Abstract
Neutralizing antibody (nAb) responses to lymphocytic choriomeningitis virus (LCMV) in mice and immunodeficiency virus and hepatitis C virus in humans are usually weak and slow to develop. This may be the result of structural properties of the surface glycoprotein, a low frequency of B cells with neutralizing specificity, and the necessity of prolonged affinity maturation of specific nAbs. In this study, we show that during LCMV infection, CD27 signaling on CD4+ T cells enhances the secretion of interferon-γ and tumor necrosis factor-α. These inflammatory cytokines lead to the destruction of splenic architecture and immunodeficiency with reduced and delayed virus-specific nAb responses. Consequently, infection with the otherwise persistent LCMV strain Docile was eliminated after CD27 signaling was blocked. Our data provide a novel mechanism by which LCMV avoids nAb responses and suggest that blocking the CD27–CD70 interaction may be an attractive strategy to prevent chronic viral infection.
Viruses use different strategies to evade the host immune response to establish persistent infection. These strategies include generating antibody and T cell escape variants as well as actively suppressing the immune system (1–5). In addition, neutralizing antibody (nAb) responses to certain viral infections are mounted late and inefficiently (2, 6, 7). Lymphocytic choriomeningitis virus (LCMV) infection in mice is an exemplary model characterized by low titers of nAbs, which are not detected until at least 60 d after infection (2). A similar phenomenon is observed in humans after infection with HIV and hepatitis C virus (HCV; references 6, 7). There are several possible reasons for the delayed formation of nAb, including immunopathological and suppressive effects mediated by T cells (5, 8), the prolonged time for affinity and avidity maturation of specific antibodies (9), low precursor frequency of B cells with neutralizing specificity (10), and structural features of the viral surface proteins (11, 12). Despite their late appearance, nAbs may be important in the long-term control of LCMV infection because B cell–deficient mice cannot control LCMV strain WE infection (13).
CD27 is a member of the tumor necrosis factor receptor family and acts in a costimulatory pathway to elicit T and B cell responses. CD27 is expressed on naive and activated CD4+ and CD8+ T cells as well as on subsets of B and NK cells (14). CD27 signaling activates NF-κB, promotes cell survival, enhances TCR-mediated proliferative signals, and increases T cell effector function (14, 15). However, persistent CD27 signaling leads to T cell depletion (16). Thus, CD27 signaling either leads to improved T cell function or to T cell dysfunction, probably depending on the amount, duration, and timing of the expression of the CD27 ligand CD70 (14, 16). CD70 expression is tightly regulated, and it is only transiently expressed on activated T and B cells as well as on subsets of professional antigen-presenting cells (14). In contrast to the very limited expression of CD70 in normal healthy individuals, there are various pathologies with the constitutive expression of CD70. For example, the prolonged expression of CD70 has been documented in chronic infectious diseases such as HIV (17, 18).
In this study, we have analyzed the role of CD27 signaling in the generation of nAb responses and in virus control after infection with LCMV. CD27 signaling on CD4+ T cells increased the secretion of IFNγ and TNFα, leading to the destruction of the splenic architecture, including the splenic marginal zone. CD27-dependent immunopathological destruction of lymphatic organs correlated with functional immunosuppression and impaired nAb responses. Blocking CD27 interaction with CD70 by infecting CD27-deficient mice or by administering a monoclonal αCD70 antibody after infection reduced immunopathology, and nAb responses were efficiently mounted. As a consequence of the absence of CD27 signaling, infection with the otherwise persistent LCMV strain Docile was eliminated.
RESULTS
nAb responses to viral infections in the absence of CD27 signaling
To analyze the role of CD27 signaling in the induction of specific B cell responses, we first infected CD27−/− mice and C57BL/6 (BL/6) control mice with vesicular stomatitis virus (VSV) Indiana (VSV-IND; Fig. 1 A). After infection with VSV-IND, the neutralizing T-independent IgM antibody response was comparable in CD27−/− and control mice. However, there was a fourfold increase in the T-dependent neutralizing IgG antibody titers 8 and 12 d after infection in BL/6 mice compared with CD27−/− mice. This slightly enhanced switch from IgM to IgG antibodies in BL/6 mice suggests a role for CD27 signaling in CD4+ T helper cells. However, nAbs in CD27−/− mice reached comparable titers with those in BL/6 mice 20 d after infection, and all CD27−/− mice survived VSV infection without developing lethal paralysis. Therefore, in agreement with earlier findings in influenza infection (19), our results indicate that CD4+ T helper cell–dependent and –independent B cell responses are largely independent of CD27.
Figure 1.
Primary immune response against VSV-IND and LCMV-WE. (A) nAbs after VSV-IND infection of CD27−/− and BL/6 mice. (B) CD27−/− and BL/6 mice were infected with LCMV-WE and total serum Ig, and LCMV-WE–specific nAbs were measured. Data in A and B are expressed as the mean ± SEM of n = 3–5 mice and are representative of two (A) to five (B) individual experiments except for total serum Ig, which is the mean of pooled sera of five mice per group. (C) Number of CD70+ cells within CD4+, CD8+, CD11c+, and CD19+ splenocytes after LCMV-WE infection determined by flow cytometry. Mean ± SEM (error bars) of n = 3 mice. Numbers in parentheses indicate the percentage (mean ± SEM) of CD70-expressing cells at the peak of expression (day is indicated).
nAb responses to LCMV infection in mice are low and are only mounted late after infection (2). Surprisingly, CD27−/− mice mounted high nAb responses starting 20–30 d after infection with LCMV-WE, whereas BL/6 mice had antibody titers below the detection limit (Fig. 1 B). In addition to the virus-specific antibody response, LCMV infection polyclonally activates B cells that are not virus specific, leading to hypergammaglobulinemia (20). A recent study suggested an inverse correlation between CD4+ T cell–dependent polyclonal B cell activation and the induction of nAb in LCMV infection (21). However, polyclonal B cell activation and hypergammaglobulinemia was even enhanced in the absence of CD27 signaling when compared with controls (Fig. 1 B).
Our finding suggested an important inhibitory role of CD27 signaling in the generation of LCMV-specific nAb responses. Therefore, we analyzed the expression of the CD27 ligand (CD70) in the spleen at different time points after infection of BL/6 or CD27-deficient mice with LCMV-WE. CD70 was expressed for a prolonged time on CD4+ and CD8+ T cells and on CD19+ B cells (Fig. 1 C). In addition, CD70 was detectable on a few dendritic cells at days 8 and 15 after infection.
