Abstract
B-1 B cells produce circulating natural antibodies that provide “innate-like” protection against bacterial and viral pathogens. They also provide adaptive responses to blood and air-borne pathogens. B lymphocyte–induced maturation protein 1 (Blimp-1) is a transcriptional repressor that is required for the formation of B-2–derived antibody-secreting plasma cells. In this study, we used mice lacking Blimp-1 in the B cell lineage to show that Blimp-1 is not necessary for the formation or self-renewal of B-1 B cells but that Blimp-1 is required for normal immunoglobulin (Ig) secretion by B-1 cells. B-1 cells lacking Blimp-1 do not repress Pax5 mRNA and do not induce X-box binding protein 1, and μ secreted mRNA normally, showing that B-1 and B-2 cells both use a common pathway for Ig secretion. Blimp-1–deficient B-1 B cells are also defective in providing early protection against influenza infection.
B-1 B cells represent an important and functionally distinct subset of B cells that reside predominately in pleural and peritoneal cavities, in the gut lamina propria, and to a minor extent in the spleen. They can be distinguished from their “conventional” B-2 counterparts by differences in their surface phenotype because B-1 cells are B220loIgMhiIgDloCD43+ CD21−CD23−. Cavity B-1 cells also express CD11b/CD18 (Mac1), and B-1a and B-1b subsets differ in the presence or absence of CD5, respectively (1, 2).
B-1 cells develop primarily from Lin− CD45Rlo−CD19+ precursors in the fetal BM and fetal liver but can also arise from adult BM progenitors (1, 3–5). Several genetic studies have shown B cell receptor (BCR) signal strength to be crucial for B-1 cell development. Defects in signaling molecules that decrease BCR signaling result in an increase in B-1 cell populations, and defects in those molecules that increase BCR signals reduce B-1 cells (1, 6). Thus, strong BCR antigen signals appear to be important for the decision to become a B-1 cell. Unlike B2 cells, which have limited life spans and are constantly replenished from BM progenitors, B-1 cells are maintained by homeostatic proliferation (self-renewal) as shown by adoptive transfer experiments of B-1 cells into immunodeficient recipients (7, 8). Interestingly, the spleen is required for the generation and maintenance of a large fraction of B-1a cells (9), and B-1 cells are also a major source for IgA-secreting plasma cells that inhabit the lamina propria of the gut (10, 11).
A defining feature of B-1 cells is their ability to secrete so-called “natural” antibodies in the absence of apparent infection or immunization (2, 7, 10). The repertoire of these antibodies is limited. They lack N region additions and somatic hypermutations and often recognize highly conserved, T-independent type 2 bacterial and viral antigens (1, 12–20). Self- and oxidized self-antigens are thought to be responsible for the positive selection and maintenance of B-1 cells expressing natural antibodies (21–23). In addition to providing immunity against several pathogens, B-1–specific antibodies also reduce atherosclerotic lesions, activate T cell responses, contribute to autoimmunity, and promote ischemia/reperfusion injury (23–32). Finally, important functional differences have been identified for B-1a and B-1b cells. B-1a cells spontaneously secrete protective natural antibodies, whereas B-1b cells respond to pathogens by generating long-lasting immunity independent of T cell help (32, 33).
In humans, B-1 lymphocytes are present at the time of birth and persist into adulthood. Although less is known about their function, human B-1a and B-1b cells resemble murine B-1 cells in their expression of surface CD5, in their anatomical placement within the peritoneal cavity (PerC), spleen, and peripheral blood, and in their secretion of poly-specific, autoreactive antibodies (34, 35). In spite of such diverse and important roles for natural antibodies, the mechanisms that regulate antibody secretion by B-1 cells are poorly understood.
Our current molecular understanding of antibody secretion comes almost entirely from the investigation of B-2–derived plasma cells. Recent studies have revealed a network of transcription factors that regulate plasmacytic differentiation (36, 37). One principle player in this process is the transcriptional repressor, B lymphocyte–induced maturation protein 1 (Blimp-1; reference 38). Blimp-1 orchestrates a gene expression program that drives B cells to become plasma cells through the repression of genes involved in B cell proliferation, antigen presentation, germinal center reactions, BCR signaling, and B–T cell–cell interactions (39). Importantly, Blimp-1 also promotes the Ig secretion program (39–45). A crucial direct target of Blimp-1 for inducing the secretory program is Pax5, which encodes B cell lineage-specific activator protein and represses genes encoding Ig heavy chain, J chain protein, and X-box binding protein 1 (XBP-1; references 46–48). Blimp-1 relieves Pax5-dependent repression of XBP-1, which in turn functions as the proximal transcriptional activator for most of the genes necessary for the dramatic phenotypic changes in plasma cells associated with antibody secretion, including increases in cell and ER size, ribosomal and mitochondria number and function, and expression of numerous genes involved in the secretory pathway (49). Like Blimp-1, XBP-1 is required for plasma cell formation and antibody secretion (50). Blimp-1 is also required for processing of μ heavy chain transcripts to the secreted form (μS), although the molecular mechanism is not understood (42).
Recently, Tumang et al. (51) studied Bcl-6, Pax5, Blimp-1, and XBP-1 mRNA and protein levels in purified, Ig-secreting PerC B-1 cells and compared them to those of splenic B-2 cells activated with LPS to undergo plasmacytic differentiation to antibody-secreting cells. Similar to plasmacytic development of B-2 cells, these authors found that Bcl-6 and Pax5 mRNAs were decreased in B-1 cells whereas mRNAs encoding Blimp-1 and XBP-1 were not significantly elevated when compared with LPS-treated B-2 cells. Interestingly, XBP-1 protein levels were comparable to naive B-2 cells, and levels for Bcl-6, Blimp-1, and Pax5 were completely undetected in B-1 cells relative to naive and 2-d LPS-stimulated B-2 cells. These findings suggested that B-1 cells might use a different regulatory program for Ig secretion.
In a previous study, however, we demonstrated that naive mice lacking Blimp-1 in their B cells have dramatically reduced serum IgM, of which more than half comes from B-1 cells (7, 42). To follow up this observation, we sought to determine the nature of Blimp-1's role in B-1 lymphocytes. Here we report that Blimp-1 is not required for B-1a or B-1b lymphocyte formation or for B-1 cell self-renewal. However, Blimp-1 is required for antibody secretion by B-1 cells, and in its absence, Pax5 mRNA is elevated while XBP-1 and μS mRNAs remain low compared with WT controls. Finally, we demonstrate that Blimp-1–deficient B-1 cells are also less effective at protecting reconstituted mice against influenza infection.
RESULTS
B-1 cell formation does not require Blimp-1
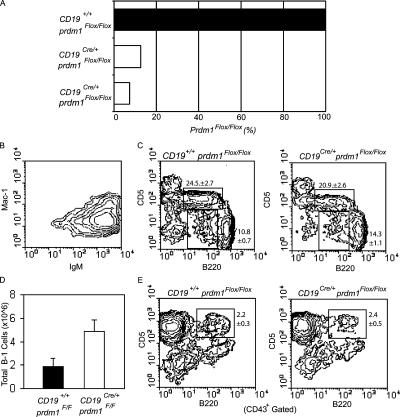
Blimp-1 is encoded by the prdm1 gene. CD19Cre/+prdm1Flox/Flox mice and littermate controls were used to assess the role of Blimp-1 in B-1 cells. CD19Cre-dependent gene deletion is very efficient in splenic B cells (52), and deletion of the prdm1Flox/Flox allele in splenic B cells is nearly complete (42). Deletion of prdm1 in PerC B-1 cells was assessed by quantitative real-time PCR performed on genomic DNA prepared from PerC B-1 cells purified from CD19Cre/+prdm1Flox/Flox and CD19 +/+ prdm1Flox/Flox animals (Fig. 1 A). Primers were designed specifically to amplify floxed but not deleted prdm1 alleles, and samples were normalized using a control single copy gene. Less than 15% of the floxed allele was detected in CD19Cre/+prdm1Flox/Flox cells. Because flow cytometry showed that the cells we analyzed were >70% Mac1+IgM+ B-1 cells (Fig. 1 B), we conclude that prdm1 is efficiently deleted in B-1 cells of CD19Cre/+prdm1Flox/Flox mice, hereafter referred to as conditional knockout (CKO) mice.
