Abstract
Proper activation of nuclear factor (NF)–κB transcription factors is critical in regulating fundamental biological processes such as cell survival and proliferation, as well as in inflammatory and immune responses. Recently, the NF-κB signaling pathways have been categorized into the canonical pathway, which results in the nuclear translocation of NF-κB complexes containing p50, and the noncanonical pathway, which involves the induced processing of p100 to p52 and the formation of NF-κB complexes containing p52 (Bonizzi, G., and M. Karin. 2004. Trends Immunol. 25:280–288). We demonstrate that loss of tumor necrosis factor (TNF) receptor–associated factor 3 (TRAF3) results in constitutive noncanonical NF-κB activity. Importantly, TRAF3−/− B cells show ligand-independent up-regulation of intracellular adhesion molecule 1 and protection from spontaneous apoptosis during in vitro culture. In addition, we demonstrate that loss of TRAF3 results in profound accumulation of NF-κB–inducing kinase in TRAF3−/− cells. Finally, we show that the early postnatal lethality observed in TRAF3-deficient mice is rescued by compound loss of the noncanonical NF-κB p100 gene. Thus, these genetic data clearly demonstrate that TRAF3 is a critical negative modulator of the noncanonical NF-κB pathway and that constitutive activation of the noncanonical NF-κB pathway causes the lethal phenotype of TRAF3-deficient mice.
The Rel/NF-κB family of transcription factors comprise critical regulators of inflammation, proliferation, and apoptosis and, collectively, lie at the center of both innate and adaptive immune responses (1, 2). Recent efforts have identified two separate NF-κB signaling pathways that lead to the activation of specific and distinct Rel dimers. In unstimulated cells, NF-κB dimers are sequestered in the cytoplasm by one of a family of inhibitory molecules, termed inhibitors of κB (IκBs) (3). The canonical pathway requires activation of the trimeric IκB kinase (IKK) complex (IKKα, IKKβ, and IKKγ/ NF-κB essential modulator), which mediates phosphorylation and degradation of IκBα and IκBβ and the release of p50:RelA and p50:cRel dimers (3). The noncanonical or “alternative” pathway requires activation of NF-κB–inducing kinase (NIK) (4), which, in association with IKKα, binds to the C terminus of p100 (also termed IκBδ), leading to p100 processing to p52 and the preferential release of p52:RelB dimers (5, 6). Although genetic studies have revealed overlapping contributions of Rel family members in inflammation, proliferation, and cell survival, they have also identified distinct functions for individual family members with the alternative NF-κB pathway components being particularly important for secondary lymphoid tissue development and propagation of adaptive immune responses (7, 8). Importantly, excessive activity of either NF-κB activation pathway contributes to an array of human pathologies, including cancer and inflammatory and autoimmune diseases (9–11).
Members of the TNF receptor (TNFR) superfamily play vital roles in inflammatory responses, lymphoid tissue development, and orchestration of adaptive immune responses via activation of both the canonical and noncanonical NF-κB signaling pathways (1). The diverse biological effects of TNFR family members are mediated, in part, via recruitment of one or more of a small family of cytoplasmic adaptor proteins called TNFR-associated factors (TRAFs) (12). TRAF proteins share a common C terminus, termed the TRAF-domain, which mediates TRAF homo- and heterotrimerization as well as interaction with the receptor and downstream signaling molecules. The N terminus of all known TRAFs (except TRAF1) contains several zinc-binding motifs that are believed to specify pathway activation potential. Although overexpression and genetic experiments have demonstrated the critical roles of TRAF2, -5, and -6 in activation of canonical NF-κB signaling, the mechanism by which TNFRs activate the noncanonical pathway has remained elusive. Interestingly, the TNFR family members, lymphotoxin-β receptor (LTβR), B cell–activation factor receptor (BAFF-R), and CD40, all of which activate the noncanonical NF-κB pathway (5, 13, 14), also share the capacity to recruit the enigmatic adaptor molecule TRAF3 (15–17). Recent studies have suggested that TRAF3 functions as a negative regulator of noncanonical NF-κB activation (18, 19). Clear genetic data defining the function of TRAF3, however, has been lacking because of the early postnatal lethality associated with loss of TRAF3 signaling (20).
