMDA) receptor, and particularly by the lipid soluble antioxidant alpha-tocopherol, but not by a calcium chelator (Reistad et al. 2006). Interestingly, addition of an S9 fraction decreased the toxicity of DE-71, suggesting that possible metabolites possess lower cytotoxicity. An analysis of nuclear morphology and DNA laddering indicated that DE-71 caused apoptotic cell death, that was judged to be caspase 3-independent (Reistad et al. 2006). BDE-99 (50 uM) was also shown to cause apoptotic cell death and to increase levels of p53 in human astrocytoma cells (Madia et al. 2004).
As previously described, Reistad et al. (2006) found that neurotoxicity of DE-71 was prevented by an antioxidant; however, as they did not find any increase in the level of reactive oxygen species (ROS), they attributed the protective effects of alpha-tocopherol to an influence on membrane properties. However, the same authors found that in human neutrophil granulocytes, DE-71, as well as BDE-47, enhanced the production of ROS (Reistad and Mariussen, 2005). They also proposed, based on results with a number of inhibitors, that the effect of PBDEs on ROS was mediated through activation of tyrosine kinase, PI-3 kinase, PKC, phospholipase C, and intracellular calcium, leading to activation of NADPH oxidase, which plays a major role in ROS formation and the activation of the respiratory burst in these cells (Reistad and Mariussen, 2005). Another brominated flame retardant, TBBPA, also caused an increase of ROS levels in human neutrophil granulocytes, that may similarly involve tyrosine kinase, PKC, calcium and NADPH oxidase (Reistad et al. 2005). This same compound was further shown to increase intracellular [Ca++], to increase ROS and to induce apoptotic cell death in rat cerebellar granule neurons (Reistad et al. 2007). The apparent discrepancy of these findings (similar effects of DE-71 and TBBPA in neutrophil granulocytes, different effects in cerebellar granule cells) prompted a re-examination of the effects of DE-71 and other PBDEs in cerebellar granule neurons. These studies indicate that DE-71 causes cytotoxicity in mouse cerebellar granule neurons, with an IC50 (in the MTT assay) of 7.7 uM, almost identical to that reported by Reistad et al. (2006). The cytotoxicity of DE-71 was antagonized by the membrane permeable glutathione (GSH) delivery agent GSH ethyl ester, and by the antioxidant melatonin (Costa et al. 2007). DE-71 also caused a time-dependent increase in the levels of ROS, measured by DCF fluorescence. Furthermore, cell death induced by DE-71 was mostly apoptotic in nature. These findings were interpreted as indicating a role for oxidative stress in DE-71 neurotoxicity. Further evidence was provided by parallel experiments carried out in cerebellar granule neurons from Gclm (−/−) mice. Gclm (−/−) mice lack the modifier subunit of glutamate-cysteine ligase, the first and limiting step in the synthesis of GSH. As a result, cerebellar granule neurons from Gclm (−/−) mice have very low GSH levels compared to wild-type [Gclm (+/+)] mice (2.4 vs. 12.4 nmol/mg protein), and are more sensitive to the toxicity of agents causing oxidative stress, such as domoic acid (Giordano et al. 2006). In accordance with the hypothesis, cerebellar granule neurons from Gclm (−/−) mice were 8.5-fold more sensitive to the toxicity of DE-71 than neurons from Gclm (+/+) mice (IC50s in the MTT assay = 0.9 vs. 7.7 uM). Further verification was provided by experiments in which cerebellar granule neurons from Gclm (+/+) mice were exposed to the GSH synthase inhibitor buthionine sulfoximine (BSO; 25 uM for 24 h), to decrease levels of GSH [from 12.5 to 3.7 nmol/mg protein, similar to the levels present in CGNs from Gclm (−/−) mice (2.4 nmol/mg protein)]. Under this condition, the toxicity of DE-71 was significantly increased (IC50 = 0.74 uM), and cerebellar granule neurons from Gclm (+/+) mice were as sensitive as CGNs from Gclm (−/−) mice, not treated with BSO. DE-71-induced increases in ROS levels and in apoptotic cell death were also more pronounced in cerebellar granule neurons from Gclm (−/−) mice than in their Gclm (+/+) counterparts.
Hippocampal neurons from wild-type mice were found to display lower GSH levels than cerebellar granule neurons (5.5 vs. 12.4 nmol/mg protein), and levels of GSH in hippocampal neurons from Gclm (−/−) mice were only 1.4 nmol/mg protein. Values of IC50 in the MTT assay for DE-71 in hippocampal neurons were 2.2 uM in Gclm (+/+) mice, and 0.31 uM in Gclm (−/−) mice. Hippocampal neurons thus appear to be more sensitive than cerebellar granule neurons to the toxicity of DE-71, possibly because of their lower GSH content. The ratio between the IC50s in the two genotypes was 7.1, comparable to that observed in cerebellar granule neurons (Costa et al. 2007). Hippocampal astrocytes, whose levels of GSH in Gclm (+/+) mice were 21.4 nmol/mg protein, proved to be less sensitive to DE-71 toxicity (IC50 = 52.3 uM, similar to what was previously obtained in astrocytoma cells with BDE-99; Madia et al. 2004). These findings suggest that the neurotoxicity of DE-71, and that of other PBDEs (Giordano and Costa, unpublished) may involve oxidative stress, and is modulated by intracellular GSH content. In SH-SY5Y human neuroblastoma cells, Zhang et al. (2007) reported that BDE-47 (2–8 ug/ml) increases ROS levels, induces lipid peroxidation, and causes apoptotic cell death. These effects are not limited to nervous system cells. Indeed, an increase in oxidative stress, evidenced by increased lipid peroxidation and increased levels of oxidized glutathione (GSSG), was also found in liver of American kestrels (Falco sparverius) treated in ovo with a mixture of BDE-47, −99, 100, and −153 (Fernie et al. 2005). Increased levels of ROS were also found in human HepG2 hepatoma cells upon exposure to 10–100 uM BDE-209 (Hu et al. 2007), while postnatal exposure of mice to BDE-209 was reported to increase oxidative stress in sperm (Tseng et al. 2006).
Additional in vitro studies with PBDEs addressed aspects related to neurotransmitter uptake and release. Mariussen and Fonnum (2003) reported that DE-71 inhibited vesicular dopamine uptake in rat brain synaptic vesicles, with an IC50 of 8 uM. In contrast, DE-71 did not affect synaptosomal uptake of dopamine, GABA or glutamate. DE-79 and the decaBDE mixture DE- 83R had no effect on vesicular or synaptosomal uptake (Mariussen and Fonnum, 2003). Somewhat in contrast with these findings, Dingemans et al. (2007) reported that BDE-47 (20 uM) increased vesicular catecholamine release in PC-12 cells. The release of the neuropeptide vasopressin from hypothalamic preparations (tissue punches from the supraoptic and paraventricular nuclei) was shown to be inhibited by DE-71, BDE-47 and BDE-77 at micromolar concentrations (Coburn et al. 2007). Given the role of vasopressin in cognitive function (Gulpinar and Yegen, 2004), in addition to regulation of body fluid and cardiovascular function, it was suggested that neuroendocrine effect of PBDEs may play some role in developmental neurotoxicity (Coburn et al. 2007). Lastly, BDE-99 was found to stimulate the glutamate-nitric oxide-cGMP pathways in rat cerebellar granule neurons, thus confirming previous in vivo observations (Llansola et al. 2007).
Altogether, these in vitro studies indicate that PBDEs can elicit a number of effects on cell signaling and cell function. Such effects occur at micromolar concentrations that are similar to, or higher than those reported for PCBs. Clearly, research on potential mechanisms of PBDE neurotoxicity is only in its infancy, and further research is undoubtedly needed.