Normal CTL induction and expansion in the absence of CD27
Titers of nAb against LCMV increased when CD8+ T cell function was absent (2). This increase may be caused by enhanced viral replication. We recently showed that CTL induction after infection with 200 pfu LCMV-WE was independent of CD27 signaling (22). Comparably, primary CTL function after infection with 106 pfu LCMV-WE, as analyzed by the direct lysis of peptide-pulsed target cells and the absolute number of gp33-specific T cells, was not impaired in CD27−/− mice (Fig. 2, A and B). More importantly, both CD27−/− and control mice eliminated the virus within 12–16 d after infection from the spleen, lung, liver, and kidney (Fig. 2 C and Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20060651/DC1). We next compared the production of inflammatory cytokines by LCMV gp33 TCR transgenic P14 and P14 x CD27−/− CD8+ T cells 8 d after infection with LCMV-WE and found similar levels of IFNγ and TNFα (Fig. 2 D). Therefore, CTL responses to LCMV infection were normal in the absence of CD27, and differences in viral control did not explain the more efficient generation of nAb in CD27−/− mice.
Figure 2.
Induction of CTL response and virus elimination in CD27−/− mice. (A) CTL activity (51Cr release assay) 8 d after LCMV-WE infection. Closed symbols, gp33-pulsed target cells; open symbols, unpulsed target cells. (B and C) The absolute number of Db-gp33–specific CD8+ T cells measured by tetramer staining (B) and virus titer (C) were assessed in the spleen after LCMV-WE infection. (D) Cytokine production of purified P14 and P14 × CD27−/− T cells 8 d after adoptive transfer to BL/6 recipients and LCMV-WE infection. Data are expressed as the mean ± SEM (error bars) of n = 3–4 animals and are representative of one of two individual experiments. ns, P ≥ 0.05.
CD27-dependent immunopathology
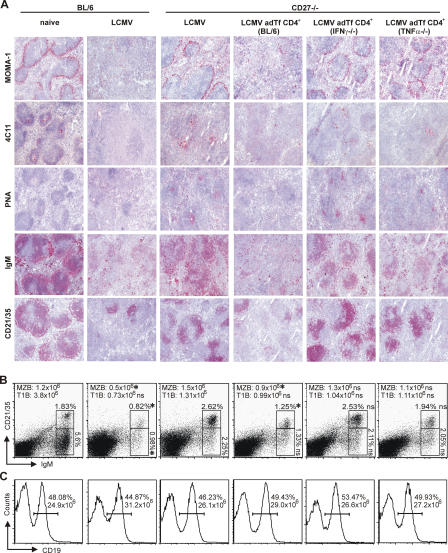
The induction of antibody and T cell responses against viral infections requires that the anatomical structures of secondary lymphoid organs are intact (23). During LCMV infection, the splenic architecture is disrupted, and the marginal zone and germinal centers are destroyed (8). Therefore, we analyzed the splenic architecture 8 d after infection with LCMV-WE in CD27−/− and BL/6 mice. Naive BL/6 (Fig. 3, A and B) and CD27−/− mice (not depicted but identical to naive BL/6 mice) had a normal splenic architecture with an intact marginal zone and germinal centers. LCMV infection of BL/6 mice induced the destruction of the marginal zone and germinal centers and a complete absence of follicular dendritic cells (Fig. 3 A). Flow cytometry analysis further revealed that marginal zone B cell (CD21+CD35+IgM+IgD−) numbers were decreased in LCMV-infected BL/6 mice when compared with noninfected BL/6 mice (Fig. 3 B). Surprisingly, the splenic architecture remained largely intact after LCMV infection of CD27−/− mice, and infected mice had clearly identifiable germinal centers containing follicular dendritic cells and a preserved marginal zone (Fig. 3 A).
Figure 3.
Spleen architecture after LCMV-WE infection. (A and B) BL/6 and CD27−/− mice and CD27−/− mice receiving purified CD4+ T cells from BL/6, IFNγ−/−, or TNFα−/− mice were infected with LCMV-WE. 8 d after infection, the spleen was analyzed. (A) Cryosections stained for metallophilic marginal zone macrophages (MOMA-1), follicular dendritic cell (4C11) networks, PNA+ germinal center clusters, surface IgM+ B cells, and CD21+CD35+ marginal zone B cells. (B) The relative frequency of CD21+CD35+IgM+IgD− marginal zone B cells and of CD21−CD35−IgM+IgD− T1B cells among total lymphocytes and their absolute number per spleen are shown. (C) The relative frequency of CD19+ B cells among total lymphocytes and their absolute number per spleen are shown. Horizontal bars indicate gates for CD19+ B cells. One representative section or dot plot out of n = 3–9 mice per group is shown. Numbers indicate the mean. Numbers of marginal zone B cells and T1B cells in BL/6 mice or CD27−/− mice receiving CD4+ T cells from BL/6, IFNγ−/−, or TNFα−/− mice were compared with CD27−/− mice. *, P < 0.05; ns, P ≥ 0.05.
In addition, flow cytometry analysis demonstrated that relative (percentage) and absolute numbers of marginal zone B cells (CD21+CD35+IgM+IgD−) were higher in LCMV-infected CD27−/− than in BL/6 mice (Fig. 3 B). The frequency (percentage) and absolute numbers of transitional type 1 B cells (T1B; CD21−CD35−IgM+IgD−) were higher in LCMV-infected CD27−/− mice. However, this only reached statistical significance if analyzed as the percentage of total lymphocytes (Fig. 3 B). In contrast, relative and absolute numbers of CD19+ B cells (Fig. 3 C) and mature IgM+IgD+ B cells in the spleen (16.4 × 106 for CD27−/− vs. 17.4 × 106 for BL/6) were similar in LCMV-infected CD27−/− and BL/6 mice. In summary, LCMV-induced immunopathology is dependent on CD27 signaling.
CD27 signaling on CD4+ T cells induces immunopathology and suppression of nAbs
To identify the cell population responsible for the CD27-dependent reduction in nAb responses, purified BL/6 CD8+ or CD4+ T cells were adoptively transferred to CD27−/− recipient mice before infection with LCMV-WE. The adoptive transfer of CD8+ T cells to CD27−/− mice did not affect the nAb response (Fig. 4 A). In contrast, the transfer of purified CD27-expressing CD4+ T cells to CD27−/− mice resulted in a reduction and delay in the nAb response when compared with CD27−/− controls (Fig. 4 A). Likewise, the adoptive transfer of CD4+ T cells from BL/6 to CD27−/− mice resulted in immunopathology comparable with BL/6 mice as analyzed by immunohistochemistry and flow cytometry (Fig. 3, A and B).
Figure 4.