In this report, we demonstrate the essential role of TRAF3 in the negative regulation of noncanonical NF-κB activation as TRAF3 deficiency leads to constitutive p100 processing caused by high NIK levels. The critical role of TRAF3 in suppression of noncanonical NF-κB activity is further illustrated by our finding that constitutive p100 processing is responsible for the lethal phenotype associated with TRAF3-null mice.
RESULTS AND DISCUSSION
Loss of TRAF3 results in constitutive processing of p100
Analysis of LTβR-mediated signal transduction in mouse embryonic fibroblasts (MEFs) has provided a powerful system for the characterization of signaling components required for noncanonical NF-κB activation (5). To gain insight into TRAF3's role in the activation of the noncanonical NF-κB pathway, WT and TRAF3 −/− MEFs were treated with agonistic anti-LTβR antibody, and processing of the p100 precursor to p52, the hallmark of noncanonical NF-κB activation, was assessed by immunoblot. Although WT MEFs exhibited the normal kinetics of p100 processing with substantial p52 accumulation by 12 h after treatment, TRAF3 −/− MEFs displayed constitutive and total processing of the p100 precursor protein without stimulation (Fig. 1 A). To rule out the possibility that this observation was a developmental manifestation of TRAF3 deficiency rather than a direct effect of TRAF3 on the noncanonical NF-κB pathway, WT TRAF3 was virally reconstituted into TRAF3 −/− MEFs, and processing of p100 to p52 was again assessed by immunoblot. As seen in Fig. 1 B, reconstitution of WT TRAF3 restored WT levels of p100 and p52 in TRAF3 −/− MEFs, indicating that the constitutive processing of p100 to p52 is caused by the loss of the TRAF3 gene.
Figure 1.
TRAF3 deficiency results in constitutive processing of p100 in MEFs. (A) WT and TRAF3 −/− MEFs were stimulated with an anti-LTβR antibody for the indicated times. Processing of p100 to p52 in whole cell extract was detected by immunoblot. Total β actin is shown as a loading control. (B) Whole cell extracts from WT and TRAF3 −/− MEFs reconstituted with either pBABEpuro-TAP or pBABEpuro-TAP-TRAF3 were assessed for basal p100 processing to p52 by immunoblot. Reconstitution of TRAF3 expression in TRAF3 −/− MEFs was confirmed by immunoblot. Total β actin is shown as a loading control. *, endogenous TRAF3.
TRAF3 −/− B cells display multiple ligand-independent activation states
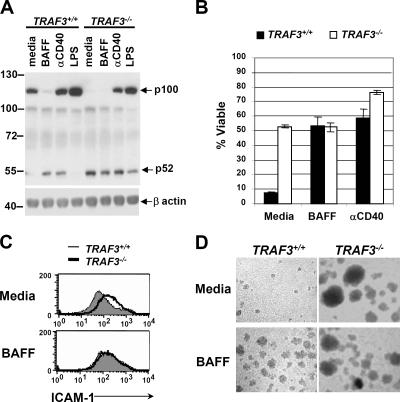
In addition to the central role of the noncanonical NF-κB pathway in LTβR orchestration of secondary lymphoid tissues, noncanonical NF-κB activity also provides vital cues for B cells in several processes, including B cell development, peripheral maintenance, and antibody production (21, 22). Therefore, to better understand the function of TRAF3 in the inhibition of p100 processing, we examined cellular responses between WT and TRAF3 −/− B cells. To obtain TRAF3 −/− B cells, lethally irradiated C57BL/6 mice were reconstituted with fetal liver cells derived from WT or TRAF3 −/− embryos. 