Toxicokinetic studies
Most aspects of the toxicokinetics of PBDEs have been summarized elsewhere (Darnerud et al. 2001; de Wit et al. 2002; Hakk and Letcher, 2003), and will not be discussed in detail. In adult rodents, lower brominated PBDEs (e.g. BDE-47, BDE-99, BDE-153) are usually well absorbed following oral exposure (Orn and Klasson-Wehler, 1998; Hakk et al. 2002; Staskal et al. 2006b). In contrast, absorption of decaBDE is much lower (10% of the dose) (Morck et al. 2003). PBDEs distribute to several tissues including the brain, and highest and more persistent concentrations are found in adipose tissue (Hakk and Letcher, 2003), however, this is not the case for BDE-209 (Morck et al. 2003). Tetra- and pentaBDEs are metabolized by mixed function oxidases to mono-and di-hydroxylated metabolites (Hakk et al. 2002; 2006; Hakk and Letcher, 2003; Malmberg et al. 2005; Marsh et al. 2004), while decaBDE is metabolized to lower brominated congeners (nona- and octaBDE) (Morck et al. 2003; Huwe and Smith, 2007). There appears to be a species difference in the rate of excretion, with a higher urinary excretion in mice (Orn and Klasson-Wehler, 1998). Gender differences are also observed, with males displaying a higher urinary excretion due to the binding of PBDEs to mouse major urinary protein (MUP), whose levels are much higher in males than females (Staskal et al. 2006b). The half-life of tetra- to hexa- PBDEs has been shown to range in mice and rats between 20 and 120 days (Hakk and Letcher, 2003; Geyer et al. 2004), with an increase proportional to the degree of bromination. In contrast, BDE-209 appears to be excreted more rapidly (Morck et al. 2003; Hakk and Letcher, 2003), though a half-life of 75 days has been reported in another study (Huwe and Smith, 2007).
Toxicokinetic studies with BDE-47 in developing mice (PND 10–28) have shown a reduced ability of young animals to excrete PBDEs, possibly due to a decreased urinary elimination, resulting in higher body burden compared to adults (Staskal et al. 2006a). In mice given BDE-47, BDE-85 or BDE-99, these compounds were shown to be secreted in milk, and suckling neonates had tissue concentrations higher than dams (Oskarsson and Moller, 2004; Darnerud and Risberg, 2006). In particular, levels of BDE-99 following maternal exposure in rats were found to be up to 17-fold higher in brain of pups compared to dams (Oskarsson and Moller, 2004). Administration of [14C]-BDE-209 to pregnant rats resulted in low levels of radioactivity in the fetus (Riu et al. 2006), an observation also made by others with BDE-47, BDE-85 and BDE-99 (Darnerud and Risberg, 2006). However, Kuriyama et al. (2006) found that after a single administration of BDE-99 (0.06 or 0.3 mg/kg) to dams on GD 6, hepatic levels were much higher in pups than in dams on PND 1–22, suggesting that gestational exposure leads to long-term body burden. In support of this finding, Ceccatelli et al. (2006) found that administration to rats of BDE-99 (1 or 10 mg/kg from GD 10 to GD 18) resulted in significant plasma and adipose tissue levels in pups at 120 days of age.
Overall, these toxicokinetics studies indicate that absorption, metabolism and excretion of PBDEs are congener-, species- and gender- dependent. Additionally, they show that substantial amounts of PBDEs are transferred to the pups through milk, and that pups have a decreased ability to eliminate PBDEs, leading to a higher body burden.
Relevance to humans
In the previous sections, as well as in Tables 7–10, the available information on PBDEs developmental neuroxicity, derived from animal and/or in vitro studies have been summarized. In contrast to the large data base on body burden (levels of PBDEs in serum, adipose tissue, breast milk), there is almost no information on possible adverse health effects in humans from PBDE exposure, including potential developmental neurotoxicity. Thus, any possible inference on potential risk for adverse nervous system effects in humans exposed to PBDEs in utero, or neonatally through breast milk or household dust, has to rely on animal data. In a traditional risk assessment approach, the applied dose would be the starting point for risk estimation. The NOEL values for PBDEs, determined in animal studies examining developmental neurotoxicity or thyroid hormone changes, range from 0.14 to 1.0 mg/kg/(day) (McDonald, 2005). In one case, the benchmark dose for BDE-99-induced changes in locomotor activity was calculated, and it was 0.31 mg/kg (Sand et al. 2004). By applying two safety factors (10x10) for interspecies extrapolation and intraspecies differences, and an additional factor (3) for insufficient data base, reference dose (RfD) values of 0.46 to 3.3 ug/kg/day (460–3300 ng/kg/day) are obtained. These values are not much dissimilar from the estimated exposure of infants to PBDEs (see Table 4). If one considers also the study by Kuriyama et al. (2005) with BDE-99, in which the dose of 0.06 mg/kg was the LOEL (low-observed-effect-level) for hyperactivity, applying all safety factors (10x10x10x3) provides a RfD of 20 ng/kg/day. By considering interspecies toxicokinetic differences, based on differences in half-lifes (Geyer et al. 2004; McDonald, 2005), which amount to about 50 fold, the safety factor for interspecies extrapolation could be 50 instead of 10. The resulting RfDs would then be 92–660 ng/kg/day, in the actual range of infant exposure (through breast milk), and close to the levels of toddler exposure through household dust and the diet.
Another approach consists in comparing body burden across species. This approach bypasses the need to quantify exposure from different routes and to consider species-specific toxicokinetics aspects, as body burden data are an integrated measure of exposure from all routes (McDonald, 2005). For example, following prenatal exposure to BDE-99 (1 mg/kg from GD 10 to GD 18) plasma levels of pups at PND 120 were 0.95 ng/ml (Ceccatelli et al. 2006). Assuming a total lipid content of rat plasma of 2 mg lipid/ml, this corresponds to 475 ng BDE-99/g lipid. It should be noted that the study by Ceccatelli et al. (2006) examined effects on gene expression in the uterus, rather than alterations in the nervous system; however, upon identical exposure, changes in sexual behavior and sweet preference were also found (Lichtensteiger et al. 2004; Lilienthal et al. 2006). Levels in human plasma as high as 580 and 460 ng/g lipid have been reported in maternal and fetal blood, respectively (Table 6; Mazdai et al. 2003). In the Fischer et al. (2006) family study, the toddler was found to have plasma PBDE levels of 418 ng/lipid (651 ng/g lipid if considering also BDE-209). These examples may represent a worst-case scenario, but indicate that adverse developmental effects are seen in animals at exposure levels relevant to humans, at least in North America. In another study, plasma levels of BDE-47 necessary to elicit a decrease of circulating T4 were found to be 421 ug/g lipid (Darnerud et al. 2007). This value would be about three orders of magnitude higher than levels found in humans. However, this study was carried out in adult rats, and sensitivity of developing animals may be higher.
As indicated earlier, an additional consideration is that in contrast to rodents, where total body half-lives of all PBDEs is in the order of several days or months, the terminal total body half -lives in humans have been estimated to be much longer, in the order of years for lower brominated congeners (e.g. BDE-47, 1.8 y; BDE-99, 2.9 y; BDE-154, 3.3 y; Geyer et al. 2004), while it is in the order of days to months for octa- to decaBDEs (Sjodin et al. 1999; Thuresson et al. 2006).