Characterization of the cell population responsible for reduced antibody production. (A) BL/6 and CD27−/− mice and CD27−/− mice receiving purified CD4+ or CD8+ T cells from BL/6 mice were infected with LCMV-WE, and nAbs were measured. (B) Cytokine production by purified SMARTA and SMARTA × CD27−/− T cells 8 d after adoptive transfer to BL/6 recipients and LCMV infection. *, P < 0.05; ns, P ≥ 0.05. (C) BL/6 and CD27−/− mice and CD27−/− mice receiving purified CD4+ T cells from BL/6, IFNγ−/−, or TNFα−/− mice were infected with LCMV-WE, and nAbs were measured 80 d later. (D) CD27−/− mice receiving purified CD4+ T cells from BL/6, IFNγ−/−, or TNFα−/− mice were infected with LCMV-WE, and, 8 d after adoptive transfer, the total number of CD27+CD4+ T cells per spleen were determined by flow cytometry. All results are shown as the mean ± SEM (error bars) of n = 3 animals per group and are representative of two independent experiments.
The constitutive expression of CD70 on B cells in transgenic mice induced IFNγ production by T cells, which led to a depletion of the CD70-expressing B cells (24). Thus, we analyzed the role of CD27 signaling on the secretion of inflammatory cytokines by LCMV gp13 TCR transgenic SMARTA and SMARTA × CD27−/− CD4+ T cells 8 d after infection with LCMV-WE (Fig. 4 B). The production of IL-6, -1β, and -10 was independent of CD27, whereas the secretion of IFNγ and TNFα was decreased in the absence of CD27 signaling on specific CD4+ T cells. To determine whether the secretion of IFNγ and TNFα by CD4+ T cells is important in the induction of immunopathology and the suppression of nAb responses, CD4+ T cells from BL/6, IFNγ−/−, and TNFα−/− mice were adoptively transferred to CD27−/− recipients before infection with LCMV-WE. The transfer of CD4+ T cells from IFNγ−/− and TNFα−/− mice to CD27−/− recipients infected with LCMV-WE did not affect specific nAb responses (Fig. 4 C). Similarly, the adoptive transfer of CD4+ T cells from IFNγ−/− or TNFα−/− mice to CD27−/− recipients before LCMV-WE infection had little effect on immunopathology (Fig. 3, A and B).
Earlier studies with B cells constitutively expressing CD70 have shown that ligation of CD70 by CD27 and consequent reverse signaling through CD70 can lead to a reduction of antibody secretion (25, 26). Therefore, the enhanced nAb response against LCMV-WE in CD27−/− mice may be caused by the absence of inhibitory signals mediated by CD70 signaling. To address this possibility, we assessed the number of CD27-expressing CD4+ T cells in CD27−/− recipients after the adoptive transfer of CD4+ T cells of BL/6, IFNγ−/−, and TNFα−/− mice (Fig. 4 D). In this experimental setup, the CD27-expressing transferred CD4+ T cells are the only cells that provide CD70 ligation. CD4+ T cells from BL/6, TNFα−/−, or IFNγ−/− mice similarly engrafted in recipient mice, and, therefore, a comparable CD27–CD70 interaction was provided by the transferred cells. Despite this similar CD27–CD70 ligation in recipient mice, only the transfer of CD4+ T cells from BL/6 mice impaired antibody production, whereas the transfer of CD4+ T cells from IFNγ−/− or TNFα−/− mice did not. This largely excludes the possibility that reverse signaling through CD70 reduces the production of nAb. Therefore, we conclude that CD27 ligation on CD4+ T cells contributes to the secretion of IFNγ and TNFα, leading to immunopathology with delayed specific nAb responses.
LCMV-induced immunosuppression of nAb responses to viral coinfections
LCMV infection has been shown to induce an acquired immunodeficiency with reduced nAb responses and increased susceptibility to coinfection with VSV (21, 27). To elucidate whether CD27-dependent immunopathology also impairs antibody responses to unrelated antigens, we infected mice with VSV New Jersey (VSV-NJ) 8 d after LCMV-WE infection. BL/6 mice immunized with LCMV-WE and coinfected with VSV-NJ had a slight reduction in the nAb IgM response, whereas the switch to IgG was virtually absent (Fig. 5 A). In contrast, the nAb response to VSV-NJ was not impaired in LCMV-infected CD27−/− mice.
Figure 5.
LCMV-induced suppression of the nAb response to viral coinfections induced by CD4+ cells. (A) CD27−/− and BL/6 mice were infected with LCMV-WE, coinfected 8 d later with VSV-NJ, and VSV-NJ nAbs were determined. (B) BL/6 (IgMb+) and CD27−/− (IgMb+) mice receiving purified CD19+ B cells from T11μMT (IgMa+) mice were infected with LCMV-WE. 8 d after adoptive transfer, the total number of VSV-specific B cells (CD19+IgMa+IgMb−) were determined in the spleen by flow cytometry. (C) BL/6, CD27−/−, and CD27−/− mice receiving purified CD4+ or CD8+ T cells from BL/6 mice were infected with LCMV-WE. 8 d later, mice were coinfected with VSV-NJ, and VSV-NJ nAbs were determined. (D) BL/6, CD27−/−, and CD27−/− mice receiving purified CD4+ T cells from BL/6, IFNγ−/−, or TNFα−/− mice were infected with LCMV-WE. 8 d later, mice were coinfected with VSV-NJ, and VSV-NJ nAbs were determined 20 d later. All experiments are shown as means ± SEM (error bars) of n = 3–6 animals per group and are representative of two independent experiments.
Similar results were obtained after challenging LCMV-infected CD27−/− and BL/6 mice with UV-inactivated VSV-NJ (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20060651/DC1). In addition, VSV-NJ only abortively replicates in immunocompetent mice and was eliminated similarly in LCMV-infected CD27−/− and BL/6 mice (Fig. S2). These controls exclude the possibility that differences in VSV replication in CD27−/− and BL/6 mice account for the observed differences in nAb responses. Although LCMV-infected BL/6 mice and CD27−/− mice possess similar numbers of B cells (Fig. 3 C), it may be possible that B cells with VSV-neutralizing specificity may have been eliminated by the CD27–CD70 interaction during LCMV infection. To analyze this, purified B cells from VSV-IgM transgenic mice crossed on a μMT background (T11μMT; reference 28) were transferred to BL/6 and CD27−/− mice before infection with LCMV-WE (Fig. 5 B). A comparable number of B cells with VSV-neutralizing specificity 8 d later excluded the possibility that the observed reduction of VSV nAbs in LCMV-immune mice coinfected with VSV was caused by a reduced number of VSV-specific B cells in the presence of a functional CD27–CD70 interaction in BL/6 mice.