8 wk after reconstitution, spleens from reconstituted mice were harvested for B cell purification and analysis (the fidelity of reconstitution and B cell purification was monitored by tracking the donor allele, Ly9.1; Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20061166/DC1).Initially, to determine whether TRAF3 was also required for suppression of p100 processing in B lymphocytes, WT and TRAF3 −/− B cells were stimulated with agonistic anti-CD40 antibody, BAFF, or LPS, and the processing of p100 to p52 was assessed by immunoblot. As expected, ligation of either CD40 or BAFF-R, but not of TLR4, resulted in the processing of p100 to p52 in WT B cells (Fig. 2 A). In TRAF3 −/− B cells, however, generation of the p52 product occurred without stimulation (Fig. 2 A), again clearly defining TRAF3 as a negative regulator of noncanonical NF-κB activation. It is also noteworthy that although CD40 and LPS stimulation of TRAF3 −/− B cells resulted in substantial induction of p100 protein, no further accumulation of p52 was observed in comparison with unstimulated TRAF3 −/− cells. Similarly, BAFF- and CD40-stimulated WT cells exhibit similar levels of p52 production even though ligation of CD40 maintains high levels of p100, whereas ligation of BAFF-R does not. This suggests that loss of TRAF3 results in the maximal rate of p100 processing.
Figure 2.
TRAF3-deficient B lymphocytes display ligand-independent activation. (A) WT and TRAF3 −/− B cells were stimulated with BAFF, anti-CD40 antibody, or LPS for 24 h, and processing of p100 to p52 was detected by immunoblot. β actin is shown as a loading control. (B) WT and TRAF3 −/− B cells were stimulated with BAFF or anti-CD40 antibody for 96 h, and cell death was measured by staining with propidium iodide. Error bars indicate ±1 SD between duplicate samples. WT and TRAF3 −/− B cells were stimulated with BAFF for 48 h, and (C) analysis of ICAM-1 protein expression was determined by FACS and (D) images of cells forming homotypic aggregates were taken (see In vitro B cell assays for details).
To further assess the biological significance of TRAF3 deficiency, WT and TRAF3 −/− B cells were cultured for 4 d in media alone or in the presence of either BAFF or agonistic anti-CD40 antibody, and viability was determined by propidium iodide exclusion. Cultured B cells undergo spontaneous apoptosis unless provided with an appropriate survival signal, including such factors as CD40L or BAFF, the latter of which provides a vital survival signal for the maintenance of peripheral B cells in vivo (21). As shown in Fig. 2 B, TRAF3 −/− B cells showed cell survival in the media control comparable with that seen in BAFF-treated WT cells. In addition to ligand-independent survival, TRAF3 −/− B cells displayed high basal levels of intracellular adhesion molecule 1 (ICAM-1) expression, similar to that seen in BAFF-stimulated WT cells (Fig. 2 C), as well as the spontaneous formation of homotypic B cell aggregates (Fig. 2 D), a phenomenon that requires ICAM-1–LFA1 interaction (23). Previous experiments indicated that certain B cell functions such as homotypic aggregation require both the canonical and noncanonical NF-κB activity (24). Therefore, the phenotypes of TRAF3 −/− B cells described in this report may not be a sole consequence of constitutive noncanonical NF-κB activity. Although BAFF-R signaling is important for proper maintenance of B cell populations, excessive BAFF signaling also contributes to deleterious B cell responses by rescuing self-reactive B cells from peripheral deletion and promoting their entry into marginal zone niches, thereby increasing the opportunity for polyclonal stimulation and enhanced autoantibody production (25). Consequently, it will be interesting to determine if mice carrying TRAF3 −/− B cells develop autoimmune phenotypes.