Whether the high levels of PBDEs in infants and children may result in adverse health effects, and specifically, in neurotoxicity, as would be predicted based on animal studies, is not known. In a study in Taiwan (Chao et al. 2007), elevated PBDE levels in breast milk were correlated with lower birth weight and length, lower head and chest circumference, and decreased Quetelet’s (body mass) index. The daily intake of PBDEs for a breastfed infant was estimated as 20.6 ng/kg/day in this study, far below the estimated exposure levels in Canada (280 mg/kg/day; Jones-Otazo et al. 2005) or in the United States (306 ng/kg/day; Schecter et al. 2006a). In another study comparing maternal and fetal blood PBDE levels, no correlation was found with serum T4 concentrations (Mazdai et al. 2003). Clearly, studies investigating the relationship between body burden of PBDEs and child development are needed, in order to validate the animal findings.
The USEPA had derived chronic oral references doses (RfD) for the commercial mixtures: penta (2 ug/kg/d), octa (3 ug/kg/d) and deca (10 ug/kg/d) (Jones-Otazo et al. 2005). However, these values were based on toxicological effects in the liver and not on developmental effects. Estimated exposure of an infant (through breast milk) is about 0.3 ug/kg/d (Table 4), with a range of 0.003 to 4.1 ug/kg/day, based on minimal and maximal concentrations of PBDEs in breast milk from Canada (Jones-Otazo et al. 2005). These values are within the current RfDs of most PBDEs. Recently, the USEPA has proposed RfD values for single congeners, based on developmental neurotoxicity (e.g. 100 ng/kg/day for BDE-47).
Overall perspective and research needs
Many issues need to be investigated before a clearer picture on possible risks of developmental neurotoxicity in children due to exposure to PBDEs can emerge. First, in this review, as in most of the literature, PBDEs are considered as a uniform class of chemicals, with possible toxicokinetic and relative potency differences, but sharing a common mechanism of action (which is still unknown). There is evidence for and against this concept. For example, all PBDEs tested so far, including higher brominated congeners, have been shown to produce alterations in locomotor behavior (Table 7). On the other hand, in vitro experiments suggest that octa-, nona-and deca-BDEs may have much lower biological activity (see Table 10). The Joint FAO/WHO Expert Committee on Food Additives stated in an evaluation of PBDEs that “data are inadequate to establish a common mechanism of action that would allow a single congener to be used as surrogate for total exposure or, alternatively, as the basis for establishing toxic equivalency factors” (JECFA, 2006).
A second issue, as pointed out by McDonald (2005), is that PBDEs may be converted to polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) when flame-retarded plastic material is subjected to thermal stress (Ebert and Bahadir, 2003). PBDDs and PBDFs have been found as impurities in commercial PBDE mixtures (Hanari et al. 2006), have been measured in adipose tissue (Choi et al. 2003), and share many of the toxicological characteristics of their chlorinated analogs (Birnbaum et al. 2003). High levels of PBDDs, exceeding those of chlorinated dioxins, have been found in fish and shellfish from the Baltic Sea, and their natural origin has been suggested (Haglund et al. 2007). Additionally, humans can also be directly exposed to hydroxylated BDEs, which are known to be a product of PBDE metabolism (Malmberg et al. 2005; Marsh et al. 2006). Hydroxylated PBDEs are found, for example, in fish such as salmon (Marsh et al. 2004), and some may also have a natural origin. As said earlier, hydroxylated-PBDEs have a much greater affinity for TTR than PBDEs (Meerts et al. 2000).
A third important issue is that PBDEs cannot be considered in isolation, as if they were the only chemicals people are exposed to. For example, the structurally similar PCBs remain a widespread class of contaminants. Exposure to PCBs causes developmental neurotoxicity, as evidenced by animal studies and observations in humans (Seegal, 1996; Carpenter, 2006). PCBs have been shown to cause effects which are similar, albeit not identical, to those observed with PBDEs in many of the in vivo and in vitro experiments summarized in this review. Indeed in several cases, the effects of PBDEs were compared with those of PCBs, which were used as a sort of “positive” control. In a study in adult rats, Hallgren and Darnerud (2002) found that BDE-47 and Aroclor 1254 had at least an additive effect on serum T4 levels. Exposure of neonatal mice to either BDE-99 or PCB-52 impaired spontaneous locomotor activity at 4 and 6 months of age (Eriksson et al. 2006). Of relevance is that while a low dose of each of these compounds did not produce any behavioral effect when given alone, upon co-exposure the same low doses of BDE-99 and PCB-52 produced significant behavioral alterations. These were equal, if not greater, than those caused by a high dose of each compound alone. This finding is suggestive of a strong synergistic effect. Verification of the potential interaction between PBDEs and PCBs is needed, before any conclusion can be drawn. Nevertheless, one should also consider that in addition to PBDEs and PCBs, exposure to several other developmental neurotoxicants (e.g. lead, methylmercury, perchlorate, dioxins etc.) can occur. The significance of these multiple co-exposures is still elusive, but their potential adverse effects on child neurodevelopment have been pointed out (Grandjean and Landrigan, 2006).
A fourth aspect relates to the possible mechanism(s) of developmental neurotoxicity of PBDEs. As evidenced by the summary of existing data, two general, and not mutually exclusive, modes of action are emerging, one related to effects of PBDEs on T4, and the other involving a direct effect of PBDEs on the developing brain. Whether the hypothesized mechanisms of PBDE- induced hypothyroxemia (induction of UDPGT and interference with TTR) are operational in humans remains to be determined. For example, as the major T4 transporter in humans is believed to be a thyroid-binding globulin (TBG), followed by TTR and albumin (Schussler, 2000; Palha, 2002), the impact of PBDE binding to TTR may be lower in humans than in rodents. Furthermore, hydroxylated PCBs bind to TBG with much lower affinity than to TTR (Lans et al. 1994), and the same may be true for hydrolated PBDEs. TTR may still facilitate the transport of OH-PBDEs in the brain (Meerts et al. 2000), though it has been shown that TTR is not indispensable for T4 entry into the brain (Palha, 2002). Biochemical studies on direct effects of PBDE on the developing brain have yet to provide convincing evidence of precise mechanisms, though some indications (e.g. interference with signaling pathways, oxidative stress) are beginning to emerge.
In conclusion, available evidence indicates a high body burden of PBDEs, particularly in the North America population, and particularly in infants and young children. Evidence is accumulating indicating that in animals PBDEs cause developmental neuroxicity, by still unknown mechanisms, at dose levels not much different from reported levels of exposure in humans. The earliest reports on PBDEs in the environment and on their health effects date back to the early 1980s (Carlson, 1980; Andersson and Blomkvist, 1981). Given that PCBs were first detected in environmental samples in 1966 (Jensen, 1966), and that they still represent a prominent health and environmental issue, increased efforts in investigating adverse health effects of PBDEs and their underlying mechanisms are a matter of great relevance and urgency.
