Abstract
The major histocompatibility complex (MHC) class Ib molecule H2-M3 primes the rapid expansion of CD8+ T cells by presenting N-formylated bacterial peptides. However, the significance of H2-M3–restricted T cells in host defense against bacteria is unclear. We generated H2-M3–deficient mice to investigate the role of H2-M3 in immunity against Listeria monocytogenes (LM), a model intracellular bacterial pathogen. H2-M3–deficient mice are impaired in early bacterial clearance during primary infection, with diminished LM-specific CD8+ T cell responses and compromised innate immune functions. Although H2-M3–restricted CD8+ T cells constitute a significant proportion of the anti-listerial CD8+ T cell repertoire, the kinetics and magnitude of MHC class Ia–restricted T cell responses are not altered in H2-M3–deficient mice. The fact that MHC class Ia–restricted responses cannot compensate for the H2-M3–mediated immunity suggests a nonredundant role of H2-M3 in the protective immunity against LM. Thus, the early H2-M3–restricted response temporally bridges the gap between innate and adaptive immune responses, subsequently affecting the function of both branches of the immune system.
The MHC contains a large number of class I genes, most of which belong to the MHC class Ib family and are distinguished from MHC class Ia molecules by their lack of polymorphism. The MHC class Ib molecules are thought to serve a more specialized function than MHC class Ia molecules, possibly through the presentation of structurally distinct, yet conserved, microbial antigens to T cells. H2-M3 is one of the best studied MHC class Ib molecules in the mouse. H2-M3 was originally shown to present a polymorphic N-formylated peptide, derived from the mitochondrially encoded ND1 protein, as a histocompatibility antigen to CD8+ T cells (1–3). Further studies have demonstrated that H2-M3 specifically binds N-formylated hydrophobic peptides, exhibiting 100–1,000-fold lower affinity for nonformylated peptides (4, 5). Like mitochondrial-encoded proteins, bacterial proteins commonly possess a formylated NH2 terminus. The unique binding specificity of H2-M3 makes it well suited to present bacterially derived NH2-terminal peptides to CTLs (6). Indeed, H2-M3–restricted CTL epitopes have been identified in both Listeria monocytogenes (LM) and Mycobacterium tuberculosis (7–10).
The supply of endogenous N-formylated mitochondrial peptides is limited, likely explaining the relatively low surface expression of H2-M3 as compared with MHC class Ia molecules (11). A significant pool of H2-M3 exists intracellularly and can be rapidly mobilized to the surface when provided with appropriate N-formylated peptides (11). This observation suggests that a rapid and effective display of bacterial peptides by H2-M3 may occur during bacteria infection. Despite low levels of surface expression and a limited repertoire of self-ligands, H2-M3 plays an active role in thymic selection (12, 13). A recent study showed that H2-M3–restricted CD8+ T cells could be efficiently positively selected by H2-M3–expressing hematopoietic cells, unlike thymic epithelial cell–mediated selection of MHC class Ia–restricted CD8+ T cells (14, 15). The disparate thymic selection may, in part, account for the distinct behavior of MHC class Ia– and class Ib–restricted T cells in response to infection.
LM is a Gram-positive facultative intracellular bacterial pathogen that causes disseminated infection in immune-compromised human hosts. Experimental listeriosis of mice has been instrumental in analyzing cellular immunity against intracellular bacteria. Investigations of this model have demonstrated that the immune response to LM proceeds in two stages: an early innate immune response that controls the initial bacterial burden (16); and then a later “sterilizing” adaptive immune response that involves antigen-specific T cells, particularly CD8+ T cells, which clear the infection and are responsible for a protective memory response (17). In addition to an MHC class Ia–restricted CD8+ T cell response, mice infected with LM also develop a robust H2-M3–restricted CD8+ T cell response. To date, three listerial peptides presented by H2-M3 have been identified, including Fr38 (fMIVIL; reference 9), LemA (fMIGWII; reference 8), and AttM (fMIVTLF; reference 7). One unique feature of the H2-M3–restricted CD8+ T cell response to LM is that it occurs earlier than the MHC class Ia–restricted response during primary infection (peaking at days 5–7 vs. days 7–9 after infection; references 18 and 19). However, upon secondary LM infection H2-M3–restricted T cells fail to undergo significant expansion while MHC class Ia–restricted T cells undergo very vigorous expansion (18–20). The distinct kinetics of H2-M3–restricted and MHC class Ia–restricted T cells during LM infection suggest that these two CD8+ T cell populations may fulfill nonoverlapping functions in host defense against intracellular bacteria. A protective role of H2-M3–restricted T cells in both primary and secondary LM infection has been implicated in studying the function of residual CD8+ T cells in mice lacking MHC class Ia molecules (Kb−/−Db−/−; references 19, 21, and 22). However, these experiments are complicated by the contribution of other MHC class Ib–restricted responses, such as the LM-specific Qa-1–restricted response (23–25).
In this study, we specifically investigated the role of H2-M3 in protective immunity against LM and examined the interrelationship between MHC class Ia– and class Ib–restricted T cells during LM infection using H2-M3–deficient (M3−/−) mice. We showed that H2-M3 is important for the protective anti-listerial response, as demonstrated by higher bacterial burdens in the liver and the spleen, as well as increased susceptibility to infection in M3−/− mice. Although naive M3−/− mice do not display an apparent defect in the CD8+ T cell compartment, we observed significantly reduced numbers of effector CD8+ T cells, concurrent with lower levels of LM-specific IFN-γ production and cytotoxicity in M3−/− mice during primary LM infection. These results suggest that H2-M3–restricted T cells comprise a substantial proportion of the anti-listerial CD8+ T cell population. In addition, lack of early activation of H2-M3–restricted T cells resulted in reduced functions of macrophages and NK cells. In contrast, H2-M3 deficiency has no significant effect on anti-listeria immunity during secondary infection. Interestingly, the kinetics and magnitude of LM-specific MHC class Ia–restricted T cell responses were not significantly altered when the H2-M3–restricted responses were absent. Our finding that MHC class Ia–restricted T cells were not able to completely recompense the CD8+ T cell compartment in M3−/− mice during LM infection suggests a unique role of H2-M3–restricted T cells in host defense against bacteria infection.
RESULTS
Generation and characterization of H2-M3–deficient (M3−/−) mice
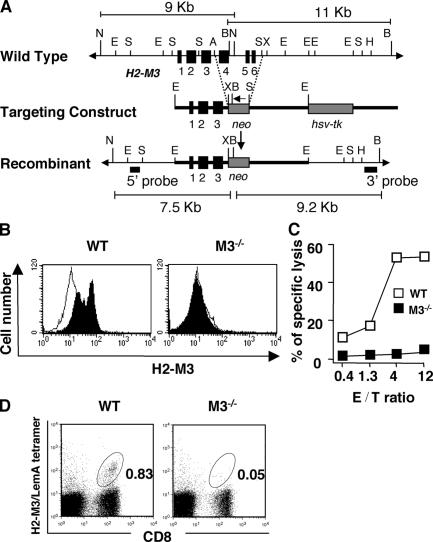
To assess the function of H2-M3, we generated M3−/− mice by a gene-targeting approach in which exons 4–6 of the H2-M3 gene were replaced by a neomycin (neo)-resistance gene (Fig. 1 A). Flow cytometric analysis and CTL assays were performed to demonstrate that mice homozygous for the H2-M3 mutation do not express H2-M3 on the cell surface. As shown in Fig. 1 B, surface expression of H2-M3 cannot be induced in splenocytes from M3−/− mice by incubating with H2-M3–restricted LemA peptide. Splenocytes from WT mice, but not from M3−/− mice, can be recognized by a mitochondrial ND1 peptide-specific, H2-M3–restricted CTL clone (Fig. 1 C). To confirm that H2-M3 deficiency affects its restricted CTL response, we infected WT and M3−/− mice with LM and measured reactivity with H2-M3/LemA tetramer at day 7 after infection. As shown in Fig. 1 D, a substantial population of tetramer+ CD8+ T cells was elicited in WT mice, but was not detectable in M3−/− mice. Thus, M3 protein and its restricted CD8+ T cells were completely absent in M3−/− mice.
