Abstract
Human immunodeficiency virus (HIV) can infect resting CD4 T cells residing in lymphoid tissues but not those circulating in peripheral blood. The molecular mechanisms producing this difference remain unknown. We explored the potential role of the tissue microenvironment and its influence on the action of the antiviral factor APOBEC3G (A3G) in regulating permissivity to HIV infection. We found that endogenous IL-2 and -15 play a key role in rendering resident naive CD4 T cells susceptible to HIV infection. Infection of memory CD4 T cells also requires endogenous soluble factors, but not IL-2 or -15. A3G is found in a high molecular mass complex in HIV infection–permissive, tissue-resident naive CD4 T cells but resides in a low molecular mass form in nonpermissive, blood-derived naive CD4 T cells. Upon treatment with endogenous soluble factors, these cells become permissive for HIV infection, as low molecular mass A3G is induced to assemble into high molecular mass complexes. These findings suggest that in lymphoid tissues, endogenous soluble factors, likely including IL-2 and -15 and others, stimulate the formation of high molecular mass A3G complexes in tissue-resident naive CD4 T cells, thereby relieving the potent postentry restriction block for HIV infection conferred by low molecular mass A3G.
Cellular activation has long been considered a requirement for HIV infection of CD4 T cells, as HIV fails to infect resting CD4 T cells from peripheral blood. Infection is aborted either during reverse transcription (1) or before nuclear import of the viral preintegration complex (2). In contrast, recent studies show that resting CD4 T cells residing in tissue, analyzed either in vivo or ex vivo, are permissive for HIV infection (3–6). Because the vast majority of CD4 T cells are present in lymphoid tissues (7), most resting CD4 T cells are permissive for HIV infection. Over half of all memory CD4 T cells are infected and killed during acute simian immunodeficiency virus (SIV) infection in rhesus macaques (4, 8). Similarly, during acute SIV/HIV chimeric virus infection, resting naive CD4 T cells are a principal target for infection and emerge as primary virus-producing cells (6). The molecular mechanisms underlying the permissivity differences between resting CD4 T cells in tissue and blood remain unknown.
Ex vivo lymphoid cultures are an attractive system to address this question, as resting CD4 T cells, both naive and memory, are permissive to HIV infection independent of exogenous stimuli (3). Although well-recognized for memory CD4 T cells (9, 10), HIV infection of naive CD4 T cells was not fully appreciated until recently. In some studies, naive CD4 T cells from peripheral blood are refractory to HIV infection in vitro (11, 12), but the physiological relevance of this finding is uncertain because circulating HIV-infected naive CD4 T cells are detectable in vivo (13, 14).
The antiviral factor APOBEC3G (A3G) plays a key role in regulating the permissivity of CD4 T cells to HIV infection. Recently, we reported that resting CD4 T cells in peripheral blood are protected from HIV infection through the action of low molecular mass (LMM) A3G (15). Small interfering RNA–mediated knock down of A3G expression rendered these cells permissive to infection, as did stimulation with mitogens that promoted the recruitment of LMM A3G into inactive high molecular mass (HMM) A3G ribonucleoprotein complexes. These findings raised the possibility that in HIV-permissive, resting CD4 T cells from tissue, A3G might be present in HMM complexes. In this study, we used lymphoid organ cultures to explore this possibility and the role of the tissue microenvironment in regulating HIV permissivity of these cells.