Acknowledgments
Studies by the authors were supported by a grant from NIEHS (P30ES07033) and a grant from the European Union (QLK4-CT-1999-01562).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akutsu K, Kitagawa M, Nakazawa H, Makino T, Iwazaki K, Oda H, Hori S. Time-trend (1973–2000) of polybrominated diphenyl ethers in Japanese mother’s milk. Chemosphere. 2003;53:645–54. doi: 10.1016/S0045-6535(03)00764-1. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Fischer C, Kultima K, Viberg H, Eriksson P, Dencker L, Stigson M. Proteomic evaluation of neonatal exposure to 2,2’,4,4’5-pentabromodiphenyl ether. Environ Health Perspect. 2006;114:254–9. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson O, Blomkvist G. Polybrominated aromatic pollutants found in fish in Sweden. Chemosphere. 1981;10:1051–60. [Google Scholar]
- Antignac JP, Maume D, Marchand P, Monteau F, Zalko D, Beerebi A, Cravedi JP, Andre F, Le Bizec B, Cariou R. Exposure assessment of fetus and newborn to brominated flame retardants in France: preliminary data. Organohalogen Compounds. 2006;68:790–3. doi: 10.1002/mnfr.200700077. [DOI] [PubMed] [Google Scholar]
- Atuma S, Aune M, Darnerud PO, Cnattingius S, Wernroth ML, Wicklund-Glynn A. Polybrominated diphenyl ethers (PBDEs) in human milk from Sweden. In: Lipnick RL, Jansson B, Mackay D, Petreas M, editors. Persistent, Bioaccumulative, and Toxic Chemicals II. Assessment of New Chemicals. Washington, DC: American Chemical Society; 2001. pp. 235–42. [Google Scholar]
- Barter RA, Klaassen CD. UDP-glucoronosyltransferase inducers reduce thyroid hormone levels in rats by and extrathyroidal mechanism. Toxicol Appl Pharmacol. 1992;113:36–42. doi: 10.1016/0041-008x(92)90006-e. [DOI] [PubMed] [Google Scholar]
- Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, Yu L, Fu J. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ Pollut. 2006;144:1024–30. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF, Diliberto JJ. Health effects of polybrominated dibenzo-p-dioxins (PBDDs) and dibenzofurans (PBDFs) Environ Internat. 2003;29:855–60. doi: 10.1016/S0160-4120(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Fensster L, Sjodin A, Jones RS, Patterson DG, Eskenazi B. Polybrominated diphenyl ether levels in the blood of pregnant women living in an agricultural community in California. Environ Health Perspect. 2007;115:71–4. doi: 10.1289/ehp.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Alleva E, Costa LG. Effects of perinatal exposure to a polybrominated diphenyl ether (PBDE 99) on mouse neurobehavioral development. Neurotoxicology. 2002;23:375–84. doi: 10.1016/s0161-813x(02)00078-5. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–62. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, Costa LG. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioral profile: interference from the administration route. Neurotoxicology. 2005;26:183–92. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Bull K, Basu N, Zhang S, Martin JW, Bursian S, Martin P, Chan LHM. Dietary and in utero exposure to a pentabrominated diphenyl ether mixture did not affect cholinergic parameters in the cerebral cortex of Ranch Mink (Mustela vison) Toxicol Sci. 2007;96:115–22. doi: 10.1093/toxsci/kfl179. [DOI] [PubMed] [Google Scholar]
- Canton RF, Sanderson JT, Nijmeijer S, Bergman A, Letcher RJ, van den Berg M. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: a novel mechanism of action? Toxicol Appl Pharmacol. 2006;216:274–81. doi: 10.1016/j.taap.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Carlson GP. Induction of xenobiotic metabolism in rats by brominated diphenyl ethers administered for 90 days. Toxicol Lett. 1980;6:207–12. doi: 10.1016/0378-4274(80)90193-9. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after develomental exposure to the polybrominated diphenyl ether PBDE 99 and PCB. Toxicology. 2006;20:104–16. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chan S, Rovet J. Thyroid hormones in the fetal central nervous system development. Fetal Matern Medi Rev. 2003;14:177–208. [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Internat. 2007;33:239–45. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Chen DA, Mai B, Song J, Sun Q, Luo Y, Luo X, Zeng EY, Hale RC. Polybrominated diphenyl ethers in birds of prey from Northern China. Environ Sci Technol. 2007;41:1828–33. doi: 10.1021/es062045r. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Polybrominated diphenyl ethers as Ah receptor agonists and antagonists. Toxicol Sci. 2003;76:310–20. doi: 10.1093/toxsci/kfg236. [DOI] [PubMed] [Google Scholar]
- Choi JW, Fujimaki S, Kitamura K, Hashimoto S, Ito H, Suzuki N, Sakai SI, morita M. Polybrominated dibenzo-p-dioxins, dibenzofurans, and diphenyl ethers in Japanese human adipose tissue. Environ Sci Technol. 2003;37:817–21. doi: 10.1021/es0258780. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PRS. Polybrominated diphenyl ethers (PBDEs) and ortho-substituted polychlorinated biphenyls (PCBs) as neuroendocrine disruptors of vasopressin release: effects during physiological activation in vitro and structure-activity relationships. Toxicol Sci. 2007;98:178–86. doi: 10.1093/toxsci/kfm086. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Kavanagh TJ. Glutathione levels modulate the neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants in mouse neurons and astrocytes. Organohalogen Compounds. 2007 in press. [Google Scholar]
- Crofton KM. Developmental disruption of thyroid hormone: correlations with hearing dysfunction in rats. Risk Analysis. 2004;24:1665–71. doi: 10.1111/j.0272-4332.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Zoeller RT. Mode of action: neurotoxicity induced by thyroid hormone disruption during development - Hearing loss resulting from exposure to PHAHs. Crit Rev Toxicol. 2005;35:757–69. doi: 10.1080/10408440591007304. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Risberg S. Tissue localization of tetra- and pentabromodiphenyl ether congeners (BDE-47, −85, −99) in perinatal and adult C57BL mice. Chemosphere. 2006;62:485–93. doi: 10.1016/j.chemosphere.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67:S386–92. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dingemans MML, Ramakers GMJ, Gardoni F, van Kleef GDM, Bergman A, Di Luca M, van den Berg M, Westerink RHS, Vijverberg HPM. Neonatal exposure to brominated flame retardant BDE-47 reduces long-tem potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–70. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 2005;88:172–80. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Dunckel AE. An updating on the polybrominated biphenyl disaster in Michigan. J Am Vet Med Ass. 1975;167:838–41. [PubMed] [Google Scholar]
- Ebert J, Bahadir M. Formation of PBDD/F from flame-retarded plastic material under thermal stress. Environ Internat. 2003;29:711–6. doi: 10.1016/S0160-4120(03)00117-X. [DOI] [PubMed] [Google Scholar]
- Ellis-Hutchings RG, Cherr GN, Hanna LA, Keen CL. Polybrominated diphenyl ether (PBDE)-induced alterations in vitamin A and thyroid hormone concentrations in the rat during lactation and early postnatal development. Toxicol Appl Pharmacol. 2006;215:135–45. doi: 10.1016/j.taap.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Erdogrul O, Covaci A, Kurtul N, Schepens P. Levels of organohalogenated persistent pollutants in human milk from Kahramanmaras region, Turkey. Environ Internat. 2004;30:659–66. doi: 10.1016/j.envint.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Fredriksson A. Neonatal exposure to 2,2’,5,5’ tetrachlorobiphenyl causes increased susceptibility in the cholinergic transmitter system at adult age. Environ Toxicol Pharmacol. 1996;1:217–20. doi: 10.1016/1382-6689(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–8. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2, 2’, 4, 4’, 5 – pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67:98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Eriksson P, von Rosen D, Viberg H, Fredriksson A. Developmental toxicology in the neonatal mouse: the use of randomly selected individuals as statistical unit compared to the litter in mice neonatally exposed to PBDE 99. Toxicol Sci. 2005;84(Suppl 1):219. [Google Scholar]