Figure 1.
Generation and characterization of M3−/− mice. (A) Genomic configuration of the H2-M3 locus before (top) and after (bottom) homologous recombination with the targeting construct (middle). Exons (black boxes), lengths of diagnostic restriction fragments, and the probes used for Southern analysis are shown. An arrow indicates the transcription orientation of the neo gene. B, BglII; N, NheI; E, EcoRI; S, SacI; A, ApaI; H, HindIII; X, XbaI. (B) Splenocytes from WT and M3−/− mice were incubated overnight with (filled histogram) or without (open histogram) 10 μM LemA peptide. Cells were stained with an anti–H2-M3 mAb 130, followed by FITC-anti–hamster IgG. (C) H2-M3–specific · CTL clone 4E3 was incubated with LPS blasts from WT and M3−/− during a 51Cr release assay. The effector–target ratio is indicated in the figure. (D) Identification of LM-specific H2-M3–restricted T cells after i.v. infection with LM. Peripheral blood lymphocytes were isolated from LM-infected M3−/− mice (N6) or WT controls on day 7 after infection. Cells were stained with FITC–anti-CD8α and PE-H2-M3/LemA-tetramer. CD8+ tetramer+ T cells are highlighted by the gate, and the proportion of gated cells is indicated.
Effect of H2-M3 on T cell development
To determine the effect of H2-M3 deficiency on T cell development, we performed flow cytometric analysis on lymphocytes isolated from the thymus, spleen, liver, bone marrow, and lymph nodes of M3−/− and control mice. We found no significant differences in the number and proportions of CD4+, CD8+, CD4−CD8−, CD4+CD8+, αβ, and γδ T cells between the two groups (not depicted). It has been shown that the residual CD8+ T cells in Kb−/−Db−/− mice are enriched for cells expressing activation markers, suggesting that MHC class Ib molecule(s) may be critical in the development of this unique subset of CD8+ T cells (19). We compared CD8+ cells from M3−/− and WT mice for the expression of various activation markers, including CD44, CD69, CD122, and Ly6C. Although there is a trend toward the frequencies of CD8+CD44hi T cells from the spleen and mesenteric lymph nodes being slightly decreased in M3−/− mice, this difference is not statistically significant with the sample size analyzed (see Table S1, available at http://www.jem.org/cgi/content/full/jem.20051866/DC1). Our data suggests that H2-M3–restricted T cells may represent only a small fraction of the total CD8+ T cell population in naive animals.
H2-M3 deficiency increases susceptibility to Listeria infection
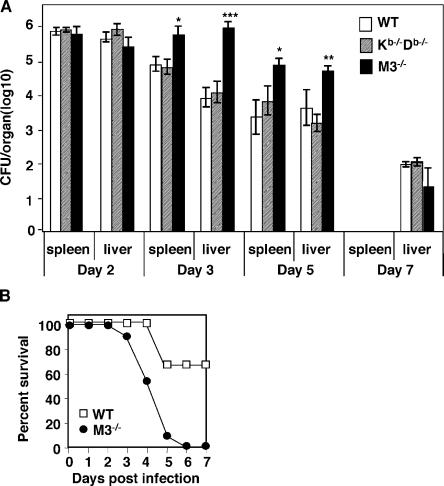
H2-M3–restricted T cells show a peak immune response that precedes their MHC class Ia–restricted counterparts after LM infection (18, 19). To investigate the relative significance of H2-M3–restricted and MHC class Ia–restricted CD8+ T cell responses in protective immunity against LM, we infected M3−/−, Kb−/−Db−/−, and WT mice with a sublethal dose of LM (5 × 103 CFU) and measured bacterial burden at various time points after infection. Compared with WT mice, M3−/− mice showed significantly higher bacterial burdens (∼10–100-fold) in both the spleen and liver at early time points (days 3 and 5) after infection (Fig. 2 A). In contrast, Kb−/−Db−/− and WT mice clear LM from the spleen and liver with similar kinetics, consistent with the previous report (22). By day 7 after infection, LM was nearly absent from all three strains of mice. This data suggests that H2-M3–restricted responses may play an important and even more dominant role than MHC class Ia–restricted responses in protective immunity against LM during the early phase of infection. In agreement with this notion, M3−/− mice were more susceptible to a higher dose (5 × 104 CFU) of LM infection than WT controls (Fig. 2 B). Thus, H2-M3–restricted responses contribute significantly to host defense against LM infection.
Figure 2.
M3−/− mice exhibit increased susceptibility to LM infection compared with WT and Kb−/−Db−/− mice. (A) Increased bacterial burdens in the spleens and livers of LM-infected M3−/− mice. M3−/−, Kb−/−Db−/−, and WT mice were infected i.v. with 5 × 103 CFU of LM. Bacterial burdens in the spleens and livers were determined at indicated time points after infection. Data are presented as the mean ± SEM from six mice per group per time point. Statistically significant differences between WT and M3−/− mice (N6) are indicated. At day 7 of infection, the bacterial burdens in the spleens of LM-infected mice were below the detection limit. (B) Enhanced susceptibility of M3−/− mice to LM. M3−/− mice (N10) and WT controls were infected with LM at the dose of 5 × 104 CFU. The survival of the mice was monitored daily up to 7 d after infection. Results are from 11 mice per group.
The frequency of activated CD8+ T cells is reduced in LM-infected M3−/− mice
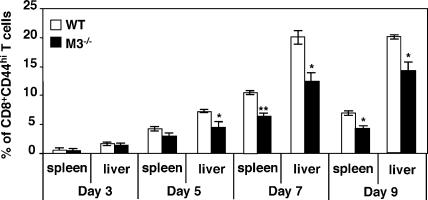
To determine whether H2-M3–restricted CD8+ T cells constitute a significant proportion of the anti-listerial CD8+ T cell repertoire, we compared the CD8+ population in M3−/− to WT mice during the course of LM infection. LM infection leads to expansion of LM-specific effector CD8+ T cells, which reach a peak number around day 7. On day 5 after LM infection, the frequency (Fig. 3) and total number (not depicted) of CD8+CD44+ T cells were slightly reduced in M3−/− mice. On day 7 after infection, the percentage of CD8+CD44+ T cells in M3−/− mice was decreased by nearly 50% compared with WT controls. The effect of H2-M3 deficiency is specific for CD8+ T cells, as no significant differences in the CD4+ population can be detected between LM-infected M3−/− and control mice (not depicted).
Figure 3.
Decreased frequency of effector CD8+ T cells in M3−/− mice during LM infection. Splenocytes and hepatic leukocytes were harvested from LM-infected M3−/− and M3+ mice (N10) on the indicated days and stained with mAbs specific for CD8α, CD4, and CD44. Percentages of CD44high CD8+ T cells were determined by flow cytometric analysis. Data shown are the mean ± SEM of three to eight animals per interval.