We also tested whether the adoptive transfer of BL/6 CD4+ T cells to CD27−/− recipients affected the nAb response in mice first infected with LCMV and coinfected 8 d later with VSV-NJ. In these mice, there was an impaired switch to VSV-NJ–specific IgG antibodies (Fig. 5 C). Mice receiving CD8+ T cells did not have an impaired antibody response (Fig. 5 C). Similar to the impaired nAb response to LCMV infection, the reduced nAb to coinfection with VSV-NJ was mediated by IFNγ and TNFα. The transfer of CD4+ T cells from IFNγ−/− and TNFα−/− mice to CD27−/− recipients infected with LCMV and coinfected with VSV did not reduce the production of specific nAb as much as CD4+ T cells from BL/6 mice (Fig. 5 D). Therefore, CD27 ligation on CD4+ T cells contributes to the secretion of IFNγ and TNFα, leading to virus-induced immunodeficiency with impaired antibody responses to related and unrelated viral antigens.
Blocking CD27 signaling eliminates an otherwise persistent infection with LCMV-Docile
Infection with the LCMV strain Docile establishes chronic infection in mice with the functional exhaustion of specific CTLs and no nAb response (29). This model of persistent infection was used to analyze the physiological role of CD27 and CD4+ T cell–dependent immunopathology and immunodeficiency. After the infection of BL/6 mice with LCMV-Docile, nAb titers were below the detection level, and the virus established persistent infection (Fig. 6, A and B). In contrast, CD27−/− mice mounted nAb within 20–30 d after infection, and high titers were reached 50 d after infection (Fig. 6 A). CD27−/− mice were also able to control and eliminate LCMV-Docile infection in the blood (Fig. 6 B) and organs within 60 d (Fig. 6 C). In contrast, high viral titers were found in BL/6 mice in all organs analyzed up to 100 d after infection (Fig. 6, B and C). Antibody-deficient Jh −/− and CD27−/− × Jh −/− mice did not control LCMV-Docile infection, indicating that nAbs were crucial for virus control (Fig. 6, A and B).
Figure 6.
Control of chronic viral infection in CD27+/+ mice. (A–E) CD27−/−, BL/6, Jh −/−, and CD27−/− × Jh −/− mice were infected with LCMV-Docile. (A) LCMV-Docile–specific nAb. (B and C) Virus titer in peripheral blood (B) and different organs (days 8, 40, and 100 after injection; C). (D) Spleen cells tested in a 51Cr release assay directly ex vivo (day 8 after injection; left) or after restimulation in vitro for 5 d (day 40 after injection, middle; and day 100 after injection, right). Closed symbols, gp33-pulsed target cells; open symbols, unpulsed target cells. Symbols in A–D represent the mean ± SEM of n = 3–8 mice. (E) Spleen cells analyzed for Db-gp33–specific T cells measured by tetramer staining and intracellular IFNγ production after gp33 stimulation. The cells' frequencies among CD8+ T cells are indicated. One representative dot plot from one mouse out of three is shown. Numbers indicate means ± SEM (error bars) of n = 3 mice. The experiment was repeated once with equivalent results.
8, 20, and 40 d (not depicted) after the infection of CD27−/− and BL/6 mice with LCMV-Docile, only a few LCMV gp33-specific CTLs were detectable by tetramer staining (Fig. 6 E). CTL function, as analyzed by the lysis of peptide-pulsed target cells or by intracellular IFNγ staining, was also impaired in BL/6 and CD27−/− mice 8 and 40 d after infection (Fig. 6, D and E). These results indicate that specific CTLs were functionally exhausted or anergic. Surprisingly, after the elimination of LCMV-Docile, CD27−/− mice regained a functional CTL response to LCMV gp33 and np396 (not depicted), as documented in a 51Cr release cytotoxicity assay (Fig. 6 D) or by intracellular IFNγ staining (Fig. 6 E).
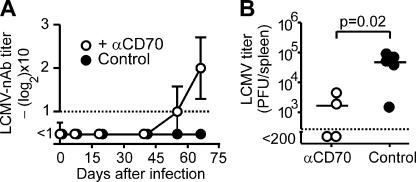
We next wanted to determine whether blocking CD27 signaling could be a therapeutic approach to prevent chronic viral infection. Because CD70 expression peaked 8 d after infection on CD8+ T cells and even later on B and CD4+ T cells (Fig. 1 C), we decided to test CD70 blockade several days after infection. BL/6 mice infected with LCMV-Docile were treated for 5 wk with a blocking monoclonal antibody to CD70 (FR70; reference 30) starting 4 d after infection. LCMV-Docile was detectable in all animals at day 4 after infection, with 1.3 × 105 (± 0.02 × 105) pfu/ml in the blood. Similar to the results obtained in CD27−/− mice, treatment with the blocking mAb to CD70 elicited a nAb response and resulted in reduced viral titers (Fig. 7, A and B).
Figure 7.
Blocking CD27–CD70 signaling after infection. (A and B) BL/6 mice were infected with LCMV-Docile and treated with αCD70 mAb or control Ig. (A) LCMV-Docile–specific nAbs were determined. Symbols represent the mean ± SEM (error bars) of n = 3–5 mice. (B) Virus titer was measured 66 d after infection in the spleen. The horizontal bars represent the mean.
Interestingly, immunohistochemistry and analysis of B cell subpopulations by flow cytometry revealed that the splenic architecture was largely restored in BL/6 mice 35 d after LCMV-Docile infection in spite of virus persistence (Fig. 8, A and B). Nevertheless, no nAbs were produced in BL/6 mice even up to 100 d after infection (Fig. 6 A). This may be explained by the fact that no functional IFNγ-producing LCMV-specific CD4+ T cells were detectable 35 d after infection, probably as a result of exhaustion or anergy (Fig. 8 C; reference 31). In addition, CD70 expression was low to nondetectable 35 d after infection with LCMV-Docile (unpublished data). Therefore, it was not surprising that treating LCMV-Docile–infected mice from day 35 to 60 after infection with mAb to CD70 neither induced protective nAb responses nor eliminated the virus (Fig. 8, D and E). In summary, these results suggest that the kinetics of CD70 expression and CD4+ T cell function determine the time frame in which blocking CD27 signaling efficiently eliminates chronic viral infections.
Figure 8.
Blocking CD27 signaling during chronic infection. (A and B) BL/6 mice were infected with LCMV-Docile, and, 35 d after infection, the spleen was analyzed. (A) Cryosections stained for metallophilic marginal zone macrophages (MOMA-1), follicular dendritic cell (4C11) networks, PNA+ germinal center clusters, surface IgM+ B cells, and CD21+CD35+ marginal zone B cells. One representative section or dot plot from 1 out of 3 mice/group is shown. (B) Relative frequency among total lymphocytes and absolute number of CD21+CD35+IgM+IgD− marginal zone B lymphocytes per spleen. Numbers indicate the mean. (C) BL/6 mice were infected with LCMV-Docile. At the indicated time points, splenocytes were restimulated in vitro with p13 and analyzed for CD4+ T cells that produce IFNγ intracellularly. (D and E) BL/6 mice were infected with LCMV-Docile and treated with αCD70 mAb or control Ig from day 35 to day 60 after infection. (E) LCMV-Docile–specific nAb. Virus titer in the spleen 66 d after infection. (C and D) Data are shown as the means ± SEM (error bars) of n = 3 animals. (E) The horizontal bars represent the mean.