- Eriksson P, Fischer C, Fredriksson A. Polybrominated diphenyl ethers, a group of brominated flame retardants, can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral effects. Toxicol Sci. 2006;94:302–9. doi: 10.1093/toxsci/kfl109. [DOI] [PubMed] [Google Scholar]
- Eslami B, Koizumi A, Ohta S, Inoue K, Aozasa O, Harada K, Yoshinaga T, Date C, Fujii S, Fujimine Y, Hachiya N, Hirosawa I, Koda S, Kusaka Y, Murata K, Nakatsuka H, Omae K, Saito N, Shimbo S, Takenaka K, Takeshita T, Todoriki H, Wada Y, Watanabe T, Ikeda M. Large-scale evaluation of the current level of polybrominated diphenyl ethers (PBDEs) in breast milk from 13 regions of Japan. Chemosphere. 2006;63:554–61. doi: 10.1016/j.chemosphere.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Evandri MG, Mastrangelo S, Costa LG, Bolle P. In vitro assessment of mutagenicity and clastogenicity of BDE-99, a pentabrominated diphenyl ether flame retardant. Environ Mol Mutagen. 2003;42:85–90. doi: 10.1002/em.10178. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Strid A, Grandjean P, Weihe P, Bergman A. A retrospective study of PBDEs and PCBs in human milk from the Faroe Islands. Environ Health: Global Acc Sci Source\ 2005;4:12. doi: 10.1186/1476-069X-4-12. ( http://www.ehjournal.net/content/4/1/12) [DOI] [PMC free article] [PubMed]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouilard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicol Sci. 2005;88:375–83. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A. Children show highest levels of polybrominated diphenyl ethers in a California family of four: a case study. Environ Health Perspect. 2006;114:1581–4. doi: 10.1289/ehp.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox WM. Reflex-ontogeny and behavioral development of the mouse. Animal Behav. 1965;13:234–40. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Fairbrother A, Baecher-Steppan L, Kerkvliet NI. Immunologic and endocrine effects of the flame retardant pentabromodiphenyl ether (DE-71) in C57BL/6 mice. Toxicology. 1994;86:49–61. doi: 10.1016/0300-483x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Furst P. Dioxins, polychlorinated biphenyls and other organohalogen compounds in human milk. Mol Nutr Food Res. 2006;50:922–33. doi: 10.1002/mnfr.200600008. [DOI] [PubMed] [Google Scholar]
- Geyer HJ, Schramm KW, Darnerud PO, Aune M, Feicht EA, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald TA. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–72. [Google Scholar]
- Gill U, Chu I, Ryan JJ, Feelry M. Polybrominated diphenyl ethers: human tissue levels and toxicology. Rev Environ Contam Toxicol. 2004;183:55–97. doi: 10.1007/978-1-4419-9100-3_3. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, McConnachie LA, Fernandez C, Kavanagh TJ, Costa LG. Neurotoxicity of domoic acid in cerebellar granule neurons in a genetic model of glutathione deficiency. Mol Pharmacol. 2006;70:2116–26. doi: 10.1124/mol.106.027748. [DOI] [PubMed] [Google Scholar]
- Gomara B, Herrero L, Gonzales MJ. Survey of polybrominated diphenyl ether levels in Spanish commercial foodstuff. Environ Sci Technol. 2006;40:7541–7. doi: 10.1021/es061130w. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Gulpinar MA, Yegen BC. The physiology of learning and memory: role of peptides and stress. Curr Protein Pep Sci. 2004;5:457–73. doi: 10.2174/1389203043379341. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. New Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Haglund P, Malmvarn A, Bergek S, Bignert A, Kautsky L, Nakano T, Wiberg K, Asplund L. Brominated dibenzo-p-dioxins: a newclass of marine toxins? Environ Sci Technol. 2007;41:3069–74. doi: 10.1021/es0624725. [DOI] [PubMed] [Google Scholar]
- Hakk H, Larsen G, Klasson-Wehler E. Tissue disposition, excretion and metabolism of 2,2’,4,4’,5-pentabromodiphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica. 2002;32:369–82. doi: 10.1080/00498250110119117. [DOI] [PubMed] [Google Scholar]
- Hakk H, Letcher RJ. Metabolism in the toxicokinetics and fate of brominated flame retardants – a review. Environ Internat. 2003;29:801–28. doi: 10.1016/S0160-4120(03)00109-0. [DOI] [PubMed] [Google Scholar]
- Hakk H, Huwe J, Low M, Rutherford D, Larsen G. Tissue disposition, excretion and metabolism of 2,2’,4,4’,6-pentabromodiphenyl ether (BDE-100) in male Sprague-Dawley rats. Xenobiotica. 2006;36:79–94. doi: 10.1080/00498250500491675. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Arch Toxicol. 2001;75:200–8. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats – Testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–43. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Keter MHA, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–73. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Hanari N, Kannan K, Miyake Y, Okazawa T, Kodavanti PRS, Aldous KM, Yamashita N. Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ethers. Environ Sci Technol. 2006;40:4400–5. doi: 10.1021/es060559k. [DOI] [PubMed] [Google Scholar]
- Hardy ML. The toxicology of the three commercial polybrominated diphenyl oxide (ether) flame retardants. Chemosphere. 2002;46:757–77. doi: 10.1016/s0045-6535(01)00240-5. [DOI] [PubMed] [Google Scholar]
- Hazrati S, Harrad S. Causes of variability in concentrations of polychlorinated biphenyls and polybrominated diphenyl ethers in indoor air. Environ Sci Technol. 2006;40:7584–9. doi: 10.1021/es0617082. [DOI] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Schwager SJ, Knuth BA, Hamilton MC, Carpenter DO. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol. 2004;38:4945–9. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- Hsu PC, Tseng LH, Lee CW. Effects of prenatal exposure of decabrominated diphenyl ether (PBDE 209) on reproductive system in male mice. Organohalogen Compounds. 2006;68:1547–50. [Google Scholar]
- Hu XZ, Xu Y, Hu DC, Hui Y, Yang FX. Apoptosis induction on human hepatoma cells HepG2 of decabrominated diphenyl ether (PBDE-209) Toxicol Lett. 2007;171:19–28. doi: 10.1016/j.toxlet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Smith DJ. Accumulation, whole-body depletion, and debromination of decabromodiphenyl ether in male Sprague-Dawley rats following dietary exposure. Environ Sci Technol. 2007;41:2371–77. doi: 10.1021/es061954d. [DOI] [PubMed] [Google Scholar]
- Ingelido AM, Ballard T, Dellatte E, Di Domenico A, Ferri F, Fulgenzi AR, Herrman T, Iacovella N, Miniero R, Papke O, Porpora MG, De Felip E. Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in milk from Italian women living in Rome and Venice. Chemosphere. 2007;207:S301–6. doi: 10.1016/j.chemosphere.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, Takasuga T, Senthilkumar K, Yamashita F, Koizumi A. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk of Japanese mothers. Environ Health Perspect. 2006;114:179–85. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, Schepens P. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Tot Environ. 2006;372:20–31. doi: 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Exper Committee on Food Additives) Evaluation of polybrominated diphenyl ethers. 2006. TRS 930-JECFA 64/44. [Google Scholar]
- Jensen S. Report of a new chemical hazard. New Sci. 1966;32:612. [Google Scholar]