Figure 1.
Prdm1 gene deletion and phenotypic analysis of PerC and splenic B-1 cells. (A) Quantitative real-time PCR for prdm1 performed on genomic DNA from two purified CD19Cre/+prdm1Flox/Flox (open bars) and one littermate control (filled bar) PerC B-1 cell cultures. Primers were designed to amplify the floxed, but not the deleted, allele (see Materials and methods), and DNA loading was normalized to the peptidyl prolyl isomerase A gene. Percentages of prdm1Flox/Flox amplified DNA are shown. (B) Representative flow cytometry analysis from one purified CD19Cre/+ prdm1Flox/Flox B-1 cell culture used for deletion analysis in A, stained with antibodies against IgM and Mac-1. (C) Total PerC cells from CD19Cre/+ prdm1Flox/Flox mice (right) and littermate control (left) mice stained with antibodies against B220 and CD5. Upper gate is B-1a, and the lower gate is B-1b. Mean ± SEM, n = 7. (D) Bar graph shows averages and SEM of total PerC B-1 cells (IgM+Mac-1+), n = 7. (E) Total splenocytes stained with antibodies for CD43, B220, and CD5. B220 and CD5 expression on CD43+ cells are shown. Mean ± SEM, n = 4.
Flow cytometry was used to study B-1 cells in 6–10-wk-old CKO and control mice. No significant differences were observed in the frequency of PerC B-1a and B-1b subsets determined by staining for B220 and CD5 (Fig. 1 C). However, CKO mice had an increase in total cellularity in the PerC resulting in a 2.5-fold increase in total numbers of B-1 cells (Fig. 1 D). When total splenocytes were examined, no differences were seen in the frequency of CD5+B220+CD43+ splenic B-1a cells (Fig. 1 E) or in the overall cellularity of total splenocytes (not depicted). From these data, we conclude that Blimp-1 is not required for the formation or maintenance of B-1 cells in either the PerC or spleen. Moreover, we surmise that the absence of serum Ig observed in our initial study of naive CKO mice (42) was not due to the absence of B-1 cells.
Blimp-1–deficient B-1 lymphocytes are defective in antibody secretion
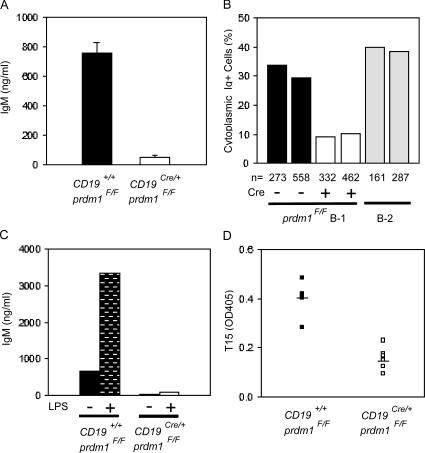
To determine directly the antibody-secreting ability of Blimp-1–deficient B-1 cells, ELISA assays were performed on supernatants from purified PerC B-1 cells cultured ex vivo. Although control B-1 cells secreted IgM in this setting, IgM secreted by B-1 cells derived from CKO mice was nearly undetectable (Fig. 2 A; WT, 756 ± 73 ng/ml; CKO, 53 ± 13 ng/ml). Similar results were obtained from sort-purified, cultured PerC B-1a and B-1b cells (not depicted).
Figure 2.
Absence of Ig secretion in vitro and in vivo by CD19Cre/+prdm1flox/Flox B-1 cells. (A) Anti-IgM ELISA performed on supernatants from B-1 cells after enrichment and in vitro culture. B-1 cells were plated at a density of 106 cells/ml for 4 d with no stimulation. Graph represents five control and eight CD19Cre/+prdm1Flox/Flox samples. (B) Summary of data from two immunohistochemical experiments for the percentages of cytoplasmic Ig+ WT B-1 (filled bars, 33.8 and 29.5%), CD19Cre/+prdm1Flox/Flox B-1 (open bars, 9.2 and 10.2%), and 4-d LPS-treated splenic B-2 (gray bars, 39.1 and 37.9%) B cells. Percentages were determined by dividing the fraction of cytoplasmic Ig+ cells (WT, 68 Ig+/273 total and 114 Ig+/558 total; CKO, 23 Ig+/332 total and 32 Ig+/462 total) by the purity of the respective cultures (WT, 73.8 and 69.3% Mac1+IgM+; CKO, 74.9 and 67.5% Mac1+IgM+). The fraction of cytoplasmic Ig+ splenocytes (C; 63 Ig+/161 total and 109 Ig+/297 total) was determined directly because the culture was assumed to be near 100% pure. (C) Bar graph showing data from one representative anti-IgM ELISA experiment for untreated (no bars) or LPS-treated (bars) control (filled bars) and CD19Cre/+prdm1Flox/Flox (open bar)-purified B-1 cell cultures. ELISA was performed as in A. (D) Anti-T15 ELISA against serum harvested from 6–10-wk-old CKO (filled squares, n = 8) and control (open squares, n = 6) mice. Filled bars represent the SEM values. Units are OD405.
To estimate the portion of cells secreting Ig in these B-1 cultures, we stained permeabilized cells for cytoplasmic Ig and calculated the fraction of cytoplasmic Ig+ B-1 cells. This analysis showed that in control B-1 cell cultures, 31.7% (average from two experiments) were secreting, as indicated by the presence of cytoplasmic Ig. In the CKO cultures, this fraction was 3.2-fold lower or 9.7% (average from two experiments), consistent with the conclusion that Blimp-1 is required for Ig secretion by B-1 cells (Fig. 2 B and Fig. S1, A and B, which is available at http://www.jem.org/cgi/content/full/jem.20060411/DC1). For comparison, ∼38.5% (average from two experiments) of LPS-activated B-2 splenocytes were found to be cytoplasmic Ig+ after 4 d in culture when similarly analyzed (Fig. 2 B and Fig. S1 C).
Treatment of PerC B-1 cells in vitro with LPS causes their proliferation (53), and in mice treated with LPS, PerC B-1 cells increase IgM secretion (28). When purified PerC B-1 cells were cultured for 3 d in LPS, the cells proliferated (on average, cell numbers doubled during the 3-d treatment) and greater than fivefold more IgM measured by ELISA was secreted into the cultures compared with untreated cultures. Similarly, when CKO B-1 cells were treated with LPS they also doubled during the 3-d LPS treatment. However, the CKO B-1 cells secreted 38-fold less IgM than LPS-treated control B-1 cells, although there was a small increase in IgM secretion comparing untreated and treated CKO cultures (Fig. 2 C).