The effector functions of CD8+ T cells are diminished in LM-infected M3−/− mice
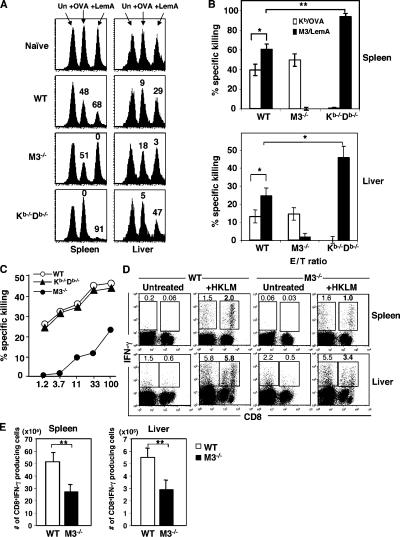
To investigate the mechanisms by which H2-M3–restricted T cells contribute to anti-listerial immunity, we compared the antigen-specific cytotoxic response and IFN-γ production between M3−/− and WT mice. For comparison purposes, we also included Kb−/−Db−/− mice in our analysis. First, an in vivo cytotoxicity assay was used to assess the cytotoxic potential of H2-M3–restricted T cells during the early phase (day 3) of LM infection, the time point when the difference in bacterial clearance was already apparent. Because MHC class Ia–restricted listerial peptides are not well defined in the H-2b background, we infected mice with a recombinant LM strain expressing OVA protein (rLM-ova) to compare H2-M3– and Kb-restricted CTL responses simultaneously. On day 3 after infection, mice were injected with B6 splenocytes pulsed with either H2-M3–restricted LemA peptide or Kb-restricted OVA peptide, and the clearance of these target cells was evaluated 15 h later by flow cytometry. H2-M3/LemA-specific and Kb/OVA-specific cytotoxic responses are readily detectable in WT mice (Fig. 4, A and B). However, the cytotoxic response to LemA-coated targets was significantly higher than that to OVA-bearing targets both in the spleens and livers, suggesting that H2-M3/LemA-specific CTLs may be more dominant than Kb/OVA-specific CTLs in WT mice at this time point (Fig. 4, A and B). In M3−/− mice, no H2-M3/LemA-specific response was detected, confirming the specificity of the assay. The cytolytic activity to OVA-coated targets was comparable between M3−/− mice and WT mice, suggesting that the generation/expansion of Kb-restricted CTLs was not affected in the absence of H2-M3–restricted responses. On the contrary, the H2-M3/LemA-specific cytotoxic response was significantly enhanced in Kb−/−Db−/− mice compared with WT mice (Fig. 4, A and B). Thus, the overall CTL activity is reduced in M3−/− mice during the early phase of LM infection due to the lack of a compensatory increase of MHC class Ia–restricted CTL responses while remaining largely unchanged in Kb−/−Db−/− mice due to augmented H2-M3–restricted responses.
Figure 4.
Impaired LM-specific cytolytic activity and the IFN-γ production in M3−/− mice. (A) LM infection elicited a potent H2-M3–restricted CTL response during the early phase of infection. M3−/−, Kb−/−Db−/−, and WT mice were infected with rLM-ova. Fluorescence-labeled peptide-pulsed or unpulsed B6 splenocytes were coinjected i.v. into day 3 LM-infected or naive mice. In vivo cytolysis was assessed 15 h later by flow cytometry. Histograms are gated on PKH26+ target cells and the numbers above each peak represent the percent of specific killing. The data are representative of eight mice per group from two independent experiments. (B) The bar graph indicates the percentage of specific lysis for OVA- and LemA-coated targets from the indicated group of mice. Data shown are the mean ± SEM of eight animals per group. (C) Diminished LM-specific cytolytic activity in M3−/− mice. 5 d after LM infection, splenocytes from indicated mice were activated with Con A and examined for CTL activity to 51Cr-labeld LM-infected J774-Kb target cells. Each data line represents the mean from two mice. Data are representative of results obtain from three independent experiments. Specific lysis for uninfected J774-Kb targets was below 5% from all effectors tested. (D and E) Decreased LM-specific, IFN-γ–producing CD8+ T cells in M3−/− mice. Splenocytes and hepatic leukocytes from indicated mice were harvested on day 7 after LM infection and stimulated overnight with HKLM. Cells were stained for CD8 expression and intracellular IFN-γ, and analyzed by flow cytometry. The percentages of CD8+IFN-γ+ and CD8−IFN-γ+ cells are indicated (D). Results are representative from eight mice per group. Bar graph depicts mean ± SEM for total number of LM-specific CD8+ cells in the spleens and livers of M3−/− and WT mice (E). M3−/− and WT controls used in these experiments are from N10 backcross.
The use of in vivo cytotoxicity assays allowed us to detect relatively small numbers of LM-specific CTLs during early LM infection. However, the analysis was limited to a single immunodominant epitope for Kb and H2-M3. To further investigate the contribution of H2-M3–restricted T cells to LM-specific cytolytic response, we performed in vitro CTL assays using LM-infected J774-Kb transfectants as targets, which allowed us to measure overall LM-specific, H2-M3– and Kb-restricted CTL responses. Splenocytes from M3−/−, Kb−/−Db−/−, or WT mice infected 5 d previously were isolated and stimulated with Con A for 3 d and then used as effectors in a 51Cr release assay. As shown in Fig. 4 C, effectors derived from WT and Kb−/−Db−/− mice lysed LM-infected J744-Kb targets to a similar extent, whereas effectors derived from M3−/− mice displayed much lower lytic activity.
To determine whether H2-M3 deficiency affects the IFN-γ production by CD8+ T cells, intracellular IFN-γ staining was performed. Splenocytes and hepatic leukocytes harvested from M3−/− or WT mice 7 d after infection were stimulated in vitro with heat-killed LM (HKLM). We found that the frequency and total numbers of LM-specific IFN-γ–producing CD8+ T cells were significantly reduced in the spleens and livers of M3−/− mice compared with WT mice (Fig. 4, D and E). Collectively, these data demonstrate that cytolytic activity and IFN-γ production by CD8+ T cells are impaired in M3−/− mice during primary LM infection.
H2-M3–deficient mice have compromised macrophage and NK cell functions
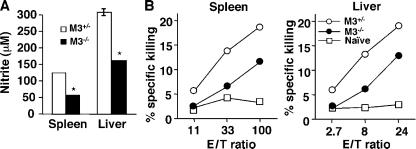
Innate immunity to LM is thought to limit the bacteria burden until the development of specific adaptive immunity. Although several studies have shown that innate immunity can influence the development of antigen-specific T cells (26, 27), the reciprocal effect of antigen-specific T cells on the innate response is less defined. As H2-M3–restricted T cells are functional before the receding of innate immune responses, we examined whether the lack of H2-M3–dependent responses can alter the intensity of the innate immune response to LM infection. At day 3 after LM infection, splenocytes and hepatic leukocytes from M3−/− and littermate controls were assayed in vitro for nitric oxide production and cytotoxicity to YAC-1 cells to assess the function of macrophages and NK cells, respectively. As shown in Fig. 5 A, splenocytes and hepatic leukocytes from LM-infected M3−/− mice produced less nitric oxide (as measured by nitrite formation) compared with that of M3+/− littermate controls. Similar results were obtained from peritoneal exudate macrophages (not depicted). Although splenocytes and hepatic leukocytes from LM- infected M3−/− mice were able to lyse NK targets, the percentage of killing was lower compared with WT mice (Fig. 5 B). Thus, H2-M3–restricted responses may potentiate the innate immune responses during the early phase of LM infection.
Figure 5.
