Abstract
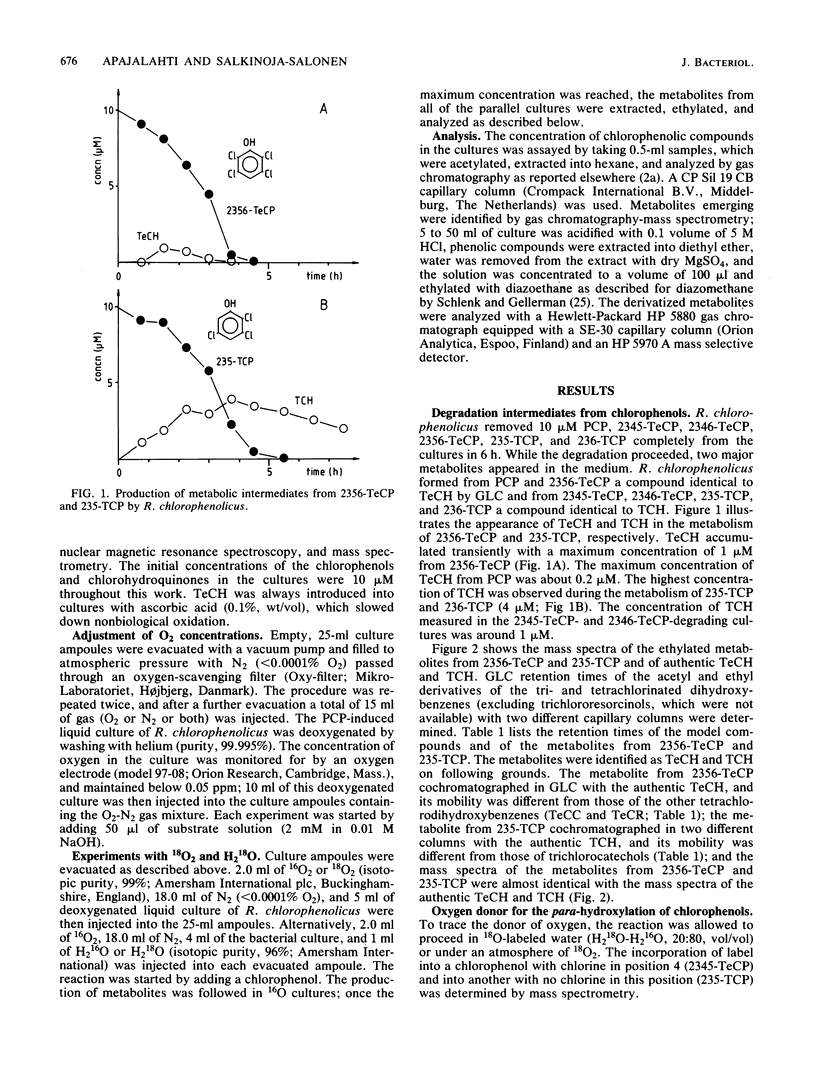
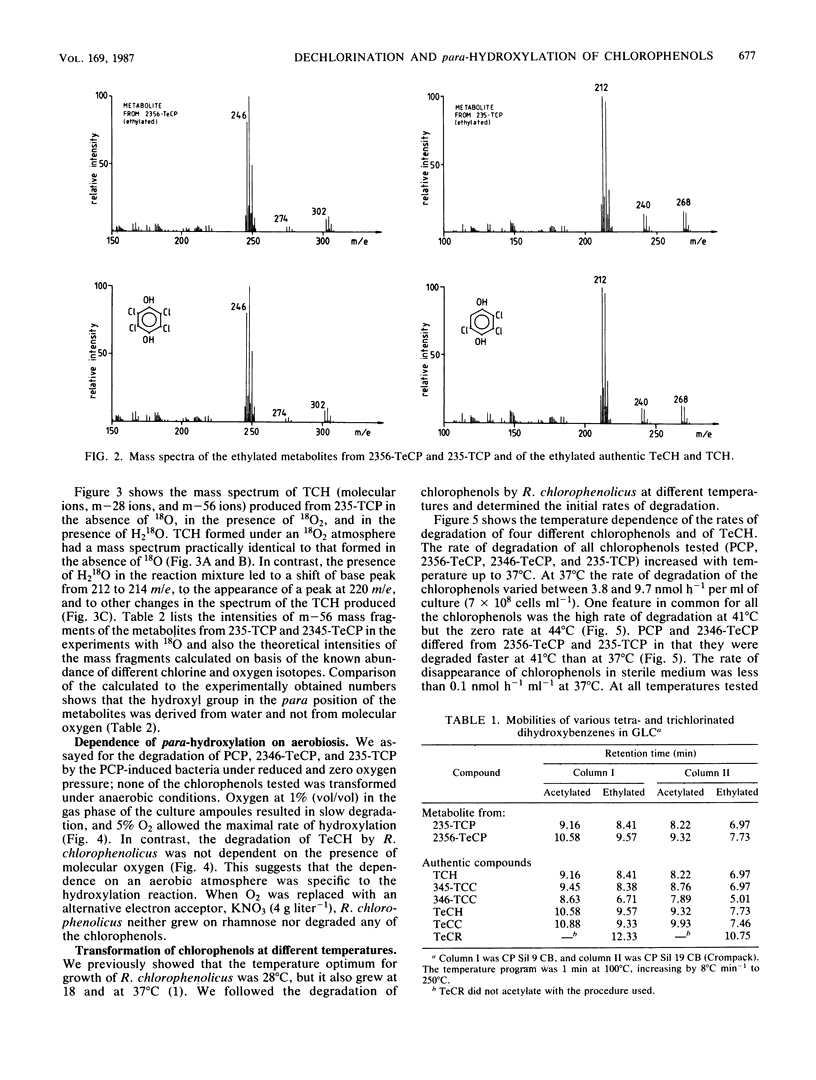
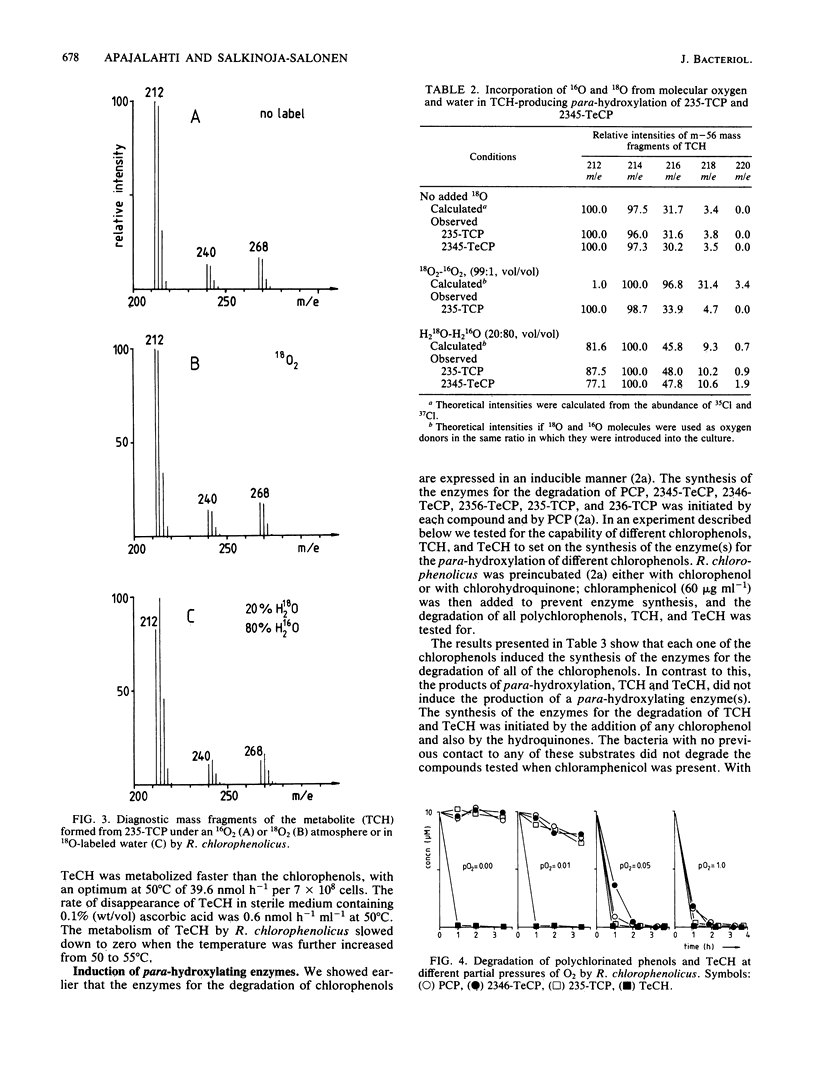
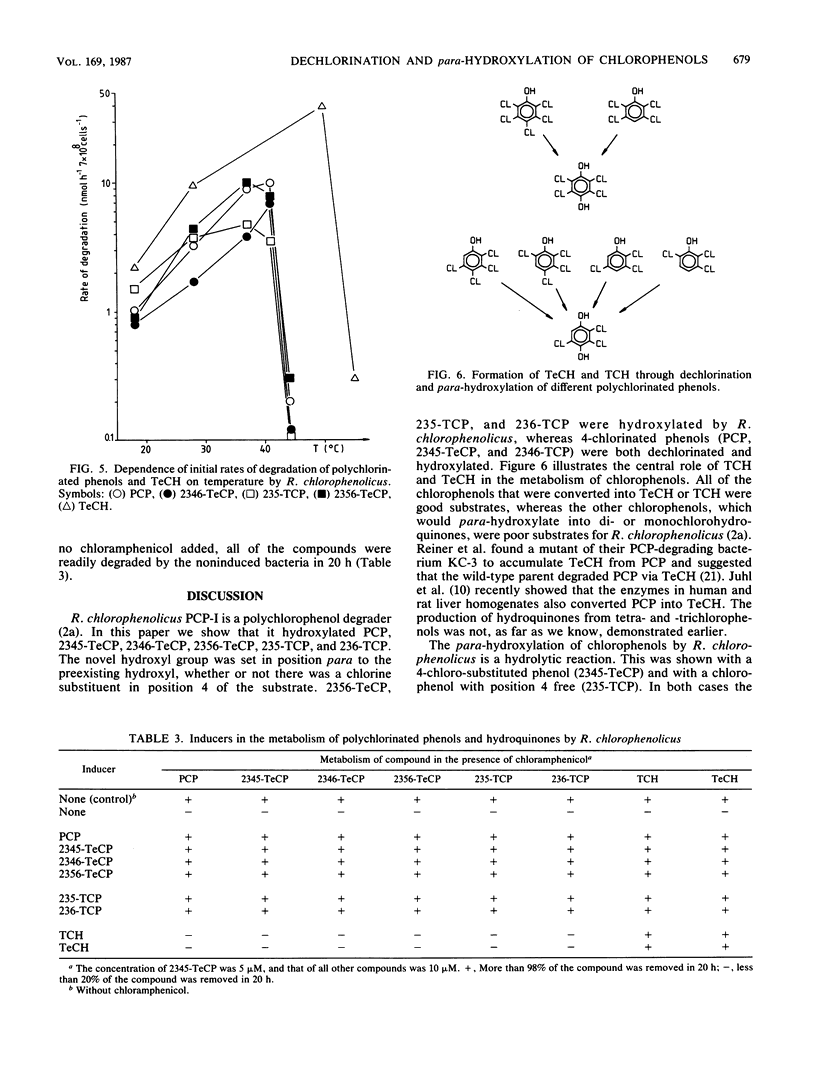
In this paper we show that a polychlorophenol degrader Rhodococcus chlorophenolicus PCP-I initially attacked polychlorinated phenols (pentachlorophenol, 2,3,4,5-, 2,3,4,6-, and 2,3,5,6-tetrachlorophenol, and 2,3,5- and 2,3,6-trichlorophenol) by tetra- or trichlorohydroquinone-producing para-hydroxylation. The novel hydroxyl group was set in position 4, whether or not a substrate had chlorine substituent in this position. The hydroxyl was in each case derived from water molecules, as was shown by following the incorporation of oxygen from H2(18)O into the reaction products. Nevertheless, the para-hydroxylation reaction required the presence of molecular oxygen, whereas further metabolism of the reaction product, tetrachlorohydroquinone, proceeded also in anaerobiosis. All polychlorinated phenols were readily transformed at 41 degrees C, but none were transformed at 44 degrees C. In contrast to this, tetrachlorohydroquinone was metabolized at a high rate at 50 degrees C, but was not metabolized at 55 degrees C. Polychlorinated phenols were specific inducers of the para-hydroxylating enzymes; para-hydroxylated reaction products did not