RESULTS AND DISCUSSION
Soluble factors in HLAC-conditioned medium render tissue-derived naive CD4 T cells permissive to HIV infection
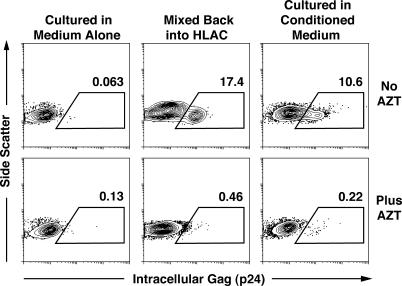
We first sought to identify the mechanisms underlying the permissivity of naive CD4 T cells in lymphoid tissues. To determine whether cell–cell contact or soluble factors produced in lymphoid tissue are required for HIV infection of tissue-resident naive CD4 T cells, these cells were purified from human lymphoid aggregate cultures (HLACs) prepared from human tonsils (3, 16) and tested under different culture conditions. Infected cells were identified by intracellular Gag (anti-p24) immunostaining and flow cytometric analysis. Naive CD4 T cells cultured in isolation in medium alone could not be infected by HIV (Fig. 1, left). However, when the cells were returned to autologous HLACs, HIV infectivity was restored (Fig. 1, middle). To determine whether endogenously produced soluble factors contributed to the acquisition of HIV permissivity, the cells were cultured in HLAC-conditioned medium. Again, the cells became permissive for HIV infection (Fig. 1, right). Addition of azidothymidine (AZT) blocked detection of intracellular Gag immunostaining, demonstrating that this signal was dependent on de novo reverse transcription. Of note, the viability of naive CD4 T cells in these cultures was comparable (unpublished data), suggesting that the infection results reflect differences in their state of permissivity rather than overall health. In addition, the activation status of both cultures was similar; each contained a small percentage of cells expressing either CD25 or CD69, typical for tissue-resident naive CD4 T cells (unpublished data).
Figure 1.
Conditioned medium from HLAC renders tissue-derived naive CD4 T cells permissive to HIV infection. Cells were cultured in medium alone (left), labeled with CFSE, and mixed back with autologous HLAC (middle) or were cultured in HLAC-conditioned medium (right). Intracellular anti–p24-Gag staining was performed 7 d after infection with NL4-3 (150 ng of p24-Gag). 50 μM AZT was included in some samples to ensure identification of productively infected cells (bottom). Data are representative of three experiments in which typically 5–10% of cells cultured in conditioned medium stained positively for intracellular Gag at the peak of infection.
These results are consistent with those of Kinter et al. (17), who showed that isolated tissue-derived CD4 T cells are permissive upon return to HLAC but not when cultured alone. Our work extends these studies by showing that permissivity of tissue-derived naive CD4 T cells can be conferred by HLAC-conditioned medium, suggesting a role for soluble factors.
IL-2 and -15 are necessary but not sufficient components of the permissivity activity in conditioned medium
To identify the factors present in conditioned medium necessary to render tissue-derived naive CD4 T cells permissive, we considered the study by Unutmaz et al. (18), which showed that 10–20 ng/ml IL-2, -4, -7, or -15 rendered otherwise unstimulated blood-derived CD4 T cells susceptible to HIV infection. Consistent with this and other studies (19, 20), we found that addition of 20 ng/ml IL-2, -4, -7, or -15 was sufficient for tissue-derived naive CD4 T cells to sustain a spreading infection (unpublished data).
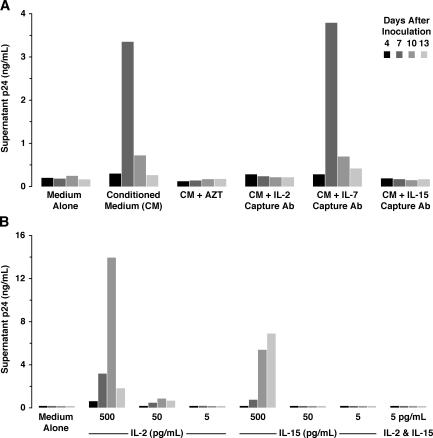
We next examined whether depletion of select cytokines from the conditioned medium altered rescue of HIV permissivity in tissue-derived naive CD4 T cells. IL-2 and -15, present at 5–10 and 2–5 pg/ml, respectively, were depleted from the conditioned medium by treatment with capture antibodies bound to insoluble bead supports. This procedure effectively reduced these cytokines to undetectable levels (unpublished data). To control for nonspecific inhibition by the depletion procedure, beads bound with antibodies recognizing IL-7 were also used, as IL-7 was undetectable in the conditioned medium. Depletion of either IL-2 or -15 markedly reduced the ability of the conditioned medium to rescue viral replication; however, as expected, depletion with antibodies recognizing IL-7 had no effect (Fig. 2 A). Further supporting a necessary role for IL-2, addition of a blocking antibody specific for the α chain of the high-affinity IL-2 receptor also inhibited viral replication, whereas a control antibody against the IL-4R did not (unpublished data).
Figure 2.