Defective nitrite production and NK cell activity in M3−/− mice during the early phase of LM infection. M3−/− and M3+/− littermate controls (N6) were infected with 5 × 103 CFU of LM. (A) Measurement of nitrite production. 3 d after infection, splenocytes and hepatic leukocytes were isolated and stimulated with HKLM. After 48 h of incubation, the concentration of nitrite as a surrogate marker for the production of reactive nitrogen was determined with Greiss reagent. Bars represent mean ± SEM of two mice per group and are representative of four independent experiments. (B) NK cell cytotoxicity assay. Splenocytes and hepatic lymphocytes were isolated from day 3 LM-infected mice and used as effectors. Varying numbers of effectors were combined with a constant number of 51Cr-labeled YAC-1 cells in a standard 4-h cytotoxicity assay. Each data line represents the mean from two mice and is representative of four independent experiments.
H2-M3 deficiency does not influence the secondary CD8+ T cell response to LM
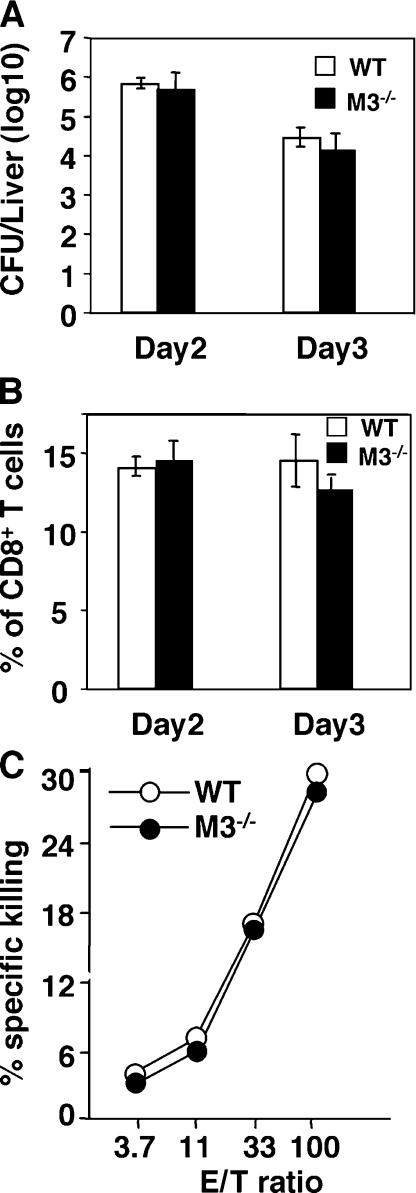
Although H2-M3–restricted T cells exhibit limited expansion during secondary LM infection, a substantial number of H2-M3–restricted T cells are detectable 3 d after rechallenging with LM (18). To determine whether an H2-M3–restricted response plays a significant role in secondary LM infection, we immunized WT and M3−/− mice with a sublethal dose of LM and rechallenged them 1 mo later with a high dose of LM. In contrast to the results seen in primary infection, M3−/− and WT mice displayed equivalent numbers of bacteria in the liver at days 2 and 3 after secondary infection (Fig. 6 A). As early as day 2 after secondary LM infection, the bacterial burdens in the spleens approached the detection limits in both WT and M3−/− mice (not depicted). In addition, the number of CD8+ T cells and anti-listerial CTL activity are comparable between M3−/− and WT controls (Fig. 6, B and C). Thus, H2-M3 deficiency does not have a significant effect on the secondary immune response to LM.
Figure 6.
H2-M3 deficiency has no effect on secondary anti-listerial response. 1 mo after primary LM infection, M3−/− (N6) and WT mice were rechallenged with 105 CFU of LM. (A) The bacterial burdens in the livers were determined on days 2 and 3 after secondary LM infection. (B) The percentage of CD8+ T cells in the spleens of LM-infected mice on the indicated days was determined by flow cytometric analysis. Data shown are mean ± SEM of five to six mice per group per time point. (C) Comparable LM-specific cytolytic activity in M3−/− and WT mice after secondary LM infection. 3 d after LM reinfection, splenocytes from indicated mice were activated with Con A and examined for CTL activity to 51Cr-labeld LM-infected J774-Kb target cells. Data are representative of results obtained from three mice per group
Interrelationship between MHC class Ia– and H2-M3–restricted CD8+ T cells
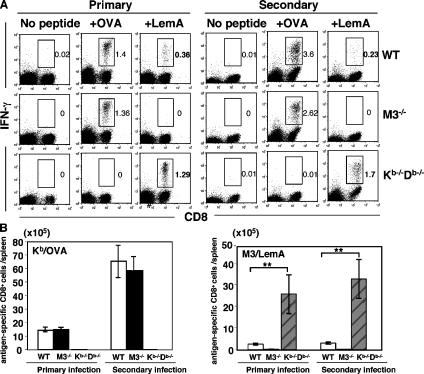
The increased LM burden during the early phase of LM infection in M3−/− mice could influence the antigenic load to which class Ia–restricted CD8+ T cells are exposed during infection, which consequently might affect the kinetics and magnitude of class Ia–restricted LM-specific CD8+ T responses. To address this question, we used Kb/OVA tetramer staining (not depicted) and intracellular IFN-γ staining to compare the Kb/OVA-specific response between WT and M3−/− mice after primary and secondary infection with rLM-ova. We found no significant differences in the number of Kb/OVA-specific IFN-γ–producing CD8+ T cells between M3−/− and WT mice either after primary or secondary LM infection (Fig. 7, A and B), indicating that H2-M3 deficiency does not affect the primary and secondary expansion of MHC class Ia–restricted T cells. In addition, comparable numbers of Kb/OVA-specific CD8+ T cells were found in WT control and H2-M3–deficient mice at day 30 after LM infection (2.13 ± 0.41 × 105 cells and 2.37 ± 0.38 × 105 cells per spleen in WT and M3−/− mice, respectively), suggesting that H2-M3 deficiency does not affect the development of the class Ia–restricted memory CD8+ T cell population We also assessed the functional avidity of Kb/OVA-specific CD8+ T cells by incubation of splenocytes from LM-infected WT or M3−/− mice with a serial dilution of OVA peptide and measured the amount of IFN-γ production by ELISA. No differences in functional avidity of Kb/OVA-specific T cells from WT or M3−/− mice were observed (not depicted). A reciprocal analysis was performed to examine the effect of the lack of MHC class Ia–restricted responses on H2-M3/LemA-specific responses. We found that the number of H2-M3/LemA-specific CD8+ T cells is drastically increased in Kb−/−Db−/− mice during primary LM infection compared with WT mice (Fig. 7, A and B). A similar trend was also observed during secondary LM infection. Thus, these data suggest that H2-M3 deficiency has no quantitative or qualitative effect on MHC class Ia–restricted T cell responses. In stark contrast, MHC class Ia–restricted responses limit the expansion of H2-M3–restricted T cells during primary as well as secondary LM infection.
Figure 7.
Comparison of MHC class Ia– and H2-M3–restricted CD8+ T cell responses in LM-infected M3−/−, Kb−/−Db−/−, and WT mice. M3−/− (N10), Kb−/−Db−/−, and WT mice were infected i.v. with 5 × 104 CFU of rLM-ova. Some of the mice were rechallenged 1 mo later with 106 CFU of the same bacteria. (A) The frequencies of Kb/OVA-specific and H2-M3/LemA-specific CD8+ T cells were determined by intracellular IFN-γ staining at day 7 after primary infection or day 3 after secondary infection. In brief, splenocytes isolated from LM-infected mice were stimulatedin vitro with either LemA or OVA peptide. 5 h later, intracellular cytokine staining for IFN-γ were performed. Antigen-specific CD8+IFN-γ+ T cells are highlighted by the gate, and the percentage of gated cells is indicated. Data are representative of results obtained from five to eight mice per group. (B) Bar graphs depict mean ± SEM for the total number of Kb/OVA-specific or H2-M3/LemA-specific CD8+ T cells in the spleens of WT, M3−/−, and Kb−/−Db−/− mice.