To study Ig secretion by B-1 cells in a more physiological context, we determined the relative serum levels of antibodies bearing the T15 idiotype in CKO and WT mice. T15 idiotype antibodies recognize phosphorylcholine-containing self-antigens derived from oxidized lipids on apoptotic cells and atherosclerotic lesions (23, 54), provide protection against the pathogen Streptococcus pneumonia (55), and are regarded as typical natural antibodies that are exclusively derived from B-1 cells (16). ELISA assays were performed for T15 antibodies in serum from WT and CKO animals using a mixture of two rat anti-T15 antibodies, T139 and Tc54 (56). Relative T15 antibody levels in the sera of unimmunized CKO mice were roughly equivalent to the level of detection of this assay in all but one of eight mice analyzed, whereas the T15 serum levels in all six unimmunized control animals were on average >2.8-fold above background (Fig. 2 D; WT, OD405 = 0.404 ± 0.11; CKO, OD405 = 0.144 ± 0.07). Collectively, these data demonstrate that Blimp-1 is required for normal antibody secretion by B-1 cells both ex vivo and in vivo.
Ig secretion by B-1 cells depends on transcriptional regulators previously identified in B-2 cells
We next explored the molecular mechanisms underlying the requirement for Blimp-1 in Ig secretion by B-1 cells. In B-2 cells, direct repression of Pax5 leads to the derepression of the activator XBP-1, which then functions as the critical proximal regulator of a complex secretory program (42, 49). Furthermore, Blimp-1 is required for the processing of primary μ transcripts to the μS form of mRNA (42).
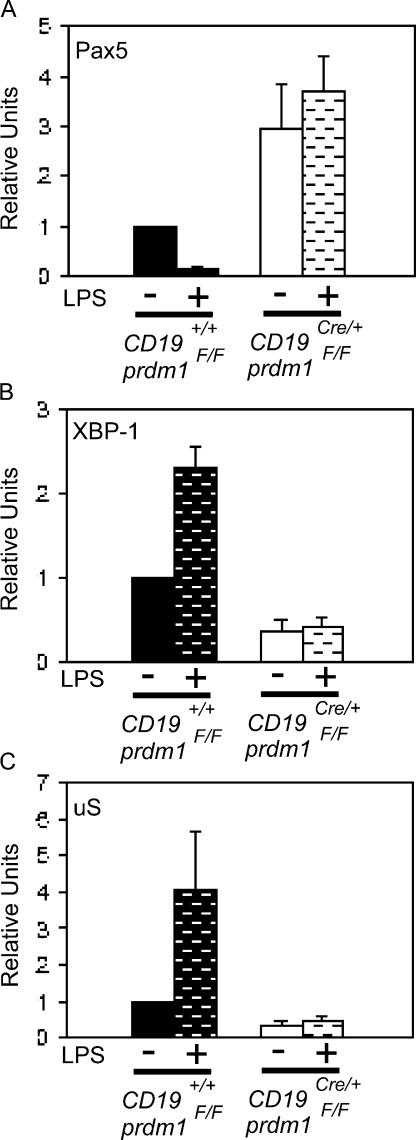
Quantitative RT-PCR was used to determine the steady-state levels of μS, Pax5, and XBP-1 mRNAs in CKO and control B-1 cells. Purified PerC B-1 cells were analyzed with and without treatment with LPS for 3 d. Pax5 mRNA was higher in CKO cells compared with WT cells, both without LPS and after LPS treatment. Furthermore, Pax5 mRNA decreased after LPS treatment in the WT but not in CKO cells (Fig. 3 A; 2.9-fold difference between unstimulated CKO/WT; 20.2-fold difference between LPS-stimulated CKO/WT). These data provide evidence that Blimp-1 is required to repress Pax5 mRNA in B-1 cells. CKO B-1 cells also had lower levels of XBP-1 mRNA without LPS treatment and failed to induce XBP-1 after LPS treatment compared with control B-1 cells (Fig. 3 B; 2.6-fold unstimulated WT/CKO; 5.6-fold LPS-stimulated WT/CKO). Finally, μS was not expressed normally in untreated cells nor was it induced normally in LPS-treated CKO B-1 cell (Fig. 3 C; 3.0-fold unstimulated WT/CKO; 8.6-fold LPS-stimulated WT/CKO) transcripts. Thus, we conclude that Blimp-1 is required in B-1 cells for Pax5 repression and XBP-1 induction, as well as for formation of μS mRNA.
Figure 3.
Misregulation of Blimp-1 targets in Blimp-1–deficient B-1 cells. Quantitative real-time PCR performed on cDNA prepared from purified, untreated (no bars) or LPS-treated (bars) control (filled bars) and CD19Cre/+prdm1Flox/Flox (open bars) B-1 cells. Mean and SEM for (A) Pax5 (n = 5; unstimulated CKO/WT, P = 0.050; LPS-stimulated CKO/WT, P = 0.002), (B) XBP-1 (n = 6; unstimulated WT/CKO, P < 0.001; LPS-stimulated WT/CKO, P < 0.001), and (C) μS (n = 6; unstimulated WT/CKO, P = 0.002; LPS-stimulated WT/CKO, P <0.040) steady-state mRNA levels are shown as relative units normalized to the untreated control samples. All values were normalized to β2 microglobulin mRNA.
Blimp-1 is not required for self-renewal/homeostatic proliferation of B-1 cells
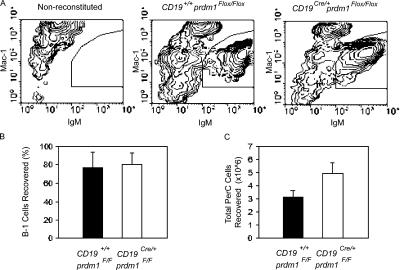
A unique feature of PerC B-1 cells, in contrast to B-2 cells, is their ability to regenerate the entire B-1 cell compartment. Adoptive transfer of peritoneal B-1 cells by i.p. injection into immunodeficient mice leads to the stable, long-term reconstitution of the PerC and IgA+ lamina propria B-1 cell pools, as well as restoration of natural IgM titers (7, 8). Peripheral B-2 cells, on the other hand, lack this ability and can only be generated from BM progenitors. To investigate a possible role for Blimp-1 in the self-renewal capacity of B-1 cells, total PerC cells from WT and CKO mice were harvested and adoptively transferred i.p. to 6–12-wk-old Rag1 −/− mice. Recipient mice were killed 6–8 wk after transfer and the frequency of PerC B-1 cells was measured by flow cytometry. A small number of mice, receiving either WT or CKO B-1 cells, failed to reconstitute. Those in which reconstitution was <10% were excluded from the study. The half-life of B-1 cells has been reported to be between 38 and 56 d (57); therefore, recovery of >50% of donor B-1 cells after 6–8 wk indicates proliferation of the transferred B-1 cells. Representative flow cytometry analyses for IgM+Mac1+-stained PerC cells harvested from nonreconstituted, WT-reconstituted, and CKO-reconstituted Rag1 −/− mice (Fig. 4 A) indicate that CKO cells can reconstitute Rag1 −/− mice. CKO B-1 cells, as well as WT B-1 cells (Fig. 4 B; WT, 66.2 ± 17.1% recovered; CKO, 71.4 ± 13.9% recovered), proliferated and self-renewed in this experimental setting, and more total PerC cells were recovered from CKO-reconstituted Rag1 −/− mice than WT-transferred Rag1 −/− mice (Fig. 4 C; WT, 3.1 × 106 ± 0.55 total cells; CKO, 5.0 × 106 ± 0.81 total cells). In addition, T15 antibodies were detected in the sera of mice reconstituted with WT PerC cells at the time recipient mice were killed, demonstrating that transferred B-1 cells were functional (not depicted). Thus, we conclude that Blimp-1 is not required for the self-renewal/homeostatic proliferation of B-1 cells.
Figure 4.