Loss of TRAF3 results in profound accumulation of NIK
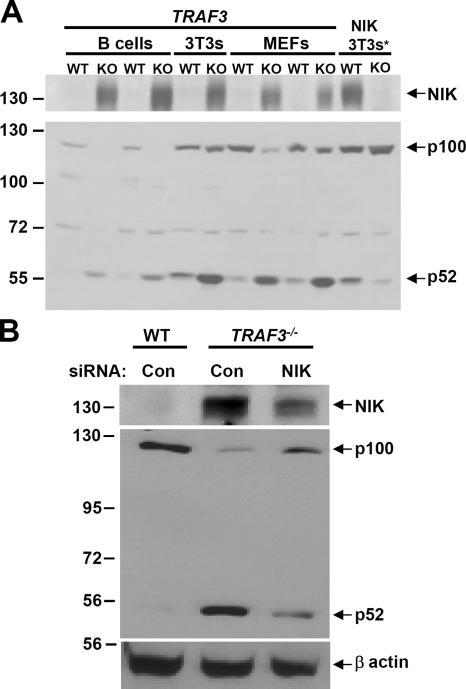
A recent study examining the regulation of NIK protein levels demonstrated that inhibition of TRAF3 resulted in the marked accumulation of NIK, which suggests that TRAF3 negatively regulates NIK stability (18). To test whether or not TRAF3 is required for suppression of NIK protein levels, immunoblot analysis of NIK was performed on multiple TRAF3-deficient cell types, including immortalized B cells, 3T3s, and MEFs. As shown in Fig. 3 A, in which NIK was undetectable in WT cells, profound accumulation of NIK was observed in all cells lacking TRAF3, which correlated well with increased processing of p100 to p52 (Fig. 3 A, bottom). Importantly, several groups have previously reported the difficulty of detecting endogenous NIK (18, 26). To make certain this band was indeed NIK, NIK +/+ and NIK −/− 3T3s were treated with the proteosome inhibitor MG132 for 2 h to serve as a control (Fig. 3 A, right lanes). To verify that NIK was responsible for the constitutive processing of p100 observed in TRAF3 −/− cells, we performed siRNA-mediated knockdown of NIK in TRAF3 −/− MEFs. As shown in Fig. 3 B, TRAF3 −/− MEFs treated with siRNA against NIK, but not with a scrambled control sequence, showed considerable reduction in NIK and p52 protein levels. Collectively, these findings indicate that TRAF3 indeed regulates activation of the noncanonical NF-κB pathway through inhibition of NIK protein levels.
Figure 3.
Enhanced NIK expression levels in TRAF3-deficient cells. (A) 150 μg of whole cell extracts from v-ABL–transformed WT and TRAF3 −/− B cells, 3T3s, and primary MEFs were assessed by immunoblotting for NIK. p100 processing to p52 in these cells was confirmed by immunoblot. *, NIK +/+ and NIK −/− 3T3s were treated with MG132 for 2 h to serve as a control. (B) WT and TRAF3 −/− MEFs were transfected with 150 nM control or NIK-siRNA. Whole cell extracts were obtained 48 h after transfection, and NIK and p100/p52 levels were detected by immunoblot. β actin is shown as a loading control.
At the present, it remains unclear how TRAF3 affects NIK protein stability. Quantitative PCR analysis of NIK mRNA levels in MEFs and B cells demonstrated that loss of TRAF3 does not affect transcription of NIK, which agrees with a published model that TRAF3 regulates NIK post-transcriptionally (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20061166/DC1) (18). TRAF3 contains a zinc RING finger motif characteristic of molecules with ubiquitin ligase activity. In the Liao et al. study, the authors showed that direct interaction between NIK and TRAF3 was required for the suppressive function of TRAF3 toward NIK, suggesting that TRAF3 may lead to the ubiquitination and degradation of NIK (18). In support of this, reconstitution of TRAF3 −/− MEFs with a TRAF3 RING finger point mutant showed defect in suppression of NIK protein levels (unpublished data). However, we and other groups have been unable to see TRAF3-mediated ubiquitination of NIK in an overexpression assay (18). Moreover, TRAF proteins have only been shown to mediate lysine 63 ubiquitin linkages, which are not associated with protein degradation (27, 28) but rather the promotion of positive signaling complexes. To add to the complexity of noncanonical NF-κB activation, Grech et al. recently reported that loss of TRAF2 also results in constitutive activation of the noncanonical NF-κB pathway (29). As such, in-depth studies are required to determine how TRAF2 and TRAF3 are involved in controlling the levels of NIK before or after receiving signals from upstream receptors. Lessons learned from the characterization of the role of TRAF6-dependent ubiquitination in activation of the canonical NF-κB pathway suggest that additional players remain to be identified to complete our understanding of TRAF-dependent suppression of NIK (28).