- Jones-Otazo HA, Clarke JP, Diamond ML, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ Sci Technol. 2005;39:5121–30. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- Kalantzi OI, Martin FL, Thomas GO, Alcock RE, Tang HR, Drury SC, Carmichael PL, Nicholson JK, Jones KC. Different levels of polybrominated diphenyl ethers (PBDEs) and chlorinated compounds in breast milk from two U.K. regions. Environ Health Perspect. 2004;112:1085–91. doi: 10.1289/ehp.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karter MJ. Fire loss in the United States during 2005. National Fire Protection Association; Quincy, MA: 2006. p. 39. [PubMed] [Google Scholar]
- Kazda R, Hajslova J, Poustka J, Cajka T. Determination of polybrominated diphenyl ethers in human milk samples in the Czech Republic. Comparative study of negative chemical ionization mass spectrometry and time-of-flight high-resolution mass spectrometry. Anal Chim Acta. 2004;520:237–43. [Google Scholar]
- Kodavanti PRS, Derr-Yellin EC. Differential effects of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68:451–57. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–62. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol Sci. 2005;88:181–92. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Chahoud I. Sex-dependent behavioral changes in rat offspring after in utero administration of a single low dose of PBDE 47. Organohalogen Compounds. 2004a;66:3893–900. [Google Scholar]
- Kuriyama SN, Fidalgo-Nieto AA, Grande SW, Akkoc Z, de Souza CAM, Chahoud I. Thyroid hormone levels and hepatic enzyme activity in lactating dams after gestational exposure to low dose of PBDE 47. Organohalogen Compounds. 2004b;66:3901–6. [Google Scholar]
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE-99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect. 2005;113:149–54. doi: 10.1289/ehp.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama SN, Wanner A, Talsness CE, Koerner W, Chahoud I. Tissue distribution after developmental exposure to low dose PBDE-99. Organohalogen Compounds. 2006;68:643–6. [Google Scholar]
- LaFranchi SH, Haddow JE, Hollowell JG. Is thyroid inadequacy during gestation a risk factor for adverse pregnancy and developmental outcomes? Thyroid. 2005;15:60–71. doi: 10.1089/thy.2005.15.60. [DOI] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame retardants mixtures. Environ Sci Technol. 2006;40:6247–54. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- Lans MC, Spiertz C, Brouwer A, Koeman JH. Different competition of thyroxine binding to transthyretin and thyroxine binding globulin by hydroxyl-PCBs, PCDDs and PCDFs. Eur J Pharmacol. 1994;270:129–36. doi: 10.1016/0926-6917(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, deBoer J, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Faass O, Ceccatelli R, Schlumpf M. Developmental exposure to PBDE 99 and PCB affects estrogen sensitivity of target genes in rat brain regions and female sexual behavior. Organohalogen compounds. 2004;66:3965–70. [Google Scholar]
- Lilienthal H, Hack A, Roth-Harer A, Wichert Grande S, Talsness CE. Effects of developmental exposure to 2,2’,4,4’,5-pentabromodiphenyl ether (PBDE99) on sex steroids, sexual development, and sexually dimorphic behavior in rats. Environ Health Perspect. 2006;114:194–201. doi: 10.1289/ehp.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind Y, Darnerud PO, Atuma S, Aune M, Becker W, Bjerselius R, Cnattingius S, Glynn A. Polybrominated diphenyl ethers in breast milk from Uppsala County, Sweden. Environ Res. 2003;93:186–94. doi: 10.1016/s0013-9351(03)00049-5. [DOI] [PubMed] [Google Scholar]
- Llansola M, Erceg S, Monfort P, Montoliu C, Felipo V. Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur J Neurosci. 2007;25:373–9. doi: 10.1111/j.1460-9568.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J Exp Sci Environ Epidemiol. 2007 doi: 10.1038/sj.jes.7500572. in press. [DOI] [PubMed] [Google Scholar]
- MacPhail R, Farmer JD, Padnos BK, Crofton KM. Lack of effect of perinatal exposure to a polybrominated diphenyl ether mixture (DE-71) on the habituation of motor activity in adult rats. Toxicol Sci. 2003;72(Suppl 1):123. [Google Scholar]
- Madia F, Giordano G, Fattori V, Vitalone A, Branchi I, Capone F, Costa LG. Differential in vitro neurotoxicity of the flame retardant PBDE-99 and of the PCB Aroclor 1254 in human astrocytoma cells. Toxicol Lett. 2004;154:11–21. doi: 10.1016/j.toxlet.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Malmberg T, Athanasiadou M, Marsh G, Brandt I, Bergman A. Identification of hydroxylated polybrominated diphenyl ether metabolites in blood plasma from polybrominated diphenyl ether exposed rats. Environ Health Technol. 2005;39:5342–8. doi: 10.1021/es050574+. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem Internat. 2003;43:533–42. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Marsh G, Athanasiadu M, Bergman A, Asplund L. Identification of hydroxylated and methoxylated polybrominated diphenyl ethers in Baltic Sea salmon (Salmo salar) blood. Environ Sci Technol. 2004;38:10–8. doi: 10.1021/es034671j. [DOI] [PubMed] [Google Scholar]
- Marsh G, Athanasiadou M, Athanassiadis I, Sandolm A. Identification of hydroxylated metabolites in 2,2’,4,4’ – tetrabromodiphenyl ether exposed rats. Chemosphere. 2006;63:690–7. doi: 10.1016/j.chemosphere.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Martin P, Mayne G, Bursian S, Palace V, Tomy G. Altered thyroid status and vitamin A levels in mink (Mustela vison) exposed to a commercial PBDE mixture. Proceedings of the 25th Annual Meeting of the Society of Environmental Toxicology and Chemistry; Portland, OR. 2004. p. 260. [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ Health Perspect. 2003;111:1249–52. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TA. A perspective on the potential health risks of PBDEs. Chemosphere. 2002;46:745–55. doi: 10.1016/s0045-6535(01)00239-9. [DOI] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1:343–54. [PubMed] [Google Scholar]
- Meerts IATM, van Zanden JJ, Luijkis EAC, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some polybrominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meironyte D, Noren K, Bergman A. Analysis of polybrominated diphenyl ethers in Swedish human milk. A time-related trend study, 1972–1997. J Toxicol Env Health Part A. 1999;58:329–41. doi: 10.1080/009841099157197. [DOI] [PubMed] [Google Scholar]
- Meironyte Guvenius D, Aronsson A, Ekman-Ordeberg G, Bergman A, Noren K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–41. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck A, Hakk H, Orn U, Klasson-Wehler E. Decabromodiphenyl ether in the rat: absorption, distribution, metabolism, and excretion. Drug Metab Dispos. 2003;31:900–7. doi: 10.1124/dmd.31.7.900. [DOI] [PubMed] [Google Scholar]
- Muir T. Are thyroid and neuodevelopmental health effects in North America related to rising PBDE levels? Organohalogen Compounds. 2005;67:2487–92. [Google Scholar]
- Mundy WR, Inglefield JR, Shafer TJ. Activation of MAP kinase by the persistent bioaccumulative toxicants PCBs and PBDEs in developing cortical cultures. Tox Sci. 2002;66(Suppl 1):18. [Google Scholar]
- Mundy WR, Freudenrich TM, Crofton KM, DeVito MJ. Accumulation of PBDE-47 in primary cultures of rat neocortical cells. Toxicol Sci. 2004;82:164–9. doi: 10.1093/toxsci/kfh239. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Sekiguchi S, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y. Attention-deficit and hyperactive neurobehavioral characteristics induced by perinatal hypothyroidism in rats. Behav Brain Res. 2005;159:323–31. doi: 10.1016/j.bbr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program) Toxicology and Carcinogenesis Studies of Decabromodiphenyl Oxide (CAS no. 1163-19-5) in F344/N and B6c3F1 Mice (Fed Studies) National Toxicology Program; Research Triangle Park, NC: 1986. NTP TR 309. [PubMed] [Google Scholar]