IL-2 and -15 are necessary but not sufficient components in conditioned medium for HIV infection of tissue-derived naive CD4 T cells. (A) Cells were cultured in medium alone, conditioned medium, or conditioned medium previously incubated with anti–IL-2, anti–IL-7, or anti–IL-15 capture antibodies and inoculated with NL4-3 (150 ng of p24-Gag). After extensive washing, virus-containing supernatants were monitored for p24-Gag content after 4, 7, 10, or 13 d of culture. Select cultures were treated with 50 μM AZT as a control. (B) Cells were cultured in medium with or without IL-2 or -15 (5, 50, or 500 pg/ml). One sample was cultured with IL-2 and -15 (5 pg/ml). Cells were infected as described in A. p24-Gag was measured by FLAQ assay. Data are representative of three independent experiments.
Next, we tested whether the addition of recombinant IL-2 or -15 at levels found in conditioned medium was sufficient to render tissue-derived naive CD4 T cells permissive. By comparison, these experiments were performed with doses 99.999% lower than those previously tested by Unutmaz et al. (18). At 5 pg/ml, IL-2 and -15 alone or in combination were not sufficient to promote infection of tissue-derived naive CD4 T cells (Fig. 2 B). Similarly, the combination of IL-2 and -15 at 20 pg/ml was ineffective (not depicted). At 500 pg/ml, IL-2 and -15 each conferred permissivity (Fig. 2 B). Thus, the endogenous levels of IL-2 and -15 are necessary but probably not sufficient for the permissivity activity found in conditioned medium.
Other examples of paracrine signaling conferring permissivity to HIV infection have been described. Using cultures from peripheral blood, Swingler et al. (21) found that HIV-infected or activated macrophages produce soluble CD23 and soluble ICAM (intercellular adhesion molecule), which act on B cells to render T cells permissive by cell–cell contacts. As IL-2 and -15 act directly on purified naive CD4 T cells, effects on intermediate cells do not appear to be involved. Others have highlighted a role for paracrine signaling by certain proinflammatory cytokines. Endogenous IL-1β, IL-6, and TNF-α are necessary for HIV infection in suboptimally stimulated PBMCs (22, 23). In ex vivo lymphoid cultures, neutralization of endogenous IL-1β or -6 decreases HIV replication when the tissue is cultured on collagen rafts but not when dispersed in HLAC (16). Because HIV replication in HLAC is unaffected by neutralization of these cytokines, it is unclear what role, if any, they play in regulating the permissivity of tissue-derived naive CD4 T cells.
Soluble factors render tissue-derived memory CD4 T cells permissive to HIV infection
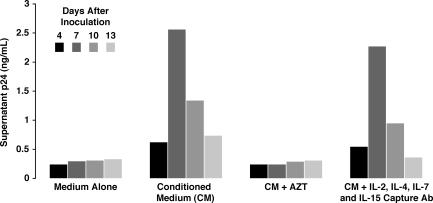
Next, we investigated the role of IL-2, IL-15, and other soluble factors in HIV infection of memory CD4 T cells. As tissue-resident memory CD4 T cells are a major target for HIV infection in vivo (4, 5, 8), understanding the molecular basis for permissivity in these cells could advance our understanding of HIV pathogenesis. Like tissue-derived naive CD4 T cells, tissue-derived memory CD4 T cells were refractory to HIV infection unless cultured in the presence of conditioned medium (Fig. 3). Surprisingly, conditioned medium depleted with antibodies specific for IL-2, -4, -7, and -15 retained the ability to render the memory CD4 T cells permissive to infection (Fig. 3). As expected, the depleted conditioned medium did not confer permissivity on tissue-derived naive CD4 T cells (unpublished data). Thus, memory CD4 T cells in lymphoid tissue appear to acquire permissivity for HIV infection through soluble factors different from those that confer permissivity on naive CD4 T cells.
Figure 3.