Normal self-renewal/homeostatic proliferation of Blimp-1–deficient B-1 cells. Equal numbers of total PerC cells from control and CD19Cre/+prdm1Flox/Flox mice were transferred i.p. to Rag1-deficient hosts. 6–8 wk after transfer, PerC cells were harvested from recipient mice and the efficiency of B-1 cell reconstitution was determined by dividing the number of B-1 cells recovered by the number transferred. (A) Representative flow cytometry plots for anti-IgM– and Mac-1–stained PerC cells from Rag1−/− recipient mice either untreated (left) or receiving WT PerC cells (middle) or CD19Cre/+prdm1Flox/Flox PerC cells (right) are shown. IgM+Mac1+ B-1 population is shown. (B) Bar graph showing the mean and SEM of the percentage of WT (filled bar) and CD19Cre/+Prdm1Flox/Flox (open bar) B-1 cells recovered for five WT and six CD19Cre/+prdm1Flox/Flox samples. (C) Total PerC cells harvested from mice in B.
Blimp-1 is required for B-1 cells to protect against influenza virus infection in vivo
Baumgarth et al. (26) have elegantly demonstrated that both B-1 and B-2 cells are required for effective early immunity against influenza infection in mice. Specifically, B-1 cell– derived natural antibodies, present before infection, promote subsequent B-2 cell IgG2b responses and reduce mortality. This is probably because natural antibodies trap viruses and fix complement (58–60). Because Blimp-1 is required for natural antibody secretion by B-1 cells (Fig. 2), we hypothesized that B-1 cells lacking Blimp-1 would be defective in their ability to provide protection to influenza infection. To test this hypothesis, 4–6-wk-old irradiated muMT − mice were reconstituted i.v. with B-2 cells from BM from WT mice. Mice were also given PerC B-1 cells i.p. from either WT or CKO mice. B-2 cell reconstitution was confirmed by flow cytometric analysis performed for peripheral blood B220+ cells. 3 wk after reconstitution, mice were infected intranasally with an <LD50 dosage of A/WSN/33 influenza virus, and then monitored for 2 wk.
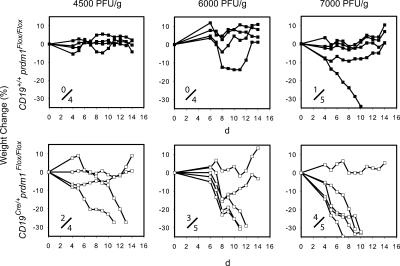
Weight loss was used as a criterion for susceptibility to influenza infection. In more than three independent experiments in which 27 mice were intranasally infected with influenza virus ranging from 4,500 to 7,000 PFU/g, we found only 1/13 WT-reconstituted mice (Fig. 5, top), but 9/14 CKO-reconstituted mice (Fig. 5, bottom) lost at least 30% of total body weight. Mice were killed when they lost 30% of their body weight to prevent excessive suffering, or on day 14 for analysis of the efficiency of B-1 cell reconstitution. Every mouse successfully reconstituted donor PerC B-1 cells as determined by flow cytometry analysis for surface IgM and Mac1 expression (not depicted). The increased susceptibility to influenza infection of mice receiving CKO B-1 cells demonstrates the physiological relevance of the requirement for Blimp-1 in antibody secretion by B-1 cells.
Figure 5.
Sensitivity to influenza virus by mice reconstituted with Blimp-1–deficient B-1 cells. Lethally irradiated muMT − mice received WT bone marrow cells and either WT (top) or CD19Cre/+prdm1Flox/Flox (bottom) PerC cells. 3 wk after reconstitution, anesthetized mice were intranasally infected with 4,500, 6,000, or 7,000 PFU/g body weight with A/WSN/33 influenza virus. Mice were monitored daily and weighed from days 4–14. The percentage of weight change over 14 d as compared with the initial weight on the day of infection is shown. B-2 cell reconstitution was confirmed 1 d before influenza infection, and B-1 cell reconstitution was determined on the final day of the experiment. The fraction of susceptible mice is shown.
DISCUSSION
Blimp-1 is required for Ig secretion in B-1 cells
Our data reveal an essential role for Blimp-1 in antibody secretion by B-1 cells, both ex vivo and in vivo (Fig. 2). A requirement for Blimp-1 in antibody secretion by B-2 cells has been established previously (42). Earlier studies have shown that Blimp-1 is necessary for full induction of IgH, J chain, and XBP-1 mRNAs in B-2 cells, presumably due to direct repression of Pax5 by Blimp-1 (44) and subsequent derepression of these genes that are repressed by Pax5 (46–50), although recent papers disagree on whether or not Pax5 represses XBP-1 (61, 62). XBP-1 then functions as the proximal regulator of the Ig secretion program, inducing genes encoding proteins responsible for targeting proteins to the ER, cleavage of signal peptides, proper protein folding, degradation of misfolded proteins, and protein glycosylation, as well as proteins needed for ER and other organelle biogenesis and increased cell size (49, 63). In addition, Blimp-1 is required for the formation of μS mRNA, although the mechanistic basis for this requirement is not currently understood (42).
We therefore compared Pax5 mRNA repression, XBP-1 mRNA induction, and formation of μS mRNA in control versus CKO B-1 cells to determine if similar mechanisms were involved in Ig secretion by B-1 cells and B-2 plasma cells. We found that B-1 cells lacking Blimp-1 failed to repress Pax5 mRNA, failed to induce XBP-1 mRNA, and failed to form μS mRNA when compared with control B-1 cells (Fig. 3). These results provide strong evidence that the Blimp-1–dependent mechanisms we studied are important for Ig secretion in both B-1 and B-2 cells. This conclusion is also consistent with a previous study showing that mice lacking XBP-1 in their lymphocytes formed B-1 cells but failed to secrete IgM (50).
Why do spontaneously secreting B-1 cells have significantly lower levels of mRNA encoding Blimp-1 and XBP-1 compared with Ig-secreting B-2 cells, as reported by Tumang et al. (51)? The amount of IgM measured by ELISA in LPS-treated splenic B-2 cell supernatants is ∼18-fold higher than that in cultures of purified B-1 cells (unpublished data). Yet our data for purified B-1 cells in short-term culture (Fig. 2 B), and that of Tumang et al. for ex vivo–purified B-1 cells, show that a significant fraction (∼32 and ∼21%, respectively) of purified B-1 cells spontaneously secrete IgM. In addition, our data (Fig. 2 B and Fig. S1 C) indicate that a comparable fraction of LPS-stimulated B-2 cells are secreting, as measured by cytoplasmic Ig (∼39%). These data suggest that B-1 cells secrete less Ig per cell than B-2 cells. This conclusion is consistent with the ∼55% smaller spot sizes seen in B-1 cell ELISPOT assays, further demonstrating that B-1 cells secrete less IgM than do LPS-treated B splenocytes (51). Moreover, the morphology of B-1 cells is distinct from that of plasma cells. Although they have ample ER, B-1 cells lack the distinct arrays of rough ER characteristic of plasma cells (64). Thus, we suggest that although B-1 cells use the same regulatory mechanisms for Ig secretion, because they have less ER and secrete less Ig per cell, they may require lower amounts of Blimp-1 and XBP-1 mRNA and protein compared with B-2 plasma cells.
Our data clearly show that although Blimp-1 mRNA in B-1 cells is relatively low, it is nevertheless functionally important because it is required for normal Ig secretion. This conclusion is strengthened by the demonstration that Blimp-1–deficient B-1 cells do not secrete normal amounts of T15 natural antibodies (Fig. 2) and do not provide normal protection against influenza virus infection (Fig. 5).