The TRAF3-null phenotype results from constitutive processing of p100
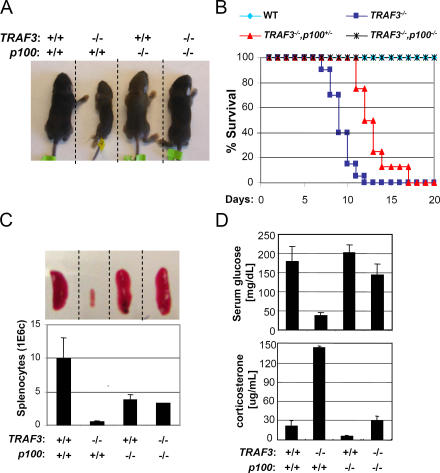
A previous study showed that constitutive activation of p52 in mice lacking the C terminus of p100 (the IκBδ portion) leads to early lethality 3–5 wk after birth (30). Interestingly, TRAF3-deficient mice cannot survive beyond 2 wk of life (20). Therefore, we hypothesized that constitutive noncanonical NF-κB activity may have contributed to the TRAF3-null phenotype. To investigate this possibility, we crossed TRAF3 +/−/p100 −/− mice in an attempt to generate double knockout animals. We found that loss of p100 rescued TRAF3 −/− mice from postnatal lethality. Although TRAF3 −/− mice die by postnatal day 12, TRAF3 −/−/p100 −/− mice grow at normal rates into adulthood (PCR and Western blot analyses were performed to confirm the TRAF3 −/−/p100 −/− genotype; Fig. 4, A and B; and not depicted). Interestingly, TRAF3 −/− mice with one disrupted copy of the p100 gene could survive as long as 18 d (Fig. 4 B). Several abnormalities had previously been reported in TRAF3 −/− mice, including drastically reduced spleen size and lymphocyte count, greatly reduced serum glucose levels, and elevated serum corticosterone (20). As shown in Fig. 4 (C and D), all of these TRAF3 −/− phenotypes are rescued with compound loss of the p100 gene. Collectively, these data demonstrate that constitutive activation of the noncanonical NF-κB pathway is an essential contributor to the TRAF3-null phenotype.
Figure 4.
Rescue of TRAF3-null phenotypes by combined deletion of the p100 gene. The rescue of the TRAF3-null phenotype was examined by body size (A), survival (B), spleen size and total splenocyte count (C; error bars indicate ±1 SD between three mice), and serum glucose and corticosterone concentration (D; error bars indicate ±1 SD between two and five mice).
Efforts to define the physiological role of TRAF3 began more than 10 yr ago. Unlike other TRAF family members, overexpression of TRAF3 did not result in the activation of the NF-κB pathway. As TRAF3-null mice died soon after birth, identification of the function of TRAF3 had remained a mystery. In this report, we provide genetic data that TRAF3 functions as a critical negative regulator of the noncanonical NF-κB pathway through inhibition of NIK protein levels. With our improved understanding of the bifurcation of the NF-κB signaling pathways, we are now able to reassess the role of TRAF3 function in B lymphocytes. Our finding that TRAF3 −/− B cells receive a de facto BAFF signal suggests that TRAF3 plays an important role in the inhibition of inappropriate B cell activity and, therefore, may play a pivotal role in the suppression of B cell–mediated autoimmune disease. Most strikingly, we demonstrate that the lethal phenotype caused by TRAF3 deficiency results from constitutive processing of p100. Ultimately, the generation of a tissue-specific strategy in the targeted disruption of TRAF3 may be required to define the mechanism of the early postnatal lethality that comes with loss of TRAF3. It is also worth noting that mice lacking the C-terminal IκBδ portion of p100 (30), which has constitutively activated p52, survive as long as 5 wk, suggesting that the exacerbated phenotype of TRAF3 −/− mice may result from deregulation of additional pathways.