DISCUSSION
Emerging evidence suggests that MHC class Ib molecules can contribute to the host immune response by presenting conserved microbial antigens to T cells. However, the relative contributions and dynamic interactions between the MHC class Ia– and class Ib–restricted responses to infections have yet to be defined. In this study, we investigated the role of H2-M3 in T cell development and protective immunity against intracellular bacteria using M3−/− mice in a Listeria infection model. We found that significantly higher numbers of bacteria were recovered from both the spleens and livers of M3−/− mice than from WT and Kb−/−Db−/−mice at days 3 and 5 after LM infection, suggesting that H2-M3–restricted CD8+ T cells may play a dominant role in protective immunity against LM during the early phase of infection. The increased susceptibility of M3−/− mice to LM infection is associated with a decreased number of LM-specific IFN-γ–producing CD8+ T cells and reduced cytolytic activity. In addition, innate immunity to Listeria infection is lessened in the absence of H2-M3–restricted responses. Lack of H2-M3–restricted T cells, however, does not alter the kinetics and magnitude of MHC class Ia–restricted T cell responses during LM infection. Conversely, lack of MHC class Ia–restricted T cells results in an increased number of LM-specific H2-M3–restricted T cells after LM infection. This differential ability in compensating for the loss of anti-listerial CD8+ T cell responses could be one of the factors underlying the increased susceptibility of M3−/− mice, but not Kb−/−Db−/− mice, to LM infection. Collectively, our results suggest that H2-M3–restricted responses play a unique role in early host defense against listerial infection by contributing to adaptive immunity as well as by modulating the innate response to Listeria in infected mice.
As the number of CD8+ T cells is reduced by 90% in the periphery of naive Kb−/−Db−/− mice (28), one may expect that only a small fraction of the CD8+ T cell repertoire is selected by MHC class Ib molecules. Thus, it is not surprising that we were not able to detect significant differences in the proportions of T cell subsets between M3−/− and WT mice. Although H2-M3–restricted T cells only represent a small proportion of the total pool of CD8+ T cells in naive animals, we found that H2-M3–restricted T cells contribute substantially to the overall anti-listerial CD8+ T cell responses during primary infection. The fact that the number of CD8+CD44hi cells and LM-specific IFN-γ–producing CD8+ T cells is reduced by 50% in LM-infected M3−/− mice suggests that the small pool of naive M3-restricted T cells are quite capable of expanding to a magnitude similar to their MHC class Ia–restricted counterparts after LM infection. This could be attributed to the accelerated expansion of H2-M3–restricted T cells in response to LM infection as a result of their “memory-like” phenotype (14, 19). Another possible explanation could be that the precursor frequency of LM-specific T cells within the H2-M3–restricted T cell repertoire is relatively high. It has been shown that some H2-M3–restricted LM-specific CTLs are cross-reactive with antigens derived from a variety of Gram-positive and Gram-negative bacteria (29). In addition, deletion of H2-M3–restricted epitopes from LM does not prevent the priming of H2-M3–restricted T cells with specificity for the deleted epitope, suggesting that promiscuous antigen recognition may be a feature common to many H2-M3–restricted T cells (30–32). The promiscuous recognition and cross-reactivity to common environmental bacteria may lead to the priming and expansion of LM-reactive H2-M3–restricted T cells in “naive” animals, resulting in increased precursor frequency as well as a “primed” state.
CD8+ CTLs exert their protective functions against intracellular pathogens through cytolytic activity and secretion of cytokines, such as IFN-γ. Both activities are diminished in H2-M3–deficient mice. To address whether IFN-γ or cytolysis or both are important for M3-dependent immunity to Listeria, we treated LM-infected M3−/− and WT mice with neutralizing antibody against IFN-γ. CFU data obtained from both the spleens and livers at day 3 after infection showed that anti–IFN-γ–treated M3−/− mice are still more susceptible to LM infection when compared with anti–IFN-γ–treated WT mice (see Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20051866/DC1). Thus, neutralization of IFN-γ does not significantly affect the protection mediated by H2-M3–restricted response, suggesting that cytolytic activity may play an important role in H2-M3–mediated early protection during LM infection.
Our results have shown that the LM-specific CD8+ T cell population is significantly reduced in M3−/− mice, suggesting that MHC class Ia–restricted LM-specific CD8+ T cells did not undergo compensatory expansion during LM infection. This is further supported by the observations that CD8+ T cell responses to two Kb-restricted epitopes, OVA257–264 and LLO296–304, despite the difference in magnitude, were not affected by the H2-M3 deficiency (Fig. 7 and not depicted). One possible explanation is that the majority of naive LM-specific MHC class Ia–restricted CD8+ T cell precursors have been recruited in our experimental settings. Thus, MHC class Ia–restricted LM-specific CD8+ T cells were not able to contribute further, even when a sizable amount of H2-M3–restricted T cells were absent. Recent studies have shown that the magnitude of LM-specific CD8+ T cell responses is determined within the first 24 h of an infection and is independent of the severity and duration of in vivo bacterial infection (33). This notion may further explain the lack of a concordant increase in LM-specific class Ia–restricted T cells in M3−/− mice despite the presence of higher bacterial titers in the spleens and livers of M3−/− mice after 3–5 d of LM infection. However, it is conceivable that in an infection model where not all naive antigen-specific MHC class Ia–restricted T cells were recruited, either due to the presence of a large pool of antigen-specific MHC class Ia–restricted T cell precursors or due to the limited supplies of antigens/inflammatory cytokines, the presence or absence of an H2-M3–restricted response may affect the magnitude of MHC class Ia–restricted T cell responses.
Our finding that the number of LM-specific H2-M3–restricted T cells is significantly higher in Kb−/−Db−/− mice than in that of WT controls after primary infection is in accordance with previous observations using an in vitro cytotoxicity assay (22). It is possible that the precursor frequency of H2-M3–restricted CD8+ T cells is increased in Kb−/−Db−/− mice. Another possible explanation, although not mutually exclusive, is that lack of MHC class Ia–restricted T cells may allow for maximal expansion of LM-specific H2-M3–restricted T cells during infection by providing a favorable environment with more access to APCs, cytokines, and growth factors. A recent study has shown that the expansion of LM-specific H2-M3–restricted memory T cells can be prevented by LM-specific MHC class Ia–restricted memory T cells, possibly though rapid removal of antigen-presenting DCs (34). Comparing the numbers of H2-M3/LemA-specific T cells elicited during primary and secondary LM infection, we found that H2-M3–restricted T cells did not experience a similar degree of secondary expansion as observed for MHC class Ia–restricted T cells even in Kb−/−Db−/− mice, consistent with the previous finding using IFN-γ ELISPOT assays (19). Thus, these data suggest that lack of memory expansion of H2-M3–restricted response during infection may be, at least in part, due to intrinsic differences between MHC class Ia– and H2-M3–restricted T cells. Alternatively, the presence of other LM-specific non–MHC class Ia–restricted memory T cells might also be able to limit the secondary expansion of H2-M3–restricted T cells. Similar to H2-M3–restricted T cells, lack of memory expansion is also evident for Qa-1–restricted T cells during LM infection (23). Thus, MHC class Ib–restricted responses against infectious organisms may play a unique and important role during the early stages of infection, but participate only minimally in the protective memory immune response.
In addition to H2-M3–restricted T cells, LM infection has been shown to activate/induce T cells restricted by other MHC class Ib molecules, such as CD1d and Qa-1b (22, 24, 35). To see whether lack of H2-M3–restricted responses has an effect on other MHC class Ib–restricted responses, we have examined the CD1d- and Qa-1b–restricted T cell responses in LM-infected WT and M3−/− mice. We found no significant differences in the number of CD1d-restricted NKT cells or the magnitude of Qa-1b–specific cytolytic activity between LM-infected M3−/− and control mice at all time points examined (not depicted). This finding further supports the nonredundant role of H2-M3–restricted T cells in anti-listerial immunity.