Infection of tissue-derived memory CD4 T cells also requires soluble factors. Tissue-derived memory CD4 T cells were cultured in medium alone, conditioned medium, or conditioned medium previously incubated with anti–IL-2, anti–IL-4, anti–IL-7, and anti–IL-15 capture antibodies. Cultures were inoculated with NL4-3 (150 ng of p24-Gag); after extensive washing, virus-containing supernatants were monitored for p24-Gag content after 4, 7, 10, or 13 d of culture. Select cultures were treated with 50 μM AZT as a control. p24-Gag was measured by FLAQ assay. The depleted conditioned medium failed to support HIV replication in tissue-derived naive CD4 T cells from the same donor. Data are representative of three independent experiments.
Naive CD4 T cells in the absence of conditioned medium exhibit an early postentry block to HIV infection
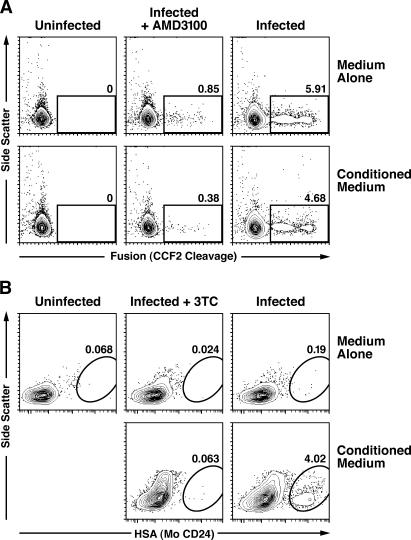
To determine whether conditioned medium alters the ability of tissue-derived naive CD4 T cells to support HIV env–mediated entry, we used a virion-based fusion assay (24). Equivalent levels of fusion were observed in the presence and absence of conditioned medium (Fig. 4 A), consistent with an effect occurring after virion fusion.
Figure 4.
Conditioned medium relieves an early postentry block of HIV infection in tissue-derived naive CD4 T cells. (A) Cells cultured in medium alone or conditioned medium were exposed to NL4-3 virions (250 ng of p24-Gag) containing BlaM-Vpr for 2 h before performing the virion-based fusion assay (reference 24 ). As a negative control, 250 nM of AMD3100, an inhibitor of CXCR4-mediated entry, was included. Data are representative of three experiments, during which levels of fusion ranged from 5 to 12% in the cell population. (B) Cells were exposed to VSV-G–pseudotyped NL4-3 HSA R-E- (700 ng of p24-Gag) for 3 h, washed extensively, and stained for surface expression of HSA 4 d later. Select cultures were treated with 10 μM 3TC as a control. Similar results were obtained in four additional experiments, with levels of HSA infection ranging from 0.7 to 4.0% in the cell population.
If conditioned medium affects early postentry steps in the viral life cycle, cells cultured in the absence of conditioned medium would be refractory to infection with single-cycle reporter viruses. However, if the block occurs after integration and gene expression, cells should be permissive in the presence or absence of conditioned medium. In tissue-derived naive CD4 T cells exposed to a single-cycle reporter virus, vesicular stomatitis virus G (VSV-G)–pseudotyped NL4-3 heat-stable antigen (HSA), reporter gene expression was detected only in the presence of conditioned medium (Fig. 4 B). Thus, conditioned medium appears to overcome an early postentry block to HIV infection in tissue-derived naive CD4 T cells.
Naive CD4 T cells contain HMM A3G when isolated from tonsil or when cultured in conditioned medium
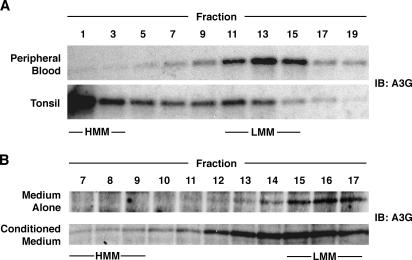
The early block to HIV infection in the absence of conditioned medium is reminiscent of the block observed in unstimulated CD4 T cells in peripheral blood (1, 2), which we have shown are protected from HIV infection by expression of LMM A3G (15). If LMM A3G is eliminated by small interfering RNA treatment or is converted to an HMM complex by mitogenic stimulation, these cells are rendered permissive. To determine whether HMM A3G is present in tissue-resident, HIV-permissive CD4 T cells, we used velocity sedimentation analysis to compare A3G complexes in purified naive CD4 T cells from peripheral blood or tonsil (Fig. 5 A). In these gradients, HMM A3G accumulated near the bottom, whereas LMM A3G sedimented near the middle, similar in size to tubulin (unpublished data). In blood-derived naive CD4 T cells, A3G was in the LMM form, consistent with results in unfractionated CD4 T cells (15). In naive CD4 T cells from tonsil tissue, A3G was detected principally in the HMM form, consistent with the permissivity of these cells to HIV infection (3). Similar results were obtained when A3G complexes were analyzed in purified CD4 T cells from either peripheral blood or tissue by size-exclusion chromatography (unpublished data), as described by Chiu et al. (15).