induce these enzymes. On the other hand, the degradation of tri- and tetrachlorohydroquinone was induced by any of the chlorophenols and also by hydroquinones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu J. P., Kirsch E. J. Metabolism of pentachlorophenol by an axenic bacterial culture. Appl Microbiol. 1972 May;23(5):1033–1035. doi: 10.1128/am.23.5.1033-1035.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal D., You I. S., Chatterjee D. K., Chakrabarty A. M. Microbial degradation of halogenated compounds. Science. 1985 Apr 12;228(4696):135–142. doi: 10.1126/science.228.4696.135. [DOI] [PubMed] [Google Scholar]
- Hartmann J., Reineke W., Knackmuss H. J. Metabolism of 3-chloro-, 4-chloro-, and 3,5-dichlorobenzoate by a pseudomonad. Appl Environ Microbiol. 1979 Mar;37(3):421–428. doi: 10.1128/aem.37.3.421-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl U., Witte I., Butte W. Metabolism of pentachlorophenol to tetrachlorohydroquinone by human liver homogenate. Bull Environ Contam Toxicol. 1985 Nov;35(5):596–601. doi: 10.1007/BF01636560. [DOI] [PubMed] [Google Scholar]
- Karns J. S., Duttagupta S., Chakrabarty A. M. Regulation of 2,4,5-trichlorophenoxyacetic acid and chlorophenol metabolism in Pseudomonas cepacia AC1100. Appl Environ Microbiol. 