Previous studies have shown that H2-M3–restricted T cells share some characteristics with CD1d-restricted NKT cells, such as the capacity to elicit rapid effector function and ability to be positively selected by hematopoietic cells (36, 37). However, unlike CD1d-restricted NKT cells that express a limited TCR repertoire (38–41), the H2-M3–restricted T cells studied to date have diverse TCR usage (42). Consistent with this notion, analysis of TCR Vα and Vβ chains in M3−/− mice does not reveal significant alterations in Vα and Vβ usage in CD8+ T cell populations compared with control (not depicted). In addition to the differences in TCR Vα and Vβ repertoire diversity, the kinetics of H2-M3–restricted T cell responses are also different from that of CD1d-restricted NKT cells in LM infection. Although we can detect H2-M3–restricted cytotoxic responses as early as day 2 after LM infection using an in vivo CTL assay (not depicted), H2-M3–restricted T cell response peaks at days 5–7 after infection (18, 19). In contrast, the majority of CD1d-restricted NKT cells are activated within 24 h after LM infection (35 and not depicted). Thus, the distinct kinetics of H2-M3–restricted T cells positions them to fill the temporal gap between “innate” T cells and conventional adaptive T cells.
In summary, our study demonstrates that H2-M3–restricted responses play a significant and nonredundant role in protective immunity against intracellular bacterial infection. Similar to H2-M3, other MHC class Ib molecules may serve a specialized role in antimicrobial immunity by selective presentation of conserved molecular signatures of microbial antigens and/or stress-induced ligands. The recognition of specific molecular patterns and rapid acquisition of effector functions may provide the unique niche for the MHC class Ib–restricted T cells in host defense against microbial pathogens.
MATERIALS AND METHODS
Generation of H2-M3-deficient (M3−/−) mice.
A DNA fragment containing H2-M3 was isolated from a 129/sv genomic DNA phage library (Stratagene). The targeting construct was generated by insertion of a 3.8-kb fragment containing exons 1–3 of the H2-M3 and a 5.5-kb fragment containing the 3′ end of H2-M3 into the EcoRI–BamHI and NotI–XhoI sites of pPNT, respectively (43). The targeting construct was designed to delete a 2.5-kb ApaI–SacI fragment containing exons 4–6 of the H2-M3 and replace these coding regions with the neo-resistance gene (Fig. 1 A). Strain 129/sv-derived R-1 embryonic stem cells were electroporated with 30 μg of NotI-linearized targeting construct, and G418-resistant colonies were screened for homologous recombination by Southern blot hybridization. Embryonic stem clones were injected into blastocysts of B6 mice, and chimeras were mated to B6 females. Offspring heterozygous for the mutation were intercrossed to produce M3−/− mice. Most of the M3−/− mice used in this study were backcrossed on to a B6 background (between 6 to 10 generations, N6-N10). The H2-M3 genotype was determined with PCR using the following three primers: M3-forward-CAGCGATGGAACCACCCACAATGA, M3-reverse-CAGACTAGCAACGATGACCATGATGAC, and neo-GATTCGCAGCGCATCGCCTTCTA, which yield a 550-bp fragment for the mutant allele and a 700-bp fragment for WT allele.
Mice.
Kb−/−Db−/− mice were provided by J. Forman (Texas Southwestern Medical Center, Dallas, TX) and were backcrossed up to 10 generations onto a B6 background. B6 mice were purchased from The Jackson Laboratory. All experiments involving animals were performed in compliance with institutional guidelines and have been approved by the Institute Animal Care Use Committee.
Peptides, antibodies, and flow cytometry.
LemA (f-MIGWII), OVA257–264 (SIINFEKL), and LLO296–304 (VAYGRQVYL; reference 44) peptides were synthesized at the University of Chicago Peptide Core facility. The following antibodies were used in this study: FITC–anti-Ly6C, FITC-anti–TCR-β, FITC–anti-CD3, FITC–anti-CD8α, FITC–anti-CD122, FITC-anti–hamster IgG, PE–anti-CD8β, PE–anti-CD44, PE–anti-CD69, PE–anti-NK1.1, PE–anti-F4/80, PE–anti-CD11c, PE–anti-Gr1, PE-anti–IFN-γ, CyChrome–anti-CD8α, and CyChrome–anti-CD4 (BD Biosciences). H2-M3/LemA tetramers were prepared as described previously (18). For flow cytometric analysis, single cell suspensions from the thymus, spleen, lymph node, and liver were prepared by standard procedures. For detecting H2-M3 surface expression, splenocytes from M3−/− and WT mice were incubated in RPMI 10 (RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, 20 mM Hepes, 50 μM 2-mercaptoethanol, penicillin, and streptomycin) with or without 10 μM LemA peptide overnight at 37°C. Cells were harvested and stained with anti–H2-M3 antibody (mAb130), followed by FITC-anti–hamster IgG (11). To examine T cell subsets and various immune cell types, cells were stained with combinations of fluorescent-conjugated antibodies for 30 min on ice in HBSS (Life Technologies) containing 2% FBS and 0.1% sodium azide (Sigma-Aldrich), followed by washing with the same buffer. The stained cells were analyzed by flow cytometry using a FACSCaliber with CELLQuest (Becton Dickinson) or Flowjo (Tree Star) software.
Bacteria, listeria infections, and CFU assays.
LM strain EGD was provided by R. Kurlander (National Institutes of Health, Bethesda, MD) and was grown in brain–heart infusion broth (Difco Laboratories). The recombinant LM strain rLM-ova, which secretes OVA and contains erythromycin marker, was provided by L. Lefrançois (University of Connecticut Health Center, Farmington, CT; reference 45). rLM-ova was grown in brain–heart infusion broth supplemented with 5 μg/ml erythromycin. For quantitation of bacterial titers from tissues, 12–20-wk-old mice were infected i.v. with 5 × 103 CFU LM (EGD) for primary infection. Recall infection with 105 CFU LM was performed 1 mo after primary infection. The bacterial dose was verified by plating dilutions of the inoculum on BH agar plates. At the indicated times after infection, mice were killed and specimens of liver and spleen were examined for bacterial titers. In brief, organs were homogenized and lysed in sterile water with 0.2% NP-40, serial dilutions of homogenates were plated on BH agar plates, and colonies were counted after incubation at 37°C for 24 h. For comparing the MHC class Ia–restricted and H2-M3–restricted T cell responses, mice were infected i.v. with 5 × 104 CFU rLM-ova for the primary infection and 106 CFU rLM-ova for the secondary infection.
51Cr release CTL assays.
ND1α-specific, H2-M3–restricted CTL clone (4E3; provided by K.F. Lindahl, Texas Southwestern Medical Center, Dallas, TX) was used as effector in CTL assays to determine the expression of H2-M3 on LPS blast from M3−/− and WT mice. To measure LM-specific CTL activity, splenocytes from LM-infected mice (either 5 d after primary infection or 3 d after secondary infection) were cultured in RPMI 10 with 1 μg/ml Con A. After 3 d, cells were harvested and used as effectors in a 51Cr release CTL assay. LM-infected and noninfected J774 transfectant-expressing H2-Kb (J774-Kb; provided by J. Forman) were used as targets. In brief, monolayers of J774-Kb targets grown in antibiotic-free medium were infected with LM for 1 h at a multiplicity of infection of 5:1. Cells were washed three times with warm PBS and cultured in DMEM containing 40 μg/ml gentamicin for an additional 3 h. All target cells used in CTL assays were labeled with 51Cr for 1 h at 37°C. Target cells (104) were added to round-bottom microtiter wells containing serial dilutions of effector cells. After 4 h of incubation at 37°C, 100 μl of supernatant from each well was collected to determine the amount of 51Cr release. The percent-specific lysis was calculated as 100 × (experimental cpm − spontaneous cpm) / (maximal cpm − spontaneous cpm). Data presented are the mean of triplicate wells.