Figure 5.
Naive CD4 T cells contain HMM A3G when isolated from tonsil or when blood-derived cells are cultured in conditioned medium. (A) Lysates of naive CD4 T cells from peripheral blood or tonsil were subjected to velocity sedimentation analysis. Equal volumes of collected fractions were subjected to SDS-PAGE and immunoblotting for endogenous A3G using a peptide-specific rabbit anti-hA3G antibody (reference 26 ). Comparable data were obtained in two additional experiments. (B) FPLC analysis was performed on lysates of blood-derived naive CD4 T cells that were cultured for 6 d in medium alone or conditioned medium. Fractions were concentrated 10-fold before immunoblot analysis. These results are representative of three independent experiments.
These findings suggest that conditioned medium might render cells permissive to HIV infection by promoting the assembly of HMM A3G complexes. As it was difficult to purify enough naive CD4 T cells from tissue for the needed biochemical analysis, experiments were instead performed using cells isolated from the peripheral blood. When cultured in conditioned medium but not medium alone, 1–3% of cells became permissive, as measured by single-cycle infection with HSA-reporter viruses (unpublished data). This finding is consistent with the small number of cells expressing CD25 in the conditioned medium–treated cultures (unpublished data), indicating low levels of activation. As permissive cells are rare, a highly sensitive biochemical technique must be used to analyze the A3G complexes present in these cultures. In this regard, size-exclusion chromatography is preferable to the velocity sedimentation gradients used earlier. When naive CD4 T cells from the peripheral blood were cultured in medium alone, A3G remained in LMM complexes (Fig. 5 B). In contrast, when cultured in conditioned medium, both the levels of A3G increased (unpublished data) and, importantly, a fraction of the LMM A3G was recruited into HMM complexes (Fig. 5 B). It is likely that the modest shift of A3G into these HMM complexes reflects a response occurring in the small percentage of cells that are permissive for HIV infection, although direct proof of such a fractional cellular response is lacking. Given the clear but modest ability of soluble factors alone to drive HMM complex formation and the high levels of HMM A3G present in cells isolated directly from tissue, it is possible that the assembly of A3G complexes in vivo is regulated by both soluble factors and cell–cell contacts.
In summary, our findings show that endogenously produced IL-2 and -15 play an important role in rendering tissue-resident resting, naive CD4 T cells permissive to HIV infection. Investigation is currently under way to identify other soluble factors that contribute to infection of both naive and memory CD4 T cells. In terms of the molecular basis underlying permissivity of tissue-resident naive CD4 T cells to HIV infection, these cells display enzymatically inactive HMM A3G complexes, whereas nonpermissive naive CD4 T cells from peripheral blood contain LMM A3G, which functions as a potent postentry restriction factor (15). Further, the addition of HLAC-conditioned medium to resting naive CD4 T cells from the peripheral blood is sufficient to induce modest recruitment of LMM A3G into HMM complexes when measured across the entire population. We suspect that HMM A3G complex assembly actually occurs only within the small subset of the cells that are permissive to HIV infection in single-cycle infection experiments. Because the vast majority of CD4 T cells reside in tissues (7), our findings help explain how massive numbers of resting CD4 T cells can be infected and depleted during acute lentiviral infections (4, 8) while circulating resting CD4 T cells remain entirely refractory to HIV infection (1, 2).
MATERIALS AND METHODS
HLAC and conditioned medium.