1983 Nov;46(5):1182–1186. doi: 10.1128/aem.46.5.1182-1186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns J. S., Kilbane J. J., Chatterjee D. K., Chakrabarty A. M. Microbial biodegradation of 2,4,5-trichlorophenoxyacetic acid and chlorophenols. Basic Life Sci. 1984;28:3–21. doi: 10.1007/978-1-4684-4715-6_2. [DOI] [PubMed] [Google Scholar]
- Karns J. S., Kilbane J. J., Duttagupta S., Chakrabarty A. M. Metabolism of Halophenols by 2,4,5-trichlorophenoxyacetic acid-degrading Pseudomonas cepacia. Appl Environ Microbiol. 1983 Nov;46(5):1176–1181. doi: 10.1128/aem.46.5.1176-1181.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbane J. J., Chatterjee D. K., Karns J. S., Kellogg S. T., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Jul;44(1):72–78. doi: 10.1128/aem.44.1.72-78.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J. Xenobiotic degradation in industrial sewage: haloaromatics as target substrates. Biochem Soc Symp. 1983;48:173–190. [PubMed] [Google Scholar]
- Marks T. S., Wait R., Smith A. R., Quirk A. V. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984 Oct 30;124(2):669–674. doi: 10.1016/0006-291x(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Müller R., Thiele J., Klages U., Lingens F. Incorporation of [18O]water into 4-hydroxybenzoic acid in the reaction of 4-chlorobenzoate dehalogenase from pseudomonas spec. CBS 3. Biochem Biophys Res Commun. 1984 Oct 15;124(1):178–182. doi: 10.1016/0006-291x(84)90933-1. [DOI] [PubMed] [Google Scholar]
- Reddy C. C., Vaidyanathan C. S. Purification, properties and induction of a specific benzoate-4-hydroxylase from Aspergillus niger (UBC 814). Biochim Biophys Acta. 1975 Mar 28;384(1):46–57. doi: 10.1016/0005-2744(75)90094-7. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber D. L., Crawford R. L. Isolation and characterization of Flavobacterium strains that degrade pentachlorophenol. Appl Environ Microbiol. 1985 Dec;50(6):1512–1518. doi: 10.1128/aem.50.6.1512-1518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton D. R., Tiedje J. M. Isolation and partial characterization of bacteria in an anaerobic consortium that mineralizes 3-chlorobenzoic Acid. Appl Environ Microbiol. 1984 Oct;48(4):840–848. doi: 10.1128/aem.48.4.840-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanlake G. J., Finn R. K. Isolation and characterization of a pentachlorophenol-degrading bacterium. Appl Environ Microbiol. 1982 Dec;44(6):1421–1427. doi: 10.1128/aem.44.6.1421-1427.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T. Metabolism of pentachlorophenol by a soil microbe. J Environ Sci Health B. 1977;12(2):113–127. doi: 10.1080/03601237709372057. [DOI] [PubMed] [Google Scholar]