DISCUSSION
The induction of nAbs represents a central protective mechanism of the immune system and is the basis of most currently available vaccines. Unfortunately, attempts to improve protective antibody responses against persisting viruses such as HIV in humans or LCMV in mice have repeatedly failed (12, 32). Different mechanisms may contribute to the inefficient generation of nAbs. For LCMV, one study (12) showed that the kinetics of nAb responses are crucially dependent on intrinsic factors of the surface antigen and that LCMV glycoprotein expressed on the immunogenic VSV backbone did not induce LCMV-specific nAb. In addition, B cells with a receptor of neutralizing specificity against LCMV may exist at very low frequencies and may only be generated by substantial somatic hypermutation. More potent LCMV-specific nAb responses in mice genetically engineered to express LCMV-neutralizing B cell receptors argue in favor of such mechanisms (10). In this study, we define an additional and novel mechanism by which a virus avoids the efficient production of nAb to establish persistent infection. The virus-induced expression of CD70, which was mainly on B and T cells, resulted in CD27-dependent destruction of the splenic architecture and suppression of nAb responses.
Intact secondary lymphatic organ structure is important in the generation of antibody responses (23). The marginal zone and marginal zone B cells seem to be important in the initiation of antibody responses against blood-borne pathogens (33, 34). We found that the splenic marginal zone and germinal centers were virtually absent and that numbers of marginal zone B cells were reduced in BL/6 mice during acute LCMV infection. Immunopathology in the spleen was provoked by IFNγ and TNFα secreted by CD4+ T cells. Moreover, CD27 ligation on LCMV-specific CD4+ T cells increased the secretion of IFNγ and TNFα, suggesting that CD27-dependent immunopathology is induced by the increased secretion of these inflammatory cytokines. This conclusion is supported by the observation that B cell numbers in CD70 transgenic mice progressively decrease in primary and secondary lymphoid organs as a result of the CD27-induced production of IFNγ by T cells (24). Likewise, cytokines that bind to receptors for TNF and IFN type I or type I/II are involved in the destruction of the splenic architecture after repetitive CpG treatment (35). TNFα as well as IFNγ have cytotoxic action when secreted in the vicinity of target cells (36). In addition, IFNγ may render cells more sensitive to TNFα-induced apoptosis (37). Similarly, a TNFα-dependent destruction of splenic architecture has been observed after Leishmania infection (38). Recent data have suggested that after Leishmania infection, the TNFα-mediated loss of stromal cells leads to the reduced production of CCL19 and CCL21, which are both important for maintenance of the marginal zone (39).
Interestingly, it has been demonstrated that B cells are crucial for the maintenance of the splenic marginal zone and that the depletion of B cells in a Cre/lox P system resulted in destruction of the integrity of the marginal zone (40). In our study, LCMV infection resulted in a selective depletion of marginal zone B cells and T1B cells without reducing the number of other B cell subsets or total B cells. Therefore, the destruction of the splenic architecture and marginal zone after LCMV infection is not caused by reduced total B cell numbers. However, the destruction of the splenic architecture may be initiated indirectly by the elimination of B cell subsets, macrophages, or stromal cells, which provide chemokines, integrin ligands, and receptors that are important for the splenic architecture (39, 41, 42).
LCMV infection has been shown to induce an acquired immunosuppression with temporally impaired antibody responses to viral coinfections (21, 27). Preservation of the splenic architecture after LCMV infection in CD27−/− mice also enhanced the nAb response to coinfection with VSV. This indicates that CD27-dependent destruction of the splenic architecture causes a general immunodeficiency, resulting in impaired nAb responses against both LCMV and unrelated viral coinfections. In addition, as a result of polyclonal B cell activation, 90% of the antibodies produced after LCMV infection are not specific for the virus (20). This hypergammaglobulinemia is also observed after HIV and HCV infections (18, 43). Because polyclonal B cell activation is dependent on B cell–T cell interactions (20), the preservation of the splenic architecture in CD27-deficient mice facilitated this interaction and augmented hypergammaglobulinemia.
This study provides evidence that blocking the CD27–CD70 interaction may be an attractive strategy to prevent chronic infection after virus inoculation. This finding was surprising because, as outlined in the Introduction, different mechanisms, including structural aspects of LCMV glycoprotein, contribute to the delayed and inefficient generation of nAbs. The time frame in which blocking CD27 signaling reduces immunopathology and improves antibody responses may be dependent on characteristics of the viral infection, such as replication kinetics and infectious dose. For LCMV infection, we showed that CD70 is expressed at high levels up to 20 d after infection. At later time points, CD70 was no longer detectable, and the splenic architecture was restored. Therefore, in this infectious model, αCD70 mAb treatment had to be started during acute infection and was inefficient when started 35 d after infection. Nevertheless, αCD70 mAb treatment was efficient when started several days after infection at a time point when LCMV replicated to high titers and was clearly detectable in blood samples.
Further studies will be necessary to evaluate whether our findings can be generalized to other types of viral infections that are characterized by a late and inefficient induction of nAbs, such as HIV and HCV (6, 7). In analogy to LCMV, structural factors of the surface glycoproteins limit the efficient induction of nAb against HIV (4, 44). Furthermore, the frequency of B cells with neutralizing B cell receptors to HIV is low, probably as a result of cross-reactivity to self-antigens, leading to the depletion of specific B cells from the repertoire (45). Interestingly, CD70 is overexpressed on B cells and T cells in active HIV infection, suggesting a possible role for this costimulatory molecule in the induction of immunopathology and suppression (17, 18). In fact, immunopathology of the splenic architecture with the reduction of marginal zone B cells has been shown in HIV infection (46).
In summary, blocking CD27 interaction is a novel strategy for therapeutically influencing persistent virus infections by reducing immunopathology and acquired immunosuppression and, thereby, enhancing nAb production. Our findings may affect the treatment of clinically relevant chronic infections that are characterized by the delayed production of nAbs in conjunction with the prolonged expression of CD70.
MATERIALS AND METHODS
Mice.
BL/6 mice were obtained from Harlan, and CD27−/− (47) mice were obtained from the Netherlands Cancer Institute. SMARTA (48), P14 (49), T11μMT (28), and Jh −/− (50) mice were obtained from the Institute for Laboratory Animals (Zurich, Switzerland), and TNFα−/− (51) mice were obtained from C. Müller (Division of Immunopathology, University of Berne, Bern, Switzerland). IFNγ−/− (B6.129S7-Ifngtms1Ts) was purchased from the Jackson ImmunoResearch Laboratories. All animal experiments were performed with 6–8-wk-old age- and sex-matched mice at the central animal facility of the University of Berne (Bern, Switzerland) and were approved by the Experimental Animal Committee of the Canton of Berne.