Human tonsil tissue from routine tonsillectomies was obtained from the National Disease Research Interchange and the Cooperative Human Tissue Network and processed for HLAC as described previously (3, 16). In brief, tonsils were minced, passed through a 40-μm cell strainer, and cultured in 96-well U-bottomed polystyrene plates (2 × 106 cells/well) in medium (200 μl/well) consisting of RPMI 1640 supplemented with 15% FCS, 100 μg/ml gentamicin, 200 μg/ml ampicillin, 1 mM sodium pyruvate, 1% nonessential amino acids (Mediatech), 2 mM l-glutamine, and 1% fungizone (Invitrogen). After 2–4 d of culture, conditioned medium was collected and passed through a 0.2-μm nylon filter. Before use, conditioned medium was mixed with an equal volume of fresh medium to ensure an adequate supply of nutrients and neutral pH.
Cytokine depletion, receptor blocking, and quantitation of conditioned medium.
Cytokines were depleted from conditioned medium with capture antibodies specific for IL-2, -4, -7, and -15 (MAB602, -604, -207, and -647; R&D Systems). The antibodies were bound to insoluble bead supports (Seize X protein G immunoprecipitation kit; Pierce Chemical Co.) and incubated with the conditioned medium for 4 h at room temperature with constant agitation. The cytokine-depleted conditioned medium was collected after sedimentation of the antibody-containing beads. Blocking antibodies specific for IL-2Rα (2 μg/ml AF-223-NA; R&D Systems) or IL-4R (5 μg/ml MAB230; R&D Systems) were used to inhibit receptor binding. Cytokine levels in conditioned medium were quantitated by ELISA (sensitivity: IL-2, 1.6 pg/ml; IL-7, 0.156 pg/ml; IL-15, 0.3 pg/ml; R&D Systems).
Purification of naive and memory CD4 T cells.
Naive and memory CD4 T cells in buffy coats (Stanford Blood Bank) or HLAC were first enriched by Ficoll-Hypaque density gradient separation of mononuclear cells. These cells were then sorted for either CD4+ CD45RA+ CD62L+ (naive) or CD4+ CD45RA− (memory) using a FACS DiVa (BD Biosciences). Alternatively, naive cells were isolated from mononuclear cells by magnetic negative depletion (naive CD4 T cell isolation kit; Miltenyi Biotec) supplemented with microbeads against CD8, -14, -19, and -45RO. Purity was routinely >95% by flow cytometry and >90% using microbeads. After isolation, naive or memory CD4 T cells from tissue (1–2 × 105 in 96-well U-bottomed polystyrene plates) or from peripheral blood (2 × 106/ml in 6-well polystyrene plates) were cultured with medium alone, medium supplemented with recombinant IL-2 or -15 (R&D Systems), or conditioned medium. For experiments requiring CFSE labeling, 5 × 106/ml cells were washed once with PBS and incubated with 1 μM CFSE (Invitrogen) in PBS for 5 min at room temperature. To quench the labeling reaction, cells were washed in fresh medium.
HIV viral stocks.
The proviral expression plasmids pNL4-3 (M. Martin, National Institute of Allergy and Infectious Diseases [NIAID], Bethesda, MD) and pNLS4-3 HSA R-E- (N. Landau, The Salk Institute, La Jolla, CA) were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, at NIAID. The expression plasmid pVSV-G was obtained from J. Burns (University of California, San Diego, La Jolla, CA). Replication-competent NL4-3 (X4-tropic) and VSV-G–pseudotyped reporter virus (NL4-3 HSA R-E-) encoding HSA (mouse CD24) were prepared by calcium phosphate transfection of 293T cells. Virus-containing supernatants were collected 48 h later, clarified by sedimentation, filtered, and concentrated by ultracentrifugation. For fusion studies, NL4-3 virions containing BlaM-Vpr were prepared as described previously (24). All viral stocks were quantitated by measuring p24-Gag levels by ELISA (PerkinElmer) or FLAQ (25).
Viral infections.
For spreading infections, cells were cultured with or without a reverse transcriptase inhibitor (50 μM AZT) for 24 h, incubated with NL4-3 (150 ng of p24-Gag) for 24 h, washed extensively, and cultured. The medium was replaced every 3 d. For single-cycle infections, cells were cultured with or without a reverse transcriptase inhibitor (10 μM 3TC) for 48 h, inoculated with VSV-G–pseudotyped NL4-3 HSA R-E- (200–1,000 ng of p24-Gag) in the presence of 5 μg/ml polybrene for 3 h, washed extensively, and returned to culture. For virion-based fusion assays, cells were incubated with NL4-3 virions containing BlaM-Vpr (250 ng of p24-Gag) for 2 h, and assays were performed as described previously (24). Negative controls were pretreated with a CXCR4-specific entry inhibitor (250 nM AMD3100), obtained from D. Schols (Katholieke Universiteit Leuven, Leuven, Belgium).