MATERIALS AND METHODS
Mice colony.
C57BL/6 (The Jackson Laboratory) mice aged 6–12 wk were used as recipients in fetal liver transplant experiments. Targeted disruption of the TRAF3 allele and the p100 allele has been described previously (20, 24). The TRAF3 mice colony is maintained by mating TRAF3+/− mice, which are in a mixed C57BL/6-129 background. All mice were maintained and bred under specific pathogen-free conditions in the University of California, Los Angeles Life Sciences mouse facility, and experiments were conducted within the parameters of our approved protocol by the Animal Research Committee.
Antibodies and reagents.
The anti-p100/p52 and anti-NIK antibodies were purchased from Cell Signaling Technology, the anti-TRAF3 (M-20) antibody was purchased from Santa Cruz Biotechnology, Inc., and the anti-LTβR antibody was purchased from Qbiogene. The anti–β actin antibody, propidium iodide, and LPS were obtained from Sigma-Aldrich.
Fetal liver reconstitution.
Fetal livers were isolated from E14.5–15.5 embryos in a mixed C57BL/6-129 background. After genotyping, TRAF3 +/+ and TRAF3 −/− fetal livers were disrupted by passing cells through an 18-gauge needle multiple times. After filtration with a 70-μM cell strainer, 106 cells were injected intravenously into irradiated C57BL/6 mice. 4–6 wk after reconstitution, cells from the blood of recipient mice were genotyped and analyzed by FACS for the presence of the Ly9.1 marker, which is expressed on the C57BL/6-129 donor cells but not on the C57BL/6 recipient cells.
B cell purification and transformation.
Splenocytes were harvested from fetal liver–reconstituted mice 6–8 wk after reconstitution. To obtain pure naive B cells, total splenocytes were stained with a biotin-conjugated anti-CD43 antibody (BD Biosciences), followed by streptavidin-conjugated magnetic microbeads (Miltenyi Biotec), and passed through a depletion-type magnetic sorting column (Miltenyi Biotec). Unbound cells were collected as the purified naive B cell sample and analyzed by FACS for Ly9.1, B220, and IgM markers. B cells from the reconstituted mice were retrovirally transformed using v-ABL.
Cell culture condition.
B cells were cultured in RPMI medium 1640 supplemented with 10% FBS, 50 μM β-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies) at 37°C under 10% CO2. MEFs isolated from E14.5–15.5 embryos were cultured in DMEM (Mediatech Inc.), supplemented with 5% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. TRAF3-null MEFs were infected with the Moloney murine leukemia virus ΨA-MLV, containing either the pBABEpuro-tandem affinity purification tag (TAP) or pBABEpuro-TAP-TRAF3 vector, and selected with 2.5 μg/ml puromycin.
In vitro B cell assays.
Purified splenic B cells were stimulated with 15 μg/ml anti-CD40 mAb (FgK-45), 5 μg/ml LPS, or 100 ng /ml human BAFF (Amgen Biologicals). For the B cell survival assay, 2 × 105 B cells were stimulated for 96 h in a flatbottom 96-well plate, and cell death was measured after staining with propidium iodide and analyzed using a flow cytometer (FACScan; Becton Dickinson). In the homotypic aggregation assay, cells were cultured in a flatbottom 96-well microtiter plate at 5 × 106 cells/ml for 48 h, and cell images were taken using a microscope (Eclipse TE300; Nikon) with a 10× objective.