Viruses and peptides.
LCMV-WE and -Docile isolates were obtained from R.M. Zinkernagel (Institute of Experimental Immunology, University of Zurich, Zurich, Switzerland) and were propagated in L929 fibroblasts and Madin–Darby canine kidney cells, respectively. Mice were infected i.v. with 106 pfu of LCMV-WE or 2 × 107 pfu LCMV-Docile in all experiments. VSV-IND and VSV-NJ isolates were obtained from R.M. Zinkernagel and were grown in BHK21 cells. Mice were infected i.v. with 2 × 106 pfu VSV-IND or -NJ in all experiments. For UV inactivation, VSV-NJ was irradiated (G15W; Osram Sylvania) for 30 s at a distance of 5 cm in a thin layer of liquid in a 35-mm petri dish. UV inactivation was tested in a focus-forming assay. The LCMV glycoprotein peptide amino acids gp33 (KAVYNFATM), np396 (FQPQNGQFI), and p13 (GLNGPDIYKGVYQFKSVEFD) were purchased from NeoMPS SA.
Detection of virus and nAb titers.
The detection of LCMV virus titer and nAbs has been described previously (21, 52). Neutralizing total (IgM and IgG) and IgG antibodies to VSV as well as virus titer were measured as described previously (23).
51Cr release assay.
51Cr release assays directly ex vivo or after restimulation in vitro were performed as described previously (23).
Administration of αCD70.
Mice were treated i.p. with 300 μg αCD70 (FR70; reference 30) twice a week from day 4 until day 35 or from day 35 until day 60. Rat IgG (Sigma-Aldrich) was used as a control.
Immunohistochemistry.
Freshly removed organs were immersed in Hank's balanced salt solution and snap frozen in liquid nitrogen. Tissue sections of 5-μm thickness were cut in a cryostat and treated as described previously (23). Tissue sections were stained with rat monoclonal antibodies against the following antigens: IgM (clone R6-60.2), CD21/35 (clone 7G6), follicular dendritic cells (clone FDC-M1; all from BD Biosciences), and metallophilic marginal zone macrophages (MOMA-1; BMA Biomedicals AG). Lectin staining of follicular center cells was performed with biotinylated peanut agglutinin (PNA; Vector Laboratories) followed by preformed alkaline phosphatase–labeled avidin–biotin complexes (DakoCytomation).
Flow cytometry.
All antibodies were obtained from eBioscience except MHC class I (H-2Db) tetramers complexed with gp33 or np396, which were purchased from ProImmune, and αVα2, αCD21/35, and αIgD, which were obtained from BD Biosciences. For intracellular staining, lymphocytes (106/well) were stimulated for 5 h with gp33 or np396 peptide (10−6 M/well) in the presence of recombinant 25 U/ml IL-2 and 5 μg/ml brefeldin A (Sigma-Aldrich). Cells were stained for surface molecules, fixed with 4% paraformaldehyde, and cell membranes were permeabilized with Perm buffer (PBS, 2% FCS, 5 mM EDTA, 0.1% saponin, and 0.2% NaN3) and stained with αIFNγ-FITC. Relative fluorescence intensities were measured using a flow cytometer (FACScan or BD LSRII; Becton Dickinson) and analyzed using FlowJo software (Tree Star). Sorting of live cells was performed using a flow cytometry system (FACSVantage; Becton Dickinson).
Cytokine measurement.
106 SMARTA or SMARTA × CD27−/− splenocytes were injected i.v. into BL/6 recipients that were immunized on the following day with LCMV-WE. 8 d after infection, CD4+Vα2+ T cells were sorted by flow cytometry, and 3 × 105 CD4+Vα2+ T cells were cultivated with 5 × 105 gp13-pulsed and irradiated (1,500 rad) naive BL/6 splenocytes in a 96-well plate in a total of 200 μl IMDM supplemented with 10% FCS for 16 h. 120 μl of the supernatant was harvested and analyzed for cytokines using a mouse Fluorokine MAP Multiplex Assay (R&D Systems). An identical experimental setup was used to analyze cytokine secretion by P14 and P14 × CD27−/− splenocytes except that harvesting was performed after 6 h of cultivation.
Adoptive transfer of CD4+ and CD8+ cells.
CD4+ and CD8+ T cells were purified by MACS (Miltenyi Biotec) using αCD4 or αCD8 magnetic beads. 107 CD4+ or CD8+ T cells were injected i.v. into CD27−/− mice that were infected on the same day with LCMV-WE.
Adoptive transfer of CD19+ B cells of T11μMT mice.
CD19+ B cells were purified by MACS (Miltenyi Biotec) using αCD19 magnetic beads. 5 × 106 CD19+ B cells were injected i.v. into CD27−/− and BL/6 mice, which were then infected with LCMV-WE.
Serum protein electrophoresis.
Total serum immunoglobulin was detected by capillary zone electrophoresis using an electrophoresis system (Paragon CZE 2000; Beckman Coulter), and total serum protein was measured on an analyzer (model 917; Hitachi) according to the Biuret method.
Statistical analysis.
Statistical comparison between groups was performed with a two-tailed unpaired Student's t test using Prism 4 software (GraphPad Software). Data was considered statistically significant when the p-value was <0.05.
Online supplemental material.
Fig. S1 shows the control of LCMV-WE in the absence of CD27 signaling in the lung, kidney, and liver. Fig. S2 presents the nAb response to nonreplicating VSV-UV and replication of VSV-NJ in LCMV-WE–infected mice. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20060651/DC1.
Supplemental Material
Acknowledgments
We would like to thank J. Borst (Division of Immunology, Netherlands Cancer Institute, Amsterdam, Netherlands) for providing CD27−/− mice and R.M. Zinkernagel for critically reading the manuscript.
This work was supported by the Swiss National Foundation grant 632-066020, Oncosuisse grant 01312-02-2003, and the Cancer League of the Canton of Bern, Switzerland.
The authors have no conflicting financial interests.
Abbreviations used: HCV, hepatitis C virus; LCMV, lymphocytic choriomeningitis virus; nAb, neutralizing antibody; PNA, peanut agglutinin; VSV, vesicular stomatitis virus.