In vivo cytotoxicity assay.
The in vivo cytotoxicity assay was performed as described by Barber et al. (46). In brief, splenocytes from B6 mice were labeled with red membrane dye PKH26 and either 5 μM, 500 nM, or 50 nM of cytosolic dye CFSE. The labeled cells were then pulsed with 1 μM LemA or OVA peptide for 4 h and transferred retro-orbitally (1:1:1 ratio, 5–10 million cells for each population) into the indicated groups of recipient mice. After 15 h of in vivo killing, lymphocytes were isolated from the spleens and livers and analyzed by flow cytometry for target cell clearance. Target cells were differentiated from recipient cells based on PKH26 staining and from each other based on different intensity of CFSE staining. Gated on PKH26+ cells, the percentage of specific killing was calculated as the following: 100 − [(% peptide pulsed in LM-infected/% unpulsed in LM-infected) / (% peptide pulsed in uninfected/% unpulsed in uninfected)] × 100.
Intracellular IFN-γ staining.
Splenocytes or hepatic leukocytes from rLM-ova–infected mice were cultured in 96-well plates at a concentration of 106 cells/well and stimulated with either 5 μM LemA or 1 μM OVA peptide for 5 h, or HKLM overnight. For the last 2 h of stimulation, 10 μM monensin was added to block the secretion of cytokines. Cells were washed and stained for cell surface markers CD8 and CD4. After fixation with 4% paraformaldehyde and permeabilization with 0.15% saponin, cells were then stained with PE-anti–IFN-γ antibody. Labeled cells were analyzed on a FACSCalibur.
NK cell cytotoxicity assays.
Splenocytes and hepatic leukocytes from uninfected mice and mice infected for 3 d with LM were prepared and used as effectors. YAC-1, a mouse lymphoma cell line, was used as a target in this assay. Cells were harvested and labeled with 100 μCi of 51Cr for 1 h at 37°C. Target cells (104) were added to round-bottom microtiter wells containing serial dilutions of effectors. After 4 h of incubation at 37°C, 100 μl of supernatant from each well was collected and assayed for 51Cr release. Percent specific lysis = (experimental cpm − spontaneous cpm) / (maximal cpm − spontaneous cpm) × 100. Data presented are the means of triplicates.
Nitrite assay.
3 d after LM infection, hepatic leukocytes and splenocytes from M3−/− and WT mice were isolated and stimulated with HKLM for 48 h at 4 × 105–106 cells/well in 96-well plates. Equivalent population of macrophages and DCs were assessed using PE–anti-F4/80 and PE–anti-CD11c by FACS analysis. Accumulated nitrite in the culture supernatant was measured by a colorimetric assay based on the Griess reaction with NaNO2 as the standard (47).
Statistical analysis.
Mean values were compared using the unpaired Student's t test. All statistical analyses were performed with the Prism program (GraphPad Software, Inc.). Statistically significant differences P < 0.05, P < 0.01, and P < 0.001 are noted with *, **, and ***, respectively.
Online supplemental material.
Table S1 shows the expression of various activation markers on CD8+ T cells from M3−/− and WT mice. Fig. S1 shows the effect of a neutralizing anti–IFN-γ mAb on M3-mediated early protection to LM infection. Table S1 and Fig. S1 are available at http://www.jem.org/cgi/content/full/jem.20051866/DC1.
Supplemental Material
Acknowledgments
We thank Dr. Hongzhi Chen, Tara King, and Chunting Yang for technician assistance; Dr. Eric Pamer (Memorial Sloan Kettering, New York, NY) for M3-tetramer construct; and Dr. Mike Zimmer and Angela Colmone for critical review of the manuscript.
This work was supported by National Institutes of Health grant AI40310 (to C.-R. Wang).
The authors have no conflicting financial interests.
Abbreviations used: HKLM, heat-killed Listeria monocytogenes; LM, Listeria monocytogenes; neo, neomycin; rLM-ova, recombinant LM strain expressing OVA protein.
H. Xu and T. Chun contributed equally to this work.
T. Chun's present address is Department of Microbiology and Immunology, School of Medicine, Hanyang University, Seoul 133-791, South Korea.
B. Wang's present address is Biomedical Research Center, University of British Columbia, Vancouver, BC V5Z 1L3, Canada.
References
- 1.Lindahl, K.F., B. Hausmann, and V.M. Chapman. 1983. A new H-2-linked class I gene whose expression depends on a maternally inherited factor. Nature. 306:383–385. [DOI] [PubMed] [Google Scholar]
- 2.Loveland, B., C.R. Wang, H. Yonekawa, E. Hermel, and K.F. Lindahl. 1990. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 60:971–980. [DOI] [PubMed] [Google Scholar]
- 3.Wang, C.R., B.E. Loveland, and K.F. Lindahl. 1991. H-2M3 encodes the MHC class I molecule presenting the maternally transmitted antigen of the mouse. Cell. 66:335–345. [DOI] [PubMed] [Google Scholar]
- 4.Smith, G.P., V.M. Dabhi, E.G. Pamer, and K.F. Lindahl. 1994. Peptide presentation by the MHC class Ib molecule, H2-M3. Int. Immunol. 6:1917–1926. [DOI] [PubMed] [Google Scholar]
- 5.Shawar, S.M., J.M. Vyas, J.R. Rodgers, R.G. Cook, and R.R. Rich. 1991. Specialized functions of major histocompatibility complex class I molecules. II. Hmt binds N-formylated peptides of mitochondrial and prokaryotic origin. J. Exp. Med. 174:941–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, C.R., A.R. Castano, P.A. Peterson, C. Slaughter, K.F. Lindahl, and J. Deisenhofer. 1995. Nonclassical binding of formylated peptide in crystal structure of the MHC class Ib molecule H2-M3. Cell. 82:655–664. [DOI] [PubMed] [Google Scholar]
- 7.Princiotta, M.F., L.L. Lenz, M.J. Bevan, and U.D. Staerz. 1998. H2-M3 restricted presentation of a Listeria-derived leader peptide. J. Exp. Med. 187:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz, L.L., B. Dere, and M.J. Bevan. 1996. Identification of an H2-M3-restricted Listeria epitope: implications for antigen presentation by M3. Immunity. 5:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulden, P.H., P. Fischer III, N.E. Sherman, W. Wang, V.H. Engelhard, J. Shabanowitz, D.F. Hunt, and E.G. Pamer. 1996. A Listeria monocytogenes pentapeptide is presented to cytolytic T lymphocytes by the H2-M3 MHC class Ib molecule. Immunity. 5:73–79. [DOI] [PubMed] [Google Scholar]
- 10.Chun, T., N.V. Serbina, D. Nolt, B. Wang, N.M. Chiu, J.L. Flynn, and C.R. Wang. 2001. Induction of M3-restricted cytotoxic T lymphocyte responses by N-formylated peptides derived from Mycobacterium tuberculosis. J. Exp. Med. 193:1213–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu, N.M., T. Chun, M. Fay, M. Mandal, and C.R. Wang. 1999. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J. Exp. Med. 190:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu, N.M., B. Wang, K.M. Kerksiek, R. Kurlander, E.G. Pamer, and C.R. Wang. 1999. The selection of M3-restricted T cells is dependent on M3 expression and presentation of N-formylated peptides in the thymus. J. Exp. Med. 190:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg, R.E., M.F. Princiotta, S. Irion, J.A. Moticka, K.R. Dahl, and U.D. Staerz. 1999. Positive selection of an H2-M3 restricted T cell receptor. Immunity. 11:33–43. [DOI] [PubMed] [Google Scholar]