Role of IgM in the formation of B-1 cells
Mice that cannot secrete IgM due to mutation in the μ-secreted exon and polyA sites have 1.5–2-fold increases in the frequency and total numbers of PerC B-1 cells (65, 66). The CD19Cre/+prdm1Flox/Flox mice we studied have significantly reduced serum levels of all Ig isotypes including IgM (42). In spite of this, we did not observe an increase in the frequency of B-1a or B-1b cells in the PerC or in B220+CD5+CD43+ B-1 cells in the spleen of these mice. There were, however, more total cells in the PerC of the CKO mice, resulting in a 2.5-fold increase in the total number of B-1 cells. Hence, our data support the idea that a lack of serum IgM feeds back to cause an increase in total B-1 cells in the PerC. The mechanism responsible for this effect remains obscure.
CKO B-1 cells, although deficient in Ig secretion, are normal in their ability to proliferate and self-renew, as demonstrated by their successful reconstitution of lymphopenic Rag1−/− and muMT − hosts. We found no difference in the rate of recovery between CKO- and WT-transferred PerC B-1 cells after 6–8 wk after intraperitoneal transfer (Fig. 4), consistent with our previous observation that splenic B-2 cells from CKO mice proliferate well in response to LPS (42). Although we cannot formally rule out the possibility, we do not believe that CKO B-1 cell reconstitution was the result of preferential proliferation of CD19Cre/+prdm1Flox/Flox B-1 cells that failed to delete prdm1. In addition to finding no differences in total cell numbers in our in vitro cultures, serum from WT-reconstituted Rag1 −/− were found to have approximately ninefold greater T15 antibody levels than CKO-reconstituted mice (not depicted) and CKO-reconstituted muMT − mice were functionally inferior to WT-reconstituted mice upon challenge with influenza virus (Fig. 5).
The relationship between Ig secretion and cell division in B-1 cells
The B-1 cell compartment is heterogeneous and no single anatomical location or surface marker can define the entire population. B-1 cells are particularly uncharacterized in terms of two defining features: proliferation associated with self-renewal and Ig secretion. Further complexity is added by the fact that many B-1 cells are resting and perform neither function. Although in earlier studies Ig secretion was not detected in PerC B-1 cells (67, 68), Tumang et al. (51) showed by ELISPOT assay that ∼21% of naive, freshly sorted PerC CD5+B220+ B-1 cells secreted IgM over 3 h. Our results on primary B-1 cells in short-term culture confirm this (Fig. 2, A and B, and Fig. S1 A). Many fewer PerC B-1 cells, however, are cycling than were found to be secreting: 2.5% of CD5+ PerC B cells depleted of T cells and macrophages were found to be in cycle in vitro, and when the proliferative capacity of PerC CD5+ B cells was determined in vivo, only 0.5–1.0% were in S phase (69, 70). Thus, it is not clear whether B-1 cells that secrete Ig have lost their proliferative capability, retain it, or, after a period of secretion, can revert to cells with proliferative potential.
In B-2 cells, plasmablasts are highly proliferative and also capable of secreting Ig. However, terminally differentiated plasma cells do not divide. Blimp-1 has been shown to repress multiple genes required for cell cycle entry, DNA replication, and cell division, and it is thought to be important for establishing/maintaining the postmitotic state of plasma cells (39, 43, 71–74). Nonetheless, dividing plasmablasts also express Blimp-1, demonstrating that Blimp-1 expression is not incompatible with cell division if the cells receive strong mitogenic signals (75, 76). Although additional studies will be necessary to learn if B-1 cells are fundamentally different from B-2 cells with respect to terminal differentiation to an Ig-secreting, nonproliferating state, we suspect that the low level of Blimp-1 mRNA in B-1 cells, compared with B-2 plasma cells, does not preclude Ig-secreting B-1 cells in the PerC from dividing when they receive appropriate signals. PerC B-1 cells may simultaneously retain both secretory and proliferative abilities, or they may alternate between secretory and proliferative states, but more data will be required to test this hypothesis. It will also be interesting to learn how overexpression of Blimp-1 might affect Ig secretion and proliferation of B-1 cells.
Overall, this study has shown that B-1 cells, like B-2 cells, require Blimp-1 and Blimp-1–dependent derepression of XBP-1 to secrete Ig. Antibodies derived from B-1 cells are important for immunity to mucosal and air-borne pathogens and are also implicated in autoimmune diseases, including systemic lupus erythematosus (77), Sjorgen's syndrome (78), and rheumatoid arthritis (79). Antibodies derived from B-2 cells are similarly critical for humoral immunity and involved in autoimmunity. Understanding that both B-1 and B-2 cells use common mechanisms to secrete antibodies suggests that compounds designed to modulate the expression or activity of Blimp-1 or XBP-1 could affect both B-1 and B-2 cells and would be effective for either vaccine design or treatment of autoimmunity.
MATERIALS AND METHODS
Mice, cell transfers, and influenza infection.
Prdm1Flox/Flox mice were crossed with CD19Cre/+ mice to generate experimental (CD19Cre/+ prdm1Flox/Flox) and control (CD19+/+prdm1Flox/Flox) mice. Rag1−/− (B6.129S7-Rag1tm1Mom/J) and muMT− (B6.129S2-Igh-6tm1Cgn/J) were from The Jackson Laboratory. All mouse procedures were approved by Columbia University's Institutional Animal Care and Use Committee. PerC cells were harvested in 4% FBS, 1% BSA in PBS. 3–5 × 106 PerC cells were resuspended in 1 ml PBS and transferred i.p. to Rag1−/− or muMT− mice. The remaining PerC cells were stained with IgM and Mac-1 antibodies and analyzed by flow cytometry. For BM reconstitution, muMT− mice were lethally irradiated with 2× 700 rads separated by 4 h. Mice were rested overnight and then reconstituted via tail vein injection with 107 total BM cells in 200 uL PBS harvested from CD19+/+prdm1Flox/Flox control mice. Mice were fed water containing Baytril (enrofloxacin) for the remainder of the experiment. Influenza virus A/WSN/33 was provided by P. Palese (Mount Sinai School of Medicine, New York, NY). Influenza virus was cultured on Mardin-Darby Bovine Kidney cells in modified Eagle's medium supplemented with 0.2% BSA and tittered on Mardin-Darby Canine Kidney cells as described (80) but without trypsin. For influenza infections, mice were anesthetized with 5% isoflurane and maintained in 2% isoflurane with oxygen. 4,500–7,000 PFU/g body weight was administered to each mouse intranasally in 20 ul PBS. Mice were caged separately and weighed on days 0 and 4–14.
Lymphocyte purification and LPS cultures.
PerC cells were harvested in RPMI (10% FBS) and gentamycin sulfate and plated for 2 h to remove adherent cells. Thy1.2+ T cells were removed using magnetic beads and B-2 cells did not survive in culture. On day 4, cells were replated at a density of 106 cells/ml. B-1 cultures were treated with 1 ug/ml LPS (Sigma-Aldrich) or left untreated for 3 d when supernatants were harvested for anti-IgM ELISA assays and cells were processed for either immunohistochemistry, cDNA, or genomic DNA preparations (see below). Splenocyte cultures were prepared as described previously (42) and treated with 1 ug/ml LPS for 4 d at which time live cells were harvested for immunohistochemical analysis.
Flow cytometry.
The following unlabeled, biotinylated, fluorochrome-conjugated, and secondary detection antibodies were used: Mac-1-PE (M1/70), IgM-biotin (II/41), CD16/32 (93), and B220-APC (Ra3-6B2; all from eBioscience), and CD5-biotin (Ly-1), CD43-PE (S7), and strepavidin-APC (all from BD Biosciences). All flow cytometry stains were performed by incubating 106 cells in 10 uL of 4% FBS plus 1% BSA in PBS with Fc block for 10 min followed by primary antibodies for 45 min, and then, after a brief wash, secondary antibodies for 30 min at 4°C in darkness. Analysis was performed on an LSRII (BD Biosciences) using WinMDI software (Joseph Trotter, Scripps Research Institute).