References
- 1.Bertoletti, A., A. Sette, F.V. Chisari, A. Penna, M. Levrero, and M. De Carli. 1994. Natural variants of cytotoxic epitopes are T cells receptor antagonists for anti-viral cytotoxic T cells. Nature. 369:407–410. [DOI] [PubMed] [Google Scholar]
- 2.Ciurea, A., P. Klenerman, L. Hunziker, E. Horvath, B.M. Senn, A.F. Ochsenbein, H. Hengartner, and R.M. Zinkernagel. 2000. Viral persistence in vivo through selection of neutralizing antibody-escape variants. Proc. Natl. Acad. Sci. USA. 97:2749–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips, R.E., S. Rowland-Jones, D.F. Nixon, F.M. Gotch, J.P. Edwards, A.O. Ogunlesi, J.G. Elvin, J.A. Rothbard, C.R. Bangham, C.R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 354:453–459. [DOI] [PubMed] [Google Scholar]
- 4.Wei, X., J.M. Decker, S. Wang, H. Hui, J.C. Kappes, X. Wu, J.F. Salazar-Gonzalez, M.G. Salazar, J.M. Kilby, M.S. Saag, et al. 2003. Antibody neutralization and escape by HIV-1. Nature. 422:307–312. [DOI] [PubMed] [Google Scholar]
- 5.Lane, H.C., J.M. Depper, W.C. Greene, G. Whalen, T.A. Waldmann, and A.S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79–84. [DOI] [PubMed] [Google Scholar]
- 6.Pilgrim, A.K., G. Pantaleo, O.J. Cohen, L.M. Fink, J.Y. Zhou, J.T. Zhou, D.P. Bolognesi, A.S. Fauci, and D.C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924–932. [DOI] [PubMed] [Google Scholar]
- 7.Netski, D.M., T. Mosbruger, E. Depla, G. Maertens, S.C. Ray, R.G. Hamilton, S. Roundtree, D.L. Thomas, J. McKeating, and A. Cox. 2005. Humoral immune response in acute hepatitis C virus infection. Clin. Infect. Dis. 41:667–675. [DOI] [PubMed] [Google Scholar]
- 8.Odermatt, B., M. Eppler, T.P. Leist, H. Hengartner, and R.M. Zinkernagel. 1991. Virus-triggered acquired immunodeficiency by cytotoxic T-cell- dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA. 88:8252–8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battegay, M., D. Moskophidis, H. Waldner, M.A. Brundler, W.-P. Fung-Leung, T.W. Mak, H. Hengartner, and R.M. Zinkernagel. 1993. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 151:5408–5415. [PubMed] [Google Scholar]
- 10.Hangartner, L., B.M. Senn, B. Ledermann, U. Kalinke, P. Seiler, E. Bucher, R.M. Zellweger, K. Fink, B. Odermatt, K. Burki, et al. 2003. Antiviral immune responses in gene-targeted mice expressing the immunoglobulin heavy chain of virus-neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 100:12883–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwong, P.D. 2005. Human immunodeficiency virus: refolding the envelope. Nature. 433:815–816. [DOI] [PubMed] [Google Scholar]
- 12.Pinschewer, D.D., M. Perez, E. Jeetendra, T. Bachi, E. Horvath, H. Hengartner, M.A. Whitt, J.C. de la Torre, and R.M. Zinkernagel. 2004. Kinetics of protective antibodies are determined by the viral surface antigen. J. Clin. Invest. 114:988–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planz, O., S. Ehl, E. Furrer, E. Horvath, M.A. Brundler, H. Hengartner, and R.M. Zinkernagel. 1997. A critical role for neutralizing-antibody-producing B cells, CD4(+) T cells, and interferons in persistent and acute infections of mice with lymphocytic choriomeningitis virus: implications for adoptive immunotherapy of virus carriers. Proc. Natl. Acad. Sci. USA. 94:6874–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borst, J., J. Hendriks, and Y. Xiao. 2005. CD27 and CD70 in T cell and B cell activation. Curr. Opin. Immunol. 17:275–281. [DOI] [PubMed] [Google Scholar]
- 15.Akiba, H., H. Nakano, S. Nishinaka, M. Shindo, T. Kobata, M. Atsuta, C. Morimoto, C.F. Ware, N.L. Malinin, D. Wallach, et al. 1998. CD27, a member of the tumor necrosis factor receptor superfamily, activates NF-kappaB and stress-activated protein kinase/c-Jun N-terminal kinase via TRAF2, TRAF5, and NF-kappaB-inducing kinase. J. Biol. Chem. 273:13353–13358. [DOI] [PubMed] [Google Scholar]
- 16.Tesselaar, K., R. Arens, G.M. van Schijndel, P.A. Baars, M.A. van der Valk, J. Borst, M.H. van Oers, and R.A. van Lier. 2003. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat. Immunol. 4:49–54. [DOI] [PubMed] [Google Scholar]
- 17.Hazenberg, M.D., S.A. Otto, B.H. van Benthem, M.T. Roos, R.A. Coutinho, J.M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 17:1881–1888. [DOI] [PubMed] [Google Scholar]
- 18.De Milito, A., A. Nilsson, K. Titanji, R. Thorstensson, E. Reizenstein, M. Narita, S. Grutzmeier, A. Sonnerborg, and F. Chiodi. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 103:2180–2186. [DOI] [PubMed] [Google Scholar]
- 19.Xiao, Y., J. Hendriks, P. Langerak, H. Jacobs, and J. Borst. 2004. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J. Immunol. 172:7432–7441. [DOI] [PubMed] [Google Scholar]
- 20.Hunziker, L., M. Recher, A.J. Macpherson, A. Ciurea, S. Freigang, H. Hengartner, and R.M. Zinkernagel. 2003. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 4:343–349. [DOI] [PubMed] [Google Scholar]
- 21.Recher, M., K.S. Lang, L. Hunziker, S. Freigang, B. Eschli, N.L. Harris, A. Navarini, B.M. Senn, K. Fink, M. Lotscher, et al. 2004. Deliberate removal of T cell help improves virus-neutralizing antibody production. Nat. Immunol. 5:934–942. [DOI] [PubMed] [Google Scholar]
- 22.Matter, M., S. Mumprecht, D.D. Pinschewer, V. Pavelic, H. Yagita, S. Krautwald, J. Borst, and A.F. Ochsenbein. 2005. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur. J. Immunol. 35:3229–3239. [DOI] [PubMed] [Google Scholar]
- 23.Karrer, U., A. Althage, B. Odermatt, C.W.M. Roberts, S. Korsmeyer, S. Miyawaki, H. Hengartner, and R.M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185:2157–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arens, R., K. Tesselaar, P.A. Baars, G.M. van Schijndel, J. Hendriks, S.T. Pals, P. Krimpenfort, J. Borst, M.H. van Oers, and R.A. van Lier. 2001. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 15:801–812. [DOI] [PubMed] [Google Scholar]