FACS analysis of HIV-infected cultures.
For intracellular anti–p24-Gag immunostaining, cells were fixed in 1% paraformaldehyde and stained with an anti–p24-Gag antibody (KC57; Beckman Coulter) in PBS containing 0.1% saponin (Sigma-Aldrich) and 2% FBS. To measure surface expression of HSA, cells were stained with anti-HSA antibody (M1/69; BD Biosciences) in PBS containing 2% FBS. FACS data were analyzed with FlowJo software (Treestar). For the fusion assay, the levels of virion fusion are shown as a ratio of blue/green fluorescence reflecting the presence of cleaved versus uncleaved CCF2 substrate, with the former reflecting fusion (24).
Characterization of A3G complexes.
Linear, continuous 15–30% glycerol gradients were prepared in buffer containing 50 mM Hepes, pH 7.4, 125 mM NaCl, and 0.1% NP-40. Naive CD4 T cells were lysed in ice-cold lysis buffer containing 50 mM Hepes, pH 7.4, 125 mM NaCl, 0.2% NP-40, and 1 × EDTA-free protease inhibitor cocktail (Calbiochem). The clarified cell lysates were loaded on the gradients and centrifuged at 130,000 g for 16 h at 4°C in an SW55Ti rotor (Beckman Coulter). After sedimentation, 22 fractions (each ∼210 μl) were collected from the bottom to the top of the gradient. Fast protein liquid chromatography (FPLC) analysis was performed as described previously (15) except that because of the limited amount of material, FPLC fractions were concentrated 10-fold (Microcon YM-30; Millipore) before detection of A3G by immunoblot analysis. To prevent nonspecific loss of sample during concentration, 50–100 μg of glutathione reductase (Sigma-Aldrich) were added to each fraction.
Acknowledgments
We thank Drs. M.A. Martin, N.R. Landau, J.C. Burns, and D. Schols for reagents used in these studies; D. Arguello, V. Stepps, and M. Bigos for assistance with cell sorting; Y. Chiu, V. Soros, K. Stopak, J. Neidleman, M. Cavrois, and V. Ng for discussions; and R. Givens, S. Cammack, S. Ordway, G. Howard, and J. Carroll for assistance in preparation of the manuscript and figures.
J.F. Kreisberg was funded by the Universitywide AIDS Research Program (D03-GI-401), and W.C Greene was funded by the National Institutes of Health (P01 HD40543 and R01 AI065329).
The authors have no conflicting financial interests.
References
- 1.Zack, J.A., S.J. Arrigo, S.R. Weitsman, A.S. Go, A. Haislip, and I.S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 61:213–222. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson, M., T.L. Stanwick, M.P. Dempsey, and C.A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckstein, D.A., M.L. Penn, Y.D. Korin, D.D. Scripture-Adams, J.A. Zack, J.F. Kreisberg, M. Roederer, M.P. Sherman, P.S. Chin, and M.A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4+ T cells residing within human lymphoid tissues. Immunity. 15:671–682. [DOI] [PubMed] [Google Scholar]
- 4.Li, Q., L. Duan, J.D. Estes, Z.M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C.J. Miller, and A.T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 434:1148–1152. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K.A. Staskus, K.A. Reimann, T.A. Reinhart, M. Rogan, W. Cavert, C.J. Miller, et al. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 286:1353–1357. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura, Y., C.R. Brown, J.J. Mattapallil, T. Igarashi, A. Buckler-White, B.A. Lafont, V.M. Hirsch, M. Roederer, and M.A. Martin. 2005. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian–human immunodeficiency viruses in macaques. Proc. Natl. Acad. Sci. USA. 102:8000–8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haase, A.T. 1999. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 17:625–656. [DOI] [PubMed] [Google Scholar]
- 8.Mattapallil, J.J., D.C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 434:1093–1097. [DOI] [PubMed] [Google Scholar]