ELISA.
Anti-IgM ELISA assays were performed on supernatants from B-1 cell cultures as described previously (81). Anti-T15–expressing hybridoma lines T139.2 and Tc54.8 were provided by M. Scharff (Albert Einstein College of Medicine, New York, NY). Hybridomas were grown and antibodies were purified using standard ammonium acetate precipitation techniques. For detection of serum T15, 96-well plates were coated with 50 uL of a mixture of 25 ug each of T139.2 and TC54.8 antibodies in PBS for 60 min, and then blocked with a solution of 2% BSA in PBS overnight at 4°C. Wells were washed once with 0.05% Tween-20 in PBS and exposed to mouse serum (1:2 dilutions) for 60 min at 37°C. Wells were washed four times and incubated with a 1:500 dilution of goat anti–mouse Ig(H+L)-AP secondary antibody (SouthernBiotech) in 1% BSA in PBS for 60 min at 37°C. Four additional washes followed by development with 0.8 mg/ml of Sigma 104 phosphatase substrate (Sigma-Aldrich) in p-nitrophenyl phosphate buffer, and spectrophotometric measurements at OD405 were performed.
Immunohistochemistry.
Purified B-1 cells or splenocytes were seeded on slides by cytospin at 800 rpm for 5 min, air dried, fixed, and permeablized with 1% paraformaldehyde plus 0.2% Tween-20 in PBS for 20 min. Egg white in PBS was incubated for 1 h to block, followed by incubation with 3% human serum (Sigma-Aldrich) plus 3% FBS plus 1% BSA in PBS for 20 min. Primary goat anti–mouse Ig(H+L) antibody (SouthernBiotech) was diluted at 1:5,000 in serum block and applied to slides overnight. The slides were washed for 45 min with TBST (50 mM Tris, pH 7.5, plus 0.2% Tween-20) and incubated with 1:100 diluted rabbit anti–goat IgG(H+L) alkaline phosphatase–conjugated secondary antibody (SouthernBiotech) for 1 h in serum block. Slides were washed as described above and developed by fast blue (Sigma-Aldrich) and napthol AsBi-phosphate substrate (Sigma-Aldrich) supplemented with levamisole (Sigma-Aldrich) in 100 mM Tris-HCl, pH 9.2. A Nikon Eclipse TE300 microscope and Openlab (Improvision) software were used for photographic analysis. Cells with darkly staining rings of cytoplasm or with darkly staining cytoplasmic caps were scored positive.
Quantitative real-time PCR.
Quantitative real-time PCR was performed with a cycle of 50°C, 2 min; 95°, 10 min; 95°C, 15 s; 60°C, 1 min; and 81°C, 20 s for 40 cycles, recording data at 81°C and using primers for the unprocessed form of XBP-1 (5′-AGCACTCAGACTATGTGCACCTCT-3′, 5′-TCCAGAATGCCCAAAAGGATATC-3′), μS (5′-TCTGCCTTCACCACAGAAG-3′, 5′-TAGCATGGTCAATAGCAGG-3′), Pax5 (5′-CAACAAACGCAAGAGGG-3′, 5′-GGGCTCGTCAAGTTGG-3′), β-2 microglobulin (5′-AGACTGATACATACGCCTGCA-3′, 5′-GCAGGTTCAAATGAATCTTCAG-3′), Blimp-1 (5′-AGTAGTTGAATGGGAGC-3′, 5′-CAATGCTTGTCTAGTGTC-3′) and peptidyl prolyl isomerase A (5′-CTGAGCACTGGAGAGAAAGG-3′, 5′-CTTGCTGGTCTTGCCATTCC-3′). Quantitative real-time PCR was performed on an ABI7000 machine. Total RNA and cDNA were prepared from at least 0.15 × 106 purified B-1 cells by TRIzol and Superscript III reverse transcriptase according to the manufacture's instructions (Invitrogen). Genomic DNA was made by lysis of purified B-1 cells in 50 mM Tris-HCl, pH 8.0, 0.1 mM EDTA, and 12.5% SDS, followed by phenol/chloroform extraction and ethanol precipitation.
Statistics.
Data were expressed as the mean ± SEM. Statistical significance was determined by a two-tail, unpaired Student's t test.
Online supplemental material.
Fig. S1 shows representative photographs of cytoplasmic Ig staining of purified, cultured WT B-1 (A), CKO B-1 (B), and LPS-treated splenic B-2 (C) cells. It is available at http://www.jem.org/cgi/content/full/jem.20060411/DC1.
Supplemental Material
Acknowledgments
We thank Dr. P. Palese for the A/WSN/33 influenza virus, Dr. M. Scharff for the anti-T15 antibodies, and Dr. T. Rothstein for technical advice. We also thank Drs. Y. Zou and B. Diamond for critically reading the manuscript and members of the Calame lab for many helpful discussions.
This work was supported by RO1AI50659 and RO1AI43576 to K. Calame.
The authors have no conflicting financial interests.
Abbreviations used: BCR, B cell receptor; Blimp-1, B lymphocyte–induced maturation protein 1; CKO, conditional knockout; μs, secreted μ heavy chain; PerC, peritoneal cavity; XBP-1, X-box binding protein 1.
References
- 1.Berland, R., and H.H. Wortis. 2002. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 20:253–300. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa, K., R.R. Hardy, D.R. Parks, and L.A. Herzenberg. 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J. Exp. Med. 157:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayakawa, K., and R.R. Hardy. 2000. Development and function of B-1 cells. Curr. Opin. Immunol. 12:346–353. [DOI] [PubMed] [Google Scholar]
- 4.Wortis, H.H., and R. Berland. 2001. Cutting edge commentary: origins of B-1 cells. J. Immunol. 166:2163–2166. [DOI] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez, E., H. Leathers, and K. Dorshkind. 2006. Identification of a B-1 B cell-specified progenitor. Nat. Immunol. 7:293–301. [DOI] [PubMed] [Google Scholar]
- 6.Casola, S., K.L. Otipoby, M. Alimzhanov, S. Humme, N. Uyttersprot, J.L. Kutok, M.C. Carroll, and K. Rajewsky. 2004. B cell receptor signal strength determines B cell fate. Nat. Immunol. 5:317–327. [DOI] [PubMed] [Google Scholar]
- 7.Forster, I., and K. Rajewsky. 1987. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 17:521–528. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa, K., R.R. Hardy, L.A. Herzenberg, and L.A. Herzenberg. 1985. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 161:1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardemann, H., T. Boehm, N. Dear, and R. Carsetti. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J. Exp. Med. 195:771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos, N.A., C.G. Meeuwsen, B.S. Wostmann, J.R. Pleasants, and R. Benner. 1988. The influence of exogenous antigenic stimulation on the specificity repertoire of background immunoglobulin-secreting cells of different isotypes. Cell. Immunol. 112:371–380. [DOI] [PubMed] [Google Scholar]
- 11.Kroese, F.G., E.C. Butcher, A.M. Stall, P.A. Lalor, S. Adams, and L.A. Herzenberg. 1989. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int. Immunol. 1:75–84. [DOI] [PubMed] [Google Scholar]
- 12.Kantor, A.B., C.E. Merrill, L.A. Herzenberg, and J.L. Hillson. 1997. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J. Immunol. 158:1175–1186. [PubMed] [Google Scholar]
- 13.Hardy, R.R., C.E. Carmack, S.A. Shinton, R.J. Riblet, and K. Hayakawa. 1989. A single VH gene is utilized predominantly in anti-BrMRBC hybridomas derived from purified Ly-1 B cells. Definition of the VH11 family. J. Immunol. 142:3643–3651. [PubMed] [Google Scholar]
- 14.Su, S.D., M.M. Ward, M.A. Apicella, and R.E. Ward. 1991. The primary B cell response to the O/core region of bacterial lipopolysaccharide is restricted to the Ly-1 lineage. J. Immunol. 146:327–331. [PubMed] [Google Scholar]