- 25.Arens, R., M.A. Nolte, K. Tesselaar, B. Heemskerk, K.A. Reedquist, R.A. van Lier, and M.H. van Oers. 2004. Signaling through CD70 regulates B cell activation and IgG production. J. Immunol. 173:3901–3908. [DOI] [PubMed] [Google Scholar]
- 26.Kobata, T., S. Jacquot, S. Kozlowski, K. Agematsu, S.F. Schlossman, and C. Morimoto. 1995. CD27-CD70 interactions regulate B-cell activation by T cells. Proc. Natl. Acad. Sci. USA. 92:11249–11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roost, H., S. Charan, R. Gobet, E. Rüedi, H. Hengartner, A. Althage, and R.M. Zinkernagel. 1988. An acquired immune suppression in mice caused by infection with lymphocytic choriomeningitis virus. Eur. J. Immunol. 18:511–518. [DOI] [PubMed] [Google Scholar]
- 28.Senn, B.M., C. Lopez-Macias, U. Kalinke, A. Lamarre, A. Isibasi, R.M. Zinkernagel, and H. Hengartner. 2003. Combinatorial immunoglobulin light chain variability creates sufficient B cell diversity to mount protective antibody responses against pathogen infections. Eur. J. Immunol. 33:950–961. [DOI] [PubMed] [Google Scholar]
- 29.Moskophidis, D., F. Lechner, H. Pircher, and R.M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 362:758–761. [DOI] [PubMed] [Google Scholar]
- 30.Oshima, H., H. Nakano, C. Nohara, T. Kobata, A. Nakajima, N.A. Jenkins, D.J. Gilbert, N.G. Copeland, T. Muto, H. Yagita, and K. Okumura. 1998. Characterization of murine CD70 by molecular cloning and mAb. Int. Immunol. 10:517–526. [DOI] [PubMed] [Google Scholar]
- 31.Oxenius, A., R.M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus- specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity. 9:449–457. [DOI] [PubMed] [Google Scholar]
- 32.Burton, D.R., R.C. Desrosiers, R.W. Doms, W.C. Koff, P.D. Kwong, J.P. Moore, G.J. Nabel, J. Sodroski, I.A. Wilson, and R.T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233–236. [DOI] [PubMed] [Google Scholar]
- 33.Ochsenbein, A.F., and R.M. Zinkernagel. 2000. Natural antibodies and complement link innate and acquired immunity. Immunol. Today. 21:624–630. [DOI] [PubMed] [Google Scholar]
- 34.Pillai, S., A. Cariappa, and S.T. Moran. 2005. Marginal zone B cells. Annu. Rev. Immunol. 23:161–196. [DOI] [PubMed] [Google Scholar]
- 35.Heikenwalder, M., M. Polymenidou, T. Junt, C. Sigurdson, H. Wagner, S. Akira, R. Zinkernagel, and A. Aguzzi. 2004. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 10:187–192. [DOI] [PubMed] [Google Scholar]
- 36.Barry, M., and R.C. Bleackley. 2002. Cytotoxic T lymphocytes: all roads lead to death. Nat. Rev. Immunol. 2:401–409. [DOI] [PubMed] [Google Scholar]
- 37.Suk, K., S. Kim, Y.H. Kim, K.A. Kim, I. Chang, H. Yagita, M. Shong, and M.S. Lee. 2001. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J. Immunol. 166:4481–4489. [DOI] [PubMed] [Google Scholar]
- 38.Engwerda, C.R., M. Ato, S.E. Cotterell, T.L. Mynott, A. Tschannerl, P.M. Gorak-Stolinska, and P.M. Kaye. 2002. A role for tumor necrosis factor-alpha in remodeling the splenic marginal zone during Leishmania donovani infection. Am. J. Pathol. 161:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ato, M., H. Nakano, T. Kakiuchi, and P.M. Kaye. 2004. Localization of marginal zone macrophages is regulated by C-C chemokine ligands 21/19. J. Immunol. 173:4815–4820. [DOI] [PubMed] [Google Scholar]
- 40.Nolte, M.A., R. Arens, M. Kraus, M.H. van Oers, G. Kraal, R.A. van Lier, and R.E. Mebius. 2004. B cells are crucial for both development and maintenance of the splenic marginal zone. J. Immunol. 172:3620–3627. [DOI] [PubMed] [Google Scholar]
- 41.Lu, T.T., and J.G. Cyster. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 297:409–412. [DOI] [PubMed] [Google Scholar]
- 42.Karlsson, M.C., R. Guinamard, S. Bolland, M. Sankala, R.M. Steinman, and J.V. Ravetch. 2003. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 198:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawamoto, H., K. Sakaguchi, A. Takaki, S. Ogawa, and T. Tsuji. 1993. Autoimmune responses as assessed by hypergammaglobulinemia and the presence of autoantibodies in patients with chronic hepatitis C. Acta Med. Okayama. 47:305–310. [DOI] [PubMed] [Google Scholar]
- 44.Kwong, P.D., M.L. Doyle, D.J. Casper, C. Cicala, S.A. Leavitt, S. Majeed, T.D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, et al. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 420:678–682. [DOI] [PubMed] [Google Scholar]
- 45.Haynes, B.F., J. Fleming, E.W. St Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R.M. Scearce, K. Plonk, H.F. Staats, et al. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 308:1906–1908. [DOI] [PubMed] [Google Scholar]
- 46.Wilkins, B.S., Z. Davis, S.B. Lucas, G. Delsol, and D.B. Jones. 2003. Splenic marginal zone atrophy and progressive CD8+ T-cell lymphocytosis in HIV infection: a study of adult post-mortem spleens from Cote d'Ivoire. Histopathology. 42:173–185. [DOI] [PubMed] [Google Scholar]
- 47.Hendriks, J., L.A. Gravestein, K. Tesselaar, R.A. van Lier, T.N. Schumacher, and J. Borst. 2000. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 1:433–440. [DOI] [PubMed] [Google Scholar]
- 48.Oxenius, A., M.F. Bachmann, R.M. Zinkernagel, and H. Hengartner. 1998. Virus-specific MHC class II-restricted TCR-transgenic mice: effects on humoral and cellular immune response after viral infection. Eur. J. Immunol. 28:390–400. [DOI] [PubMed] [Google Scholar]
- 49.Pircher, H., K. Bürki, R. Lang, H. Hengartner, and R.M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 342:559–561. [DOI] [PubMed] [Google Scholar]
- 50.Chen, J., M. Trounstine, F.W. Alt, F. Young, C. Kurahara, J.F. Loring, and D. Huszar. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647–656. [DOI] [PubMed] [Google Scholar]
- 51.Marino, M.W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, et al. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA. 94:8093–8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Battegay, M., S. Cooper, A. Althage, J. Baenziger, H. Hengartner, and R.M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods. 33:191–198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.