Measurement of serum glucose and corticosterone levels.
Sera were isolated from 8-d-old mice. The glucose level was determined by applying serum to a one-touch strip and measured with a glucometer (Active; ACCU-CHEK). The corticosterone amount was determined by using the Corticosterone Immunoassay kit (R&D Systems).
Online supplemental material.
Fig. S1 shows FACScan flow cytometry analysis of B cell markers B220 and IgM and the C57BL/6-129 donor cell marker Ly9.1 on purified WT and TRAF3 −/− B cells from fetal liver–reconstituted mice. Fig. S2 shows the basal levels of NIK mRNA in WT and TRAF3 −/− MEFs and v-ABL–transformed B cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20061166/DC1.
Supplemental Material
Acknowledgments
We thank Leming Tong for his excellent technical assistance in obtaining serum from mice, Liming Pei for measuring the serum glucose level, and Shuling Guo for generating the transformed WT and TRAF3 −/− B cells.
J.Q. He is supported by a Clinical and Fundamental Immunology training grant (AI07126-30). B. Zarnegar is supported by a Warsaw Fellowship. G. Oganesyan and S.K. Saha are supported by a UCLA Medical Scientist Training Program training grant (GM 08042). G. Cheng is a Lymphoma and Leukemia Society scholar, and part of this work was supported by National Institutes of Health research grants (RO1 AI056154, RO1 CA87924, and R01 GM57559).
The authors declare that they have no competing financial interests.
J.Q. He and B. Zarnegar contributed equally to this work.
References
- 1.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280–288. [DOI] [PubMed] [Google Scholar]
- 2.Hayden, M.S., and S. Ghosh. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195–2224. [DOI] [PubMed] [Google Scholar]
- 3.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 4.Xiao, G., E.W. Harhaj, and S.C. Sun. 2001. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell. 7:401–409. [DOI] [PubMed] [Google Scholar]
- 5.Dejardin, E., N.M. Droin, M. Delhase, E. Haas, Y. Cao, C. Makris, Z.W. Li, M. Karin, C.F. Ware, and D.R. Green. 2002. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 17:525–535. [DOI] [PubMed] [Google Scholar]
- 6.Xiao, G., A. Fong, and S.C. Sun. 2004. Induction of p100 processing by NF-kappaB-inducing kinase involves docking IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated phosphorylation. J. Biol. Chem. 279:30099–30105. [DOI] [PubMed] [Google Scholar]
- 7.Caamano, J.H., C.A. Rizzo, S.K. Durham, D.S. Barton, C. Raventos-Suarez, C.M. Snapper, and R. Bravo. 1998. Nuclear factor (NF)–κB2 (p100/p52) is required for normal splenic microarchitecture and B cell–mediated immune responses. J. Exp. Med. 187:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yilmaz, Z.B., D.S. Weih, V. Sivakumar, and F. Weih. 2003. RelB is required for Peyer's patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J. 22:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fracchiolla, N.S., L. Lombardi, M. Salina, A. Migliazza, L. Baldini, E. Berti, L. Cro, E. Polli, A.T. Maiolo, and A. Neri. 1993. Structural alterations of the NF-kappa B transcription factor lyt-10 in lymphoid malignancies. Oncogene. 8:2839–2845. [PubMed] [Google Scholar]
- 10.Maeda, S., L.C. Hsu, H. Liu, L.A. Bankston, M. Iimura, M.F. Kagnoff, L. Eckmann, and M. Karin. 2005. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 307:734–738. [DOI] [PubMed] [Google Scholar]
- 11.Mackay, F., S.A. Woodcock, P. Lawton, C. Ambrose, M. Baetscher, P. Schneider, J. Tschopp, and J.L. Browning. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey, P.W., S.E. Doyle, J.Q. He, and G. Cheng. 2003. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 14:193–209. [DOI] [PubMed] [Google Scholar]