- 9.Spina, C.A., H.E. Prince, and D.D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Invest. 99:1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnittman, S.M., H.C. Lane, J. Greenhouse, J.S. Justement, M. Baseler, and A.S. Fauci. 1990. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc. Natl. Acad. Sci. USA. 87:6058–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roederer, M., P.A. Raju, D.K. Mitra, and L.A. Herzenberg. 1997. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J. Clin. Invest. 99:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou, C.S., O. Ramilo, and E.S. Vitetta. 1997. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. USA. 94:1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrowski, M.A., T.W. Chun, S.J. Justement, I. Motola, M.A. Spinelli, J. Adelsberger, L.A. Ehler, S.B. Mizell, C.W. Hallahan, and A.S. Fauci. 1999. Both memory and CD45RA+/CD62L+ naive CD4+ T cells are infected in human immunodeficiency virus type 1-infected individuals. J. Virol. 73:6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaak, H., A.B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc. Natl. Acad. Sci. USA. 97:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu, Y.L., V.B. Soros, J.F. Kreisberg, K. Stopak, W. Yonemoto, and W.C. Greene. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 435:108–114. [DOI] [PubMed] [Google Scholar]
- 16.Audige, A., E. Schlaepfer, A. Bonanomi, H. Joller, M.C. Knuchel, M. Weber, D. Nadal, and R.F. Speck. 2004. HIV-1 does not provoke alteration of cytokine gene expression in lymphoid tissue after acute infection ex vivo. J. Immunol. 172:2687–2696. [DOI] [PubMed] [Google Scholar]
- 17.Kinter, A., A. Moorthy, R. Jackson, and A.S. Fauci. 2003. Productive HIV infection of resting CD4+ T cells: role of lymphoid tissue microenvironment and effect of immunomodulating agents. AIDS Res. Hum. Retroviruses. 19:847–856. [DOI] [PubMed] [Google Scholar]
- 18.Unutmaz, D., V.N. KewalRamani, S. Marmon, and D.R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y.G., Y. Iwabu, J. Warachit, M. Kinomoto, M.S. Ibrahim, S. Tsuji, T. Mukai, M. Kameoka, K. Tokunaga, T. Sata, and K. Ikuta. 2005. Interleukin-4 up-regulates T-tropic human immunodeficiency virus type 1 transcription in primary CD4+ CD38+ T-lymphocyte subset. Microbiol. Immunol. 49:155–165. [DOI] [PubMed] [Google Scholar]
- 20.Cavalieri, S., S. Cazzaniga, M. Geuna, Z. Magnani, C. Bordignon, L. Naldini, and C. Bonini. 2003. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 102:497–505. [DOI] [PubMed] [Google Scholar]
- 21.Swingler, S., B. Brichacek, J.M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature. 424:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinter, A.L., G. Poli, L. Fox, E. Hardy, and A.S. Fauci. 1995. HIV replication in IL-2-stimulated peripheral blood mononuclear cells is driven in an autocrine/paracrine manner by endogenous cytokines. J. Immunol. 154:2448–2459. [PubMed] [Google Scholar]
- 23.Kinter, A.L., M. Ostrowski, D. Goletti, A. Oliva, D. Weissman, K. Gantt, E. Hardy, R. Jackson, L. Ehler, and A.S. Fauci. 1996. HIV replication in CD4+ T cells of HIV-infected individuals is regulated by a balance between the viral suppressive effects of endogenous β-chemokines and the viral inductive effects of other endogenous cytokines. Proc. Natl. Acad. Sci. USA. 93:14076–14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavrois, M., C. De Noronha, and W.C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151–1154. [DOI] [PubMed] [Google Scholar]
- 25.Hayden, M.S., E.H. Palacios, and R.M. Grant. 2003. Real-time quantitation of HIV-1 p24 and SIV p27 using fluorescence-linked antigen quantification assays. AIDS. 17:629–631. [DOI] [PubMed] [Google Scholar]
- 26.Stopak, K., C. de Noronha, W. Yonemoto, and W.C. Greene. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell. 12:591–601. [DOI] [PubMed] [Google Scholar]