- Ohta S, Ishizuki D, Nishimura H, Nakao T, Aozasa O, Shimidzu Y, Ochiai F, Kida T, Nishi M, Miyata H. Comparison of polybrominated diphenyl ethers in fish, vegetables, and meats and levels in human milk of nursing women in Japan. Chemosphere. 2002;46:689–96. doi: 10.1016/s0045-6535(01)00233-8. [DOI] [PubMed] [Google Scholar]
- Orn U, Klasson-Wehler E. Metabolism of 2,2’,4,4’-tetrabromodiphenyl ether in rat and mouse. Xenobiotica. 1998;28:199–211. [PubMed] [Google Scholar]
- Oskarsson A, Moller N. A method for studies on milk excretion of chemicals in mice with 2, 2’, 4, 4’, 5 - pentabromodiphenyl ether (BDE-99) as a model. Toxicol Lett. 2004;151:327–34. doi: 10.1016/j.toxlet.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Pacyniak EK, Cheng X, Cunningham M, Crofton K, Klaassen CD, Guo GL. The flame retardants, polybrominated diphenyl ethers (PBDE), are pregnane X receptor (PXR) activators. Toxicol Sci. 2007;97:94–102. doi: 10.1093/toxsci/kfm025. [DOI] [PubMed] [Google Scholar]
- Palha JA. Transthyretin as a thyroid hormone carrier: function revisited. Clin Chem Lab Med. 2002;40:1292–300. doi: 10.1515/CCLM.2002.223. [DOI] [PubMed] [Google Scholar]
- Peters AK, Nijmeijer S, Gradin K, Backlund M, Bergman A, Poellinger L, Denison MS, Van den Berg M. Interactions of polybrominated diphenyl ethers with the aryl hydrocarbon receptor pathway. Toxicol Sci. 2006;92:133–42. doi: 10.1093/toxsci/kfj186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatia R, Charles MJ. High body burdens of 2, 2’, 4, 4’-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111:1175–9. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polder A, Savinova TN, Tkatchev A, Loken K, Skaare JU. Polybrominated diphenyl ethers (PBDEs) in human breast milk samples from Mumansk and Arkhangelsk, Russia. Organohalogen Compounds. 2006;68:2170–2. [Google Scholar]
- Reistad T, Mariussen E. A commercial mixture of the brominated flame retardant pentabrominated diphenyl ether (DE-71) induces respiratory burst in human neutrophil granulocytes in vitro. Toxicol Sci. 2005;87:57–65. doi: 10.1093/toxsci/kfi222. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Fonnum F. The effect of a brominated flame retardant, tetrabromobisphenol-A, on free radical formation in human neutrophil granulocytes: the involvement of the MAP kinase pathway and protein kinase C. Toxicol Sci. 2005;83:89–100. doi: 10.1093/toxsci/kfh298. [DOI] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch Toxicol. 2006;80:785–96. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Reistad T, Mariussen E, Ring A, Fonnum F. In vitro toxicity of tetrabromobisphenol A on cerebellar granule cells: cell death, free radical formation, calcium influx and extracellular glutamate. Toxicol Sci. 2007;96:268–78. doi: 10.1093/toxsci/kfl198. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Diliberto JJ, DeVito MJ, Birnbaum LS. Effects of 2,2’4, 4’-tetrabromodiphenyl ether on nuclear receptor regulated genes: implications for thyroid organ disruption. Organohalogen Compounds. 2006;68:403–6. [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–20. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Riu A, Debrauwer L, Garcia A, Jouanin I, Cravedi JP, Zalko D. Disposition of [14C]-DBDE in pregnant rats. Organohalogen Compounds. 2006;68:639–42. [Google Scholar]
- Ryan JJ, Patri B, Mills P, Beaudoin G. Recent trends in levels of brominated diphenyl ethers in human milks from Canada. Organohalogen Compounds. 2002;58:173–6. [Google Scholar]
- Ryan JJ, Wainman BC, Schecter A, Moisey J, Kosarac I, Sun WF. Trends of the brominated flame retardants, PBDEs and HBCD, in human milks from North America. Organohalogen Compounds. 2006;68:778–81. [Google Scholar]
- Sand S, von Rosen D, Eriksson P, Fredriksson A, Viberg H, Victorin K, Falk Filipsson A. Dose-response modeling and benchmark calculations from spontaneous behavior data in mice neonatally exposed to 2,2’,4,4’5-pentabromodiphenyl ether. Toxicol Sci. 2004;81:491–501. doi: 10.1093/toxsci/kfh222. [DOI] [PubMed] [Google Scholar]
- Sandal S, Yilmaz B, Chen CH, Carpenter DO. Comparative effects of technical toxaphene, 2,5-dichloro-3-biphenylol and octabromodiphenylether on cell viability, [Ca2+]i levels and membrane fluidity in mouse thymocytes. Toxicol Lett. 2004;151:417–28. doi: 10.1016/j.toxlet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol Sci. 2005;88:127–33. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Schalock RL, Brown WJ, Smith RL. Neonatal hypothyroidism: behavioral, thyroid hormonal and neuroanatomical effects. Physiol Behav. 1977;19:489–91. doi: 10.1016/0031-9384(77)90223-2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–9. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environ Sci Technol. 2004;38:5306–11. doi: 10.1021/es0490830. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgreen J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005a;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Joseph JE, Tung KC. Polybrominated diphenyl ethers (PBDEs) in U.S. computers and domestic carpet vacuuming: possible sources of human exposure. J Toxicol Environ Health Part A. 2005b;68:501–13. doi: 10.1080/15287390590909715. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ Health Perspect. 2006a;114:1515–20. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Harris TR, Papke O, Tung KC, Musumba A. Polybrominated diphenyl ether (PBDE) levels in the blood of pure vegetarians (vegans) Toxicol Environ Chem. 2006b;88:107–12. [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health Part A. 2007;70:1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- Schussler GC. The thyroxine-binding proteins. Thyroid. 2000;10:141–9. doi: 10.1089/thy.2000.10.141. [DOI] [PubMed] [Google Scholar]
- Seegal RF. Epidemiological and laboratory evidence of PCB-induced neurotoxicity. Crit Rev Toxicol. 1996;26:709–37. doi: 10.3109/10408449609037481. [DOI] [PubMed] [Google Scholar]
- She J, Holden A, Tanner M, Sharp M, Adelsbach T, Hooper K. Highest PBDE levels (max 63 ppm) yet found in biota measured in seabird eggs from San Francisco Bay. Organohalogen Compounds. 2004;66:3939–44. [Google Scholar]
- She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere. 2007;207:S307–17. doi: 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jakobsson E, Kierkegaard A, Marsh G, Sellstrom U. Gas chromatographic identification and quantification of polybrominated diphenyl ethers in a commercial product, Bromkal 70-5DE. J Chromatogr A. 1998;822:83–9. [Google Scholar]
- Sjodin A, Hagmar L, Klasson-Wehler E, Kronholm-Diab K, Jakobsson E, Bergman A. Flame retardant exposure: polybrominated diphenyl ethers in blood from Swedish workers. Environ Health Perspect. 1999;107:643–8. doi: 10.1289/ehp.107-1566483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, Zhang Y, Turner WE, Slazyk B, Needham LL, Patterson DG. Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ Health Perspect. 2004;112:654–8. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarman E, Darnerud PO, Ohrvik H, Oskarsson A. Reduced thyroxine levels in mice perinatally exposed to polybrominated diphenyl ethers. Environ Toxicol Pharmacol. 2005;19:273–81. doi: 10.1016/j.etap.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Smolnikar K, Dehnhardt M, Wiegand H. Perturbation by PBDE99 of calcium homeostasis after in vitro treatment. The Second International Workshop on Brominated Flame Retardants, BFR 2001; Stockholm, Sweden. p. 189. [Google Scholar]