- 15.Malynn, B.A., G.D. Yancopoulos, J.E. Barth, C.A. Bona, and F.W. Alt. 1990. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J. Exp. Med. 171:843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masmoudi, H., T. Mota-Santos, F. Huetz, A. Coutinho, and P.A. Cazenave. 1990. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int. Immunol. 2:515–520. [DOI] [PubMed] [Google Scholar]
- 17.Baumgarth, N., O.C. Herman, G.C. Jager, L. Brown, L.A. Herzenberg, and L.A. Herzenberg. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA. 96:2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayakawa, K., R.R. Hardy, M. Honda, L.A. Herzenberg, A.D. Steinberg, and L.A. Herzenberg. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 81:2494–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster, I., H. Gu, and K. Rajewsky. 1988. Germline antibody V regions as determinants of clonal persistence and malignant growth in the B cell compartment. EMBO J. 7:3693–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tornberg, U.C., and D. Holmberg. 1995. B-1a, B-1b and B-2 B cells display unique VHDJH repertoires formed at different stages of ontogeny and under different selection pressures. EMBO J. 14:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayakawa, K., M. Asano, S.A. Shinton, M. Gui, D. Allman, C.L. Stewart, J. Silver, and R.R. Hardy. 1999. Positive selection of natural autoreactive B cells. Science. 285:113–116. [DOI] [PubMed] [Google Scholar]
- 22.Wang, H., and S.H. Clarke. 2004. Regulation of B-cell development by antibody specificity. Curr. Opin. Immunol. 16:246–250. [DOI] [PubMed] [Google Scholar]
- 23.Shaw, P.X., S. Horkko, M.K. Chang, L.K. Curtiss, W. Palinski, G.J. Silverman, and J.L. Witztum. 2000. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105:1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin, F., and J.F. Kearney. 2000. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol. Rev. 175:70–79. [PubMed] [Google Scholar]
- 25.Martin, F., A.M. Oliver, and J.F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 14:617–629. [DOI] [PubMed] [Google Scholar]
- 26.Baumgarth, N., O.C. Herman, G.C. Jager, L.E. Brown, L.A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell–derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuji, R.F., M. Szczepanik, I. Kawikova, V. Paliwal, R.A. Campos, A. Itakura, M. Akahira-Azuma, N. Baumgarth, L.A. Herzenberg, and P.W. Askenase. 2002. B cell–dependent T cell responses: IgM antibodies are required to elicit contact sensitivity. J. Exp. Med. 196:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami, M., T. Tsubata, R. Shinkura, S. Nisitani, M. Okamoto, H. Yoshioka, T. Usui, S. Miyawaki, and T. Honjo. 1994. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J. Exp. Med. 180:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, M., W.G. Austen Jr., I. Chiu, E.M. Alicot, R. Hung, M. Ma, N. Verna, M. Xu, H.B. Hechtman, F.D. Moore Jr., and M.C. Carroll. 2004. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. USA. 101:3886–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada, T., M. Abe, F. Takiura, S. Hirose, and T. Shirai. 1990. Distinct surface phenotypes of B cells responsible for spontaneous production of IgM and IgG anti-DNA antibodies in autoimmune-prone NZB x NZW F1 mice. Autoimmunity. 7:109–120. [DOI] [PubMed] [Google Scholar]
- 31.Szczepanik, M., M. Akahira-Azuma, K. Bryniarski, R.F. Tsuji, I. Kawikova, W. Ptak, C. Kiener, R.A. Campos, and P.W. Askenase. 2003. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J. Immunol. 171:6225–6235. [DOI] [PubMed] [Google Scholar]
- 32.Alugupalli, K.R., J.M. Leong, R.T. Woodland, M. Muramatsu, T. Honjo, and R.M. Gerstein. 2004. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 21:379–390. [DOI] [PubMed] [Google Scholar]
- 33.Haas, K.M., J.C. Poe, D.A. Steeber, and T.F. Tedder. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 23:7–18. [DOI] [PubMed] [Google Scholar]
- 34.Youinou, P., C. Jamin, and P.M. Lydyard. 1999. CD5 expression in human B-cell populations. Immunol. Today. 20:312–316. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarth, N., J.W. Tung, and L.A. Herzenberg. 2005. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 26:347–362. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro-Shelef, M., and K. Calame. 2005. Regulation of plasma-cell development. Nat. Rev. Immunol. 5:230–242. [DOI] [PubMed] [Google Scholar]
- 37.Lin, K.I., C. Tunyaplin, and K. Calame. 2003. Transcriptional regulatory cascades controlling plasma cell differentiation. Immunol. Rev. 194:19–28. [DOI] [PubMed] [Google Scholar]
- 38.Turner, C.A., Jr., D.H. Mack, and M.M. Davis. 1994. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 77:297–306. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer, A.L., K.I. Lin, T.C. Kuo, X. Yu, E.M. Hurt, A. Rosenwald, J.M. Giltnane, L. Yang, H. Zhao, K. Calame, and L.M. Staudt. 2002. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 17:51–62. [DOI] [PubMed] [Google Scholar]
- 40.Kallies, A., J. Hasbold, D.M. Tarlinton, W. Dietrich, L.M. Corcoran, P.D. Hodgkin, and S.L. Nutt. 2004. Plasma cell ontogeny defined by quantitative changes in Blimp-1 expression. J. Exp. Med. 200:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piskurich, J.F., K.I. Lin, Y. Lin, Y. Wang, J.P. Ting, and K. Calame. 2000. BLIMP-I mediates extinction of major histocompatibility class II transactivator expression in plasma cells. Nat. Immunol. 1:526–532. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro-Shelef, M., K.I. Lin, L.J. McHeyzer-Williams, J. Liao, M.G. McHeyzer-Williams, and K. Calame. 2003. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 19:607–620. [DOI] [PubMed] [Google Scholar]
- 43.Lin, Y., K. Wong, and K. Calame. 1997. Repression of c-myc transcription by Blimp-1, an inducer of terminal B cell differentiation. Science. 276:596–599. [DOI] [PubMed] [Google Scholar]
- 44.Lin, K.I., C. Angelin-Duclos, T.C. Kuo, and K. Calame. 2002. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol. Cell. Biol. 22:4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sciammas, R., and M.M. Davis. 2004. Modular nature of Blimp-1 in the regulation of gene expression during B cell maturation. J. Immunol. 172:5427–5440. [DOI] [PubMed] [Google Scholar]
- 46.Neurath, M.F., W. Strober, and Y. Wakatsuki. 1994. The murine Ig 3′ alpha enhancer is a target site with repressor function for the B cell lineage-specific transcription factor BSAP (NF-HB, S alpha-BP). J. Immunol. 153:730–742. [PubMed] [Google Scholar]
- 47.Rinkenberger, J.L., J.J. Wallin, K.W. Johnson, and M.E. Koshland. 1996. An interleukin-2 signal relieves BSAP (Pax5)-mediated repression of the immunoglobulin J chain gene. Immunity. 5:377–386. [DOI] [PubMed] [Google Scholar]