- 13.Claudio, E., K. Brown, S. Park, H. Wang, and U. Siebenlist. 2002. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 3:958–965. [DOI] [PubMed] [Google Scholar]
- 14.Coope, H.J., P.G. Atkinson, B. Huhse, M. Belich, J. Janzen, M.J. Holman, G.G. Klaus, L.H. Johnston, and S.C. Ley. 2002. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 21:5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu, H.M., K. O'Rourke, M.S. Boguski, and V.M. Dixit. 1994. A novel RING finger protein interacts with the cytoplasmic domain of CD40. J. Biol. Chem. 269:30069–30072. [PubMed] [Google Scholar]
- 16.VanArsdale, T.L., S.L. VanArsdale, W.R. Force, B.N. Walter, G. Mosialos, E. Kieff, J.C. Reed, and C.F. Ware. 1997. Lymphotoxin-beta receptor signaling complex: role of tumor necrosis factor receptor-associated factor 3 recruitment in cell death and activation of nuclear factor kappa B. Proc. Natl. Acad. Sci. USA. 94:2460–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, L.-G., and H.-B. Shu. 2002. TNFR-associated factor-3 is associated with BAFF-R and negatively regulates BAFF-R-mediated NF-kappa B activation and IL-10 production. J. Immunol. 169:6883–6889. [DOI] [PubMed] [Google Scholar]
- 18.Liao, G., M. Zhang, E.W. Harhaj, and S.C. Sun. 2004. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J. Biol. Chem. 279:26243–26250. [DOI] [PubMed] [Google Scholar]
- 19.Hauer, J., S. Puschner, P. Ramakrishnan, U. Simon, M. Bongers, C. Federle, and H. Engelmann. 2005. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc. Natl. Acad. Sci. USA. 102:2874–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu, Y., G. Cheng, and D. Baltimore. 1996. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 5:407–415. [DOI] [PubMed] [Google Scholar]
- 21.Schneider, P., and J. Tschopp. 2003. BAFF and the regulation of B cell survival. Immunol. Lett. 88:57–62. [DOI] [PubMed] [Google Scholar]
- 22.Vora, K.A., L.C. Wang, S.P. Rao, Z.Y. Liu, G.R. Majeau, A.H. Cutler, P.S. Hochman, M.L. Scott, and S.L. Kalled. 2003. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J. Immunol. 171:547–551. [DOI] [PubMed] [Google Scholar]
- 23.Barrett, T.B., G. Shu, and E.A. Clark. 1991. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J. Immunol. 146:1722–1729. [PubMed] [Google Scholar]
- 24.Zarnegar, B., J.Q. He, G. Oganesyan, A. Hoffmann, D. Baltimore, and G. Cheng. 2004. Unique CD40-mediated biological program in B cell activation requires both type 1 and type 2 NF-kappaB activation pathways. Proc. Natl. Acad. Sci. USA. 101:8108–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thien, M., T.G. Phan, S. Gardam, M. Amesbury, A. Basten, F. Mackay, and R. Brink. 2004. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 20:785–798. [DOI] [PubMed] [Google Scholar]
- 26.Qing, G., Z. Qu, and G. Xiao. 2005. Stabilization of basally translated NF-kappaB-inducing kinase (NIK) protein functions as a molecular switch of processing of NF-kappaB2 p100. J. Biol. Chem. 280:40578–40582. [DOI] [PubMed] [Google Scholar]
- 27.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z.J. Chen. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 103:351–361. [DOI] [PubMed] [Google Scholar]
- 28.Sun, L., L. Deng, C.K. Ea, Z.P. Xia, and Z.J. Chen. 2004. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 14:289–301. [DOI] [PubMed] [Google Scholar]
- 29.Grech, A.P., M. Amesbury, T. Chan, S. Gardam, A. Basten, and R. Brink. 2004. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 21:629–642. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa, H., D. Carrasco, E. Claudio, R.P. Ryseck, and R. Bravo. 1997. Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-κB2. J. Exp. Med. 186:999–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.