- 14.Urdahl, K.B., J.C. Sun, and M.J. Bevan. 2002. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat. Immunol. 3:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fink, P.J., and M.J. Bevan. 1995. Positive selection of thymocytes. Adv. Immunol. 59:99–133. [DOI] [PubMed] [Google Scholar]
- 16.Unanue, E.R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11–25. [DOI] [PubMed] [Google Scholar]
- 17.Ladel, C.H., I.E. Flesch, J. Arnoldi, and S.H. Kaufmann. 1994. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J. Immunol. 153:3116–3122. [PubMed] [Google Scholar]
- 18.Kerksiek, K.M., D.H. Busch, I.M. Pilip, S.E. Allen, and E.G. Pamer. 1999. H2-M3–restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 190:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman, M.S., C.R. Wang, and J. Forman. 2000. MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 165:5192–5201. [DOI] [PubMed] [Google Scholar]
- 20.Kerksiek, K.M., A. Ploss, I. Leiner, D.H. Busch, and E.G. Pamer. 2003. H2-M3-restricted memory T cells: persistence and activation without expansion. J. Immunol. 170:1862–1869. [DOI] [PubMed] [Google Scholar]
- 21.D'Orazio, S.E., D.G. Halme, H.L. Ploegh, and M.N. Starnbach. 2003. Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J. Immunol. 171:291–298. [DOI] [PubMed] [Google Scholar]
- 22.Seaman, M.S., B. Perarnau, K.F. Lindahl, F.A. Lemonnier, and J. Forman. 1999. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J. Immunol. 162:5429–5436. [PubMed] [Google Scholar]
- 23.Bouwer, H.G., R.A. Barry, and D.J. Hinrichs. 2001. Lack of expansion of major histocompatibility complex class Ib-restricted effector cells following recovery from secondary infection with the intracellular pathogen Listeria monocytogenes. Infect. Immun. 69:2286–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouwer, H.G., A. Bai, J. Forman, S.H. Gregory, E.J. Wing, R.A. Barry, and D.J. Hinrichs. 1998. Listeria monocytogenes-infected hepatocytes are targets of major histocompatibility complex class Ib-restricted antilisterial cytotoxic T lymphocytes. Infect. Immun. 66:2814–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouwer, H.G., M.S. Seaman, J. Forman, and D.J. Hinrichs. 1997. MHC class Ib-restricted cells contribute to antilisterial immunity: evidence for Qa-1b as a key restricting element for Listeria-specific CTLs. J. Immunol. 159:2795–2801. [PubMed] [Google Scholar]
- 26.Martin-Fontecha, A., L.L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265. [DOI] [PubMed] [Google Scholar]
- 27.Trinchieri, G. 1995. Natural killer cells wear different hats: effector cells of innate resistance and regulatory cells of adaptive immunity and of hematopoiesis. Semin. Immunol. 7:83–88. [DOI] [PubMed] [Google Scholar]
- 28.Perarnau, B., M.F. Saron, B.R. San Martin, N. Bervas, H. Ong, M.J. Soloski, A.G. Smith, J.M. Ure, J.E. Gairin, and F.A. Lemonnier. 1999. Single H2Kb, H2Db and double H2KbDb knockout mice: peripheral CD8+ T cell repertoire and anti-lymphocytic choriomeningitis virus cytolytic responses. Eur. J. Immunol. 29:1243–1252. [DOI] [PubMed] [Google Scholar]
- 29.Nataraj, C., G.R. Huffman, and R.J. Kurlander. 1998. H2M3wt-restricted, Listeria monocytogenes-immune CD8 T cells respond to multiple formylated peptides and to a variety of Gram-positive and Gram-negative bacteria. Int. Immunol. 10:7–15. [DOI] [PubMed] [Google Scholar]
- 30.Ploss, A., G. Lauvau, B. Contos, K.M. Kerksiek, P.D. Guirnalda, I. Leiner, L.L. Lenz, M.J. Bevan, and E.G. Pamer. 2003. Promiscuity of MHC class Ib-restricted T cell responses. J. Immunol. 171:5948–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Orazio, S.E., M. Velasquez, N.R. Roan, O. Naveiras-Torres, and M.N. Starnbach. 2003. The Listeria monocytogenes lemA gene product is not required for intracellular infection or to activate fMIGWII-specific T cells. Infect. Immun. 71:6721–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ploss, A., A. Tran, E. Menet, I. Leiner, and E.G. Pamer. 2005. Cross-recognition of N-formylmethionine peptides is a general characteristic of H2-M3-restricted CD8+ T cells. Infect. Immun. 73:4423–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercado, R., S. Vijh, S.E. Allen, K. Kerksiek, I.M. Pilip, and E.G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833–6839. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton, S.E., B.B. Porter, K.A. Messingham, V.P. Badovinac, and J.T. Harty. 2004. MHC class Ia-restricted memory T cells inhibit expansion of a nonprotective MHC class Ib (H2-M3)-restricted memory response. Nat. Immunol. 5:159–168. [DOI] [PubMed] [Google Scholar]
- 35.Ranson, T., S. Bregenholt, A. Lehuen, O. Gaillot, M.C. Leite-de-Moraes, A. Herbelin, P. Berche, and J.P. Di Santo. 2005. Invariant Valpha14+ NKT cells participate in the early response to enteric Listeria monocytogenes infection. J. Immunol. 175:1137–1144. [DOI] [PubMed] [Google Scholar]
- 36.Coles, M.C., and D.H. Raulet. 2000. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164:2412–2418. [DOI] [PubMed] [Google Scholar]
- 37.Bendelac, A. 1995. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J. Exp. Med. 182:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor α chain is used by a unique subset of major histocompatibility complex class I–specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arase, H., N. Arase, K. Ogasawara, R.A. Good, and K. Onoe. 1992. An NK1.1+ CD4+8− single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc. Natl. Acad. Sci. USA. 89:6506–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makino, Y., R. Kanno, T. Ito, K. Higashino, and M. Taniguchi. 1995. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int. Immunol. 7:1157–1161. [DOI] [PubMed] [Google Scholar]
- 41.Ohteki, T., and H.R. MacDonald. 1996. Stringent V beta requirement for the development of NK 1.1+ T cell receptor-alpha/beta+ cells in mouse liver. J. Exp. Med. 183:1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindahl, K.F., D.E. Byers, V.M. Dabhi, R. Hovik, E.P. Jones, G.P. Smith, C.R. Wang, H. Xiao, and M. Yoshino. 1997. H2-M3, a full-service class Ib histocompatibility antigen. Annu. Rev. Immunol. 15:851–879. [DOI] [PubMed] [Google Scholar]
- 43.Tybulewicz, V.L., C.E. Crawford, P.K. Jackson, R.T. Bronson, and R.C. Mulligan. 1991. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 65:1153–1163. [DOI] [PubMed] [Google Scholar]
- 44.Geginat, G., S. Schenk, M. Skoberne, W. Goebel, and H. Hof. 2001. A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes. J. Immunol. 166:1877–1884. [DOI] [PubMed] [Google Scholar]
- 45.Pope, C., S.K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
- 46.Barber, D.L., E.J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27–31. [DOI] [PubMed] [Google Scholar]
- 47.Ding, A.H., C.F. Nathan, and D.J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407–2412. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.