- 48.Singh, M., and B.K. Birshtein. 1993. NF-HB (BSAP) is a repressor of the murine immunoglobulin heavy-chain 3′ alpha enhancer at early stages of B-cell differentiation. Mol. Cell. Biol. 13:3611–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaffer, A.L., M. Shapiro-Shelef, N.N. Iwakoshi, A.H. Lee, S.B. Qian, H. Zhao, X. Yu, L. Yang, B.K. Tan, A. Rosenwald, et al. 2004. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 21:81–93. [DOI] [PubMed] [Google Scholar]
- 50.Reimold, A.M., N.N. Iwakoshi, J. Manis, P. Vallabhajosyula, E. Szomolanyi-Tsuda, E.M. Gravallese, D. Friend, M.J. Grusby, F. Alt, and L.H. Glimcher. 2001. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 412:300–307. [DOI] [PubMed] [Google Scholar]
- 51.Tumang, J.R., R. Frances, S.G. Yeo, and T.L. Rothstein. 2005. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J. Immunol. 174:3173–3177. [DOI] [PubMed] [Google Scholar]
- 52.Rickert, R.C., J. Roes, and K. Rajewsky. 1997. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 25:1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris, D.L., and T.L. Rothstein. 1993. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J. Exp. Med. 177:857–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Binder, C.J., and G.J. Silverman. 2005. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin. Immunopathol. 26:385–404. [DOI] [PubMed] [Google Scholar]
- 55.Briles, D.E., C. Forman, S. Hudak, and J.L. Claflin. 1982. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 156:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Desaymard, C., A.M. Giusti, and M.D. Scharff. 1984. Rat anti-T15 monoclonal antibodies with specificity for VH- and VH-VL epitopes. Mol. Immunol. 21:961–967. [DOI] [PubMed] [Google Scholar]
- 57.Deenen, G.J., and F.G. Kroese. 1993. Kinetics of peritoneal B-1a cells (CD5 B cells) in young adult mice. Eur. J. Immunol. 23:12–16. [DOI] [PubMed] [Google Scholar]
- 58.Barrington, R., M. Zhang, M. Fischer, and M.C. Carroll. 2001. The role of complement in inflammation and adaptive immunity. Immunol. Rev. 180:5–15. [DOI] [PubMed] [Google Scholar]
- 59.Ochsenbein, A.F., T. Fehr, C. Lutz, M. Suter, F. Brombacher, H. Hengartner, and R.M. Zinkernagel. 1999. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 286:2156–2159. [DOI] [PubMed] [Google Scholar]
- 60.Taylor, H.P., and N.J. Dimmock. 1985. Mechanisms of neutralization of influenza virus by IgM. J. Gen. Virol. 66:903–907. [DOI] [PubMed] [Google Scholar]
- 61.Nera, K.P., P. Kohonen, E. Narvi, A. Peippo, L. Mustonen, P. Terho, K. Koskela, J.M. Buerstedde, and O. Lassila. 2006. Loss of Pax5 promotes plasma cell differentiation. Immunity. 24:283–293. [DOI] [PubMed] [Google Scholar]
- 62.Delogu, A., A. Schebesta, Q. Sun, K. Aschenbrenner, T. Perlot, and M. Busslinger. 2006. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 24:269–281. [DOI] [PubMed] [Google Scholar]
- 63.Iwakoshi, N.N., A.H. Lee, P. Vallabhajosyula, K.L. Otipoby, K. Rajewsky, and L.H. Glimcher. 2003. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat. Immunol. 4:321–329. [DOI] [PubMed] [Google Scholar]
- 64.Abrahao, T.B., E. Freymuller, R.A. Mortara, J.D. Lopes, and M. Mariano. 2003. Morphological characterization of mouse B-1 cells. Immunobiology. 208:401–411. [DOI] [PubMed] [Google Scholar]
- 65.Boes, M., C. Esau, M.B. Fischer, T. Schmidt, M. Carroll, and J. Chen. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776–4787. [PubMed] [Google Scholar]
- 66.Ehrenstein, M.R., T.L. O'Keefe, S.L. Davies, and M.S. Neuberger. 1998. Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc. Natl. Acad. Sci. USA. 95:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawahara, T., H. Ohdan, G. Zhao, Y.G. Yang, and M. Sykes. 2003. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol. 171:5406–5414. [DOI] [PubMed] [Google Scholar]
- 68.Ohdan, H., K.G. Swenson, H.S. Kruger Gray, Y.G. Yang, Y. Xu, A.D. Thall, and M. Sykes. 2000. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J. Immunol. 165:5518–5529. [DOI] [PubMed] [Google Scholar]
- 69.Deenen, G.J., and F.G. Kroese. 1992. Murine peritoneal Ly-1 B cells do not turn over rapidly. Ann. NY Acad. Sci. 651:70–71. [DOI] [PubMed] [Google Scholar]
- 70.Kretschmer, K., J. Stopkowicz, S. Scheffer, T.F. Greten, and S. Weiss. 2004. Maintenance of peritoneal B-1a lymphocytes in the absence of the spleen. J. Immunol. 173:197–204. [DOI] [PubMed] [Google Scholar]
- 71.Ren, B., K.J. Chee, T.H. Kim, and T. Maniatis. 1999. PRDI-BF1/Blimp-1 repression is mediated by corepressors of the Groucho family of proteins. Genes Dev. 13:125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu, J., C. Angelin-Duclos, J. Greenwood, J. Liao, and K. Calame. 2000. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol. Cell. Biol. 20:2592–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gyory, I., J. Wu, G. Fejer, E. Seto, and K.L. Wright. 2004. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat. Immunol. 5:299–308. [DOI] [PubMed] [Google Scholar]
- 74.Ancelin, K., U.C. Lange, P. Hajkova, R. Schneider, A.J. Bannister, T. Kouzarides, and M.A. Surani. 2006. Blimp1 associates with Prmt5 and directs histone arginine methylation in mouse germ cells. Nat. Cell Biol. 8:623–630. [DOI] [PubMed] [Google Scholar]
- 75.Tarte, K., F. Zhan, J. De Vos, B. Klein, and J. Shaughnessy Jr. 2003. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. 102:592–600. [DOI] [PubMed] [Google Scholar]
- 76.Hasbold, J., L.M. Corcoran, D.M. Tarlinton, S.G. Tangye, and P.D. Hodgkin. 2004. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat. Immunol. 5:55–63. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg, B.J., P.A. Smathers, K. Frederiksen, and A.D. Steinberg. 1982. Ability of the xid gene to prevent autoimmunity in (NZB X NZW)F1 mice during the course of their natural history, after polyclonal stimulation, or following immunization with DNA. J. Clin. Invest. 70:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dauphinee, M., Z. Tovar, and N. Talal. 1988. B cells expressing CD5 are increased in Sjogren's syndrome. Arthritis Rheum. 31:642–647. [DOI] [PubMed] [Google Scholar]
- 79.Youinou, P., L. Mackenzie, P. Katsikis, G. Merdrignac, D.A. Isenberg, N. Tuaillon, A. Lamour, P. Le Goff, J. Jouquan, A. Drogou, et al. 1990. The relationship between CD5-expressing B lymphocytes and serologic abnormalities in rheumatoid arthritis patients and their relatives. Arthritis Rheum. 33:339–348. [DOI] [PubMed] [Google Scholar]
- 80.Tobita, K., A. Sugiura, C. Enomote, and M. Furuyama. 1975. Plaque assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med. Microbiol. Immunol. (Berl.). 162:9–14. [DOI] [PubMed] [Google Scholar]
- 81.Angelin-Duclos, C., K. Johnson, J. Liao, K.I. Lin, and K. Calame. 2002. An interfering form of Blimp-1 increases IgM secreting plasma cells and blocks maturation of peripheral B cells. Eur. J. Immunol. 32:3765–3775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.