- Soderstrom G, Sellstrom U, de Wit CA, Thysklind M. Photolytic debromination of decabromodiphenyl ether (BDE 209) Environ Sci Technol. 2004;38:127–32. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Disposition of BDE 47 in developing mice. Toxicol Sci. 2006a;90:309–16. doi: 10.1093/toxsci/kfj098. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS. Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice. Toxicol Sci. 2006b;94:28–37. doi: 10.1093/toxsci/kfl091. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Cooper RL, Lambright CS, Wilson VS, Furr J, Gray LE. In vivo and in vitro anti-androgenic effects of DE-71, a commercial polybrominated diphenyl ether (PBDE) mixture. Toxicol Appl Pharmacol. 2005;207:78–88. doi: 10.1016/j.taap.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Strandman T, Koistinen J, Vertainen T. Polybrominated diphenyl ethers (PBDEs) in placenta and human milk. Organohalogen Compounds. 2000;47:61–4. [Google Scholar]
- Sudaryanto A, Kajiwara N, Tsydenova O, Iwata H, Adibroto TA, Yu H, Chung KH, Subramanian A, Prudente M, Tana TS, Tanabe S. Global contaminantion of PBDEs in human milk from Asia. Organohalogen Compounds. 2005;67:1315–8. [Google Scholar]
- Sudaryanto A, Kajiwara N, Takahashi S, Muawanah, Tanabe S. Geographical distribution and accumulation features of PBDEs in human breast milk from Indonesia. Environ Pollut. 2007 doi: 10.1016/j.envpol.2007.02.016. in press. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Kuriyama SN, Grande WS, Andrade A, Sterner-Kock A, Schnitker P, Chahoud I. Low dose effects on the rat female reproductive system following exposure to a single administration of PBDE-47. Organohalogen Compounds. 2004;66:407–9. [Google Scholar]
- Talsness CE, Shakibaei M, Kuriyama SN, Grande SW, Sterner-Kock A, Schnitker P, de Souza C, Grote K, Chahoud I. Ultrastructural changes observed in rat ovaries following in utero and lactational exposure to low doses of a polybrominated flame retardant. Toxicol Lett. 2005;157:189–202. doi: 10.1016/j.toxlet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Hedge JM, Gilbert ME, DeVito MJ, Crofton K. Perinatal exposure to a polybrominated diphenyl ether mixture (DE-71): disruption of thyroid homeostasis and neurobehavioral development. Toxicol Sci. 2003;72(Suppl 1):124. [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ Sci Technol. 2002;36:1414–8. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Froshaug M, Broadwell SL, Becher G, Eggesbo M. Levels of brominated flame retardants in milk from the Norwegian human milk study: HUMIS. Organohalogen Compounds. 2005;67:509–12. [Google Scholar]
- Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ Health Perspect. 2006;114:176–81. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme-Laragy AR, Levin ED, Di Giulio RT. Developmental and behavioral effects of embryonic exposure to the polybrominated diphenylether mixture DE-71 in the killifish (Fundulus heteroclitus) Chemosphere. 2006;62:1097–104. doi: 10.1016/j.chemosphere.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Toms LML, Harden FA, Symons RK, Burniston D, Furst P, Muller JF. Polybrominated diphenyl ethers (PBDEs) in human milk from Australia. Chemosphere. 2007;68:797–803. doi: 10.1016/j.chemosphere.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Tseng LH, Lee CW, Pan MH, Tsai SS, Li MH, Chen JR, Lay JJ, Hsu PC. Postnatal exposure of the male mouse to 2,2’,3,3’,4,4’,6,6’-decabrominated diphenyl ether-decreased epididymal sperm function without alterations in DNA content and histology in testis. Toxicology. 2006;224:33–43. doi: 10.1016/j.tox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Tsydenova OV, Sudaryanto A, Kajiwara N, Kunisue T, Batoev VB, Tanabe S. Organohalogen compounds in human breast milk from Republic of Buryatia, Russia. Environ Pollut. 2007;146:225–32. doi: 10.1016/j.envpol.2006.04.036. [DOI] [PubMed] [Google Scholar]
- USEPA (United States Environmental Protection Agency) General Factors. Office of Research and Development. I. National Center for Environmental Assessment; Washington, DC: 1997. Exposure Factors Handbook. [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2,4,4’5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol Sci. 2002;67:104–7. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behavior, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003a;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Jakobsson E, Orn U, Eriksson P. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003b;76:112–20. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Investigations of strain and/or gender differences in developmental neurotoxic effects of polybrominated diphenyl ethers in mice. Toxicol Sci. 2004a;81:344–53. doi: 10.1093/toxsci/kfh215. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson Eriksson P. Neonatal exposure to the brominated flame retardant 2, 2’, 4, 4’, 5 – pentabromodiphenyl ether decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behavior in the adult mouse. Environ Toxicol Pharmacol. 2004b;17:61–5. doi: 10.1016/j.etap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher polybrominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92:211–8. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Changes in spontaneous behavior and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209) Neurotoxicology. 2007a;28:136–42. doi: 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson P. Neonatal developmental neurotoxicity of decabrominated diphenyl ether, PBDE 209: a summary. Toxicol Sci. 2007b;(Suppl 1):371. [Google Scholar]
- Vieth B, Herrmann T, Mielke H, Ostermann B, Papke O, Rudiger T. PBDE levels in human milk: the situation in Germany and potential influencing factors – a controlled study. Organohalogen Compounds. 2004;66:2643–8. [Google Scholar]
- WDEH (Washington State Depts. of Ecology and Health) Washington State Polybrominated Diphenyl Ether (PBDE) Chemical Action Plan: Final Plan. 2006 January 19; ( http://www.ecy.wa.gov/biblio/0507048.html)
- Wiegand H, Desaiah D, Dehnhardt M, Smolnikar K. Polyhalogenated hydrocarbon induced perturbation of intracellular calcium homeostasis; from astrocytes to human macrophages. Organohalogen Compounds. 2001;53:182–5. [Google Scholar]
- Wilford BH, Shoeib M, Harner T, Zhu J, Jones KC. Polybrominated diphenyl ethers in indoor dust in Ottawa, Canada: implications for sources and exposure. Environ Sci Technol. 2005;39:7027–35. doi: 10.1021/es050759g. [DOI] [PubMed] [Google Scholar]
- Wolff J. Perchlorate and thyroid gland. Pharmacol Rev. 1998;50:89–105. [PubMed] [Google Scholar]
- Wu N, Webster T, Herrmann T, Paepke O, Tickner J, Hale R, Harvey E, La Guardia M, Jacobs E. Associations of PBDE levels in breast milk with diet and indoor dust concentrations. Organohalogen Compounds. 2005;67:654–7. [Google Scholar]
- Wu T, Herrman T, Papke O, Tickner J, Hale R, Harvey H, La Guardia M, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ Sci Technol. 2007;41:1584–9. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- Zhang M, He WH, He P, Chen XM, Wang AG. [Effects of PBDE-47 on oxidative stress and apoptosis in SH-SY5Y cells] Zhongua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:145–7. [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol Sci. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol Sci. 2002;66:105–16. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Crofton KM. Mode of action: developmental thyroid hormone insufficiency – Neurological abnormalities resulting from exposure to propylthiouracil. Crit Rev Toxicol. 2005;35:771–81. doi: 10.1080/10408440591007313. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Tyl RW, Tan SW. Current and potential rodent screens and tests for thyroid toxicants. Crit Rev Toxicol. 2007;37:55–95. doi: 10.1080/10408440601123461. [DOI] [PubMed] [Google Scholar]