Abstract
Activated protein C (APC) reduces mortality of severe sepsis patients but increases the risk of serious bleeding. APC exerts anticoagulant activity by proteolysis of factors Va/VIIIa. APC also exerts antiinflammatory and antiapoptotic effects and stabilizes endothelial barrier function by APC-initiated cell signaling that requires two receptors, endothelial cell protein C receptor (EPCR) and protease-activated receptor 1 (PAR1). The relative importance of APC's various activities for efficacy in sepsis is unknown. We used protein engineering of mouse APC and genetically altered mice to clarify mechanisms for the efficacy of APC in mouse sepsis models. Mortality reduction in LPS-induced endotoxemia required the enzymatic active site of APC, EPCR, and PAR-1, highlighting a key role for APC's cytoprotective actions. A recombinant APC variant with normal signaling but <10% anticoagulant activity (5A-APC) was as effective as wild-type APC in reducing mortality after LPS challenge, and enhanced the survival of mice subjected to peritonitis induced by gram-positive or -negative bacteria or to polymicrobial peritoneal sepsis triggered by colon ascendens stent implantation. Thus, APC's efficacy in severe sepsis is predominantly based on EPCR- and PAR1-dependent cell signaling, and APC variants with normal cell signaling but reduced anticoagulant activities retain efficacy while reducing the risk of bleeding.
Severe sepsis is a systemic host response to microbial infection, and mortality of severe sepsis patients reaches 30–50%. Recombinant activated protein C (APC) is the only approved drug that reduces mortality of adult severe sepsis patients. Recombinant APC resembles an endogenous plasma serine protease with pleiotropic effects on coagulation, fibrinolysis, inflammation, immune cells, and vascular endothelial cells (for review see references 1–3). The antiapoptotic, antiinflammatory, and vascular permeability effects of APC are mediated through engagement of the endothelial cell protein C receptor (EPCR/procr) and secondary activation of the G protein–coupled thrombin receptor, protease-activated receptor 1 (PAR1/F2R) by the APC–EPCR complex (4–9). In contrast, the anticoagulant activity of APC is caused by receptor-independent proteolytic inactivation of coagulation factor Va. The anticoagulant effect associated with APC therapy increases the risk of severe bleeding in septic patients, which imposes an upper limit on the dose of APC administered. In addition, APC is not effective in children or in adults with less than severe sepsis (10–12). The key mechanisms mediating the protective effect of APC in severe sepsis are unclear. It is thought that the efficacy of APC is caused by the combination of its anticoagulant and cell signaling activities. APC-mediated anticoagulation and promotion of fibrinolysis may reduce thrombotic organ damage and perfusion insufficiency associated with severe sepsis, as well as suppress the inflammation-enhancing effect of activated coagulation factors. The EPCR- and PAR1-dependent signaling activity of APC may enhance the viability of lymphocytes and endothelial cells, prevent the deterioration of the microvascular permeability arrier associated with sepsis, and blunt the elaboration of proinflammatory cytokine release by immune and endothelial cells. Results from animal studies and from clinical evaluation of other natural anticoagulants (i.e., antithrombin [13] and tissue factor pathway inhibitor [12]) suggest that the anticoagulant activity of APC, which is the cause of bleeding complications, might not be absolutely necessary for its therapeutic efficacy in patients with severe sepsis. In this study, we distinguish the relative importance of anticoagulation and receptor-dependent signaling via EPCR and PAR1 for APC's efficacy in reducing sepsis mortality.
RESULTS
Protective effect of APC in mouse endotoxemia
As an initial step to clarify the mechanisms by which APC reduces mortality in severe sepsis patients, we examined how APC therapy alters the outcome of mouse endotoxemia. When i.p. doses of LPS causing 50 or 90% mortality (LD50 or LD90, respectively) were given to mice, recombinant mouse WT APC significantly reduced 7-d mortality (Fig. 1, A and B). An i.v. bolus of APC was as effective in reducing 7-d mortality as administration via a 20-min i.v. infusion of the same amount of APC. The recombinant mouse APC variant S360A-APC, which lacks proteolytic activity but retains some anticoagulant potency (14), did not reduce mortality (Fig. 1 A), showing that APC's enzymatic proteolytic activity is essential for mortality reduction. For LPS given at LD90, a single 10-μg APC dose reduced mortality from 90 to 30% (Fig. 1 B), whereas for LPS given at LD50, single 10-μg (0.33 mg/kg) or 2-μg (0.067 mg/kg) APC doses reduced mortality to ≤10% (Fig. 1 A).
Figure 1.
APC reduces the mortality of LPS-induced endotoxemia. 7-d survival of mice (n = 20 per group) infused with 10 μg APC (○), 2 μg APC (Δ), PBS (•), or 10 μg S360A-APC (□; n = 10 per group) over a 20-min period before challenge with an LD50 (A) or LD90 (B) of LPS. The statistical significance of mortality and survival time was determined by the Kaplan-Meyer log-rank test.
To examine the effects of APC treatment on endotoxin-induced apoptosis and vascular permeability, cohorts of animals receiving an LD50 dose of LPS concomitant with either 10 μg APC, 10 μg S360A-APC, or carrier solution (PBS) were killed 24 h after induction of endotoxemia. At this time point, ≥90% of animals in all experimental groups were still alive, but death occurred shortly thereafter in the control groups treated with S360A-APC or only carrier (Fig. 1 A). Treatment with APC, but not with S360A-APC, reduced the number of apoptotic cells in lung, spleen, and liver tissue sections, as detected by Tdt-mediated dUTP-biotin nick-end labeling (TUNEL) staining (Fig. 2, A–G). As confirmed in the liver, reduced apoptosis correlated with a suppression of caspase-3 activity in whole-tissue extracts (Fig. 2 H). APC treatment also preserved vascular endothelial barrier function in the kidney, as demonstrated by measuring the permeability of the vascular bed for fluorescent tracers of different molecular weights (MWs; Fig. 2, I and J). This beneficial APC effect was predominantly observed in small vessels (Fig. 2 K). Similarly, APC-treated animals exhibited significantly reduced lung inflammation, as determined by measurement of myeloperoxidase (MPO) activity in lung extracts (Fig. 2 L).
Figure 2.
APC treatment reduces apoptosis and LPS-induced vascular permeability. Nuclear HOECHST stain (A–C) and TUNEL assay (D–F) on liver sections of control mice (no LPS; A and D), LPS-challenged mice (B and E), and LPS-challenged mice treated with APC (C and F). (G) Quantitative analysis of apoptosis in liver, lung, and spleen tissue. (H) APC treatment reduces caspase-3 activity. All analyses were performed 24 h after LPS challenge. *, P ≤ 0.05 using the Student's t test. (I) Vascular permeability was determined before (no LPS) or 8 h after LPS challenge by infusion of FITC-labeled high MW dextran and rhodamine-labeled low MW dextran. (J) Quantitation of rhodamine–dextran extravasation by morphometry. (K) Permeability barrier protection by APC occurs predominantly in smaller vessels. *, P ≤ 0.05 compared with LPS, as determined by the Student's t test. (L) MPO activity as a marker for neutrophil activity in lung tissue 24 h after LPS challenge. APC reduces lung inflammation. *, P ≤ 0.01 compared with PBS control lungs; and #, P ≤ 0.03 compared with LPS challenged lungs, as determined by the Student's t test. Data represent the mean ± SD of 10 randomly chosen fields per section, with five sections per mouse and five mice each in the treatment and control groups. Bars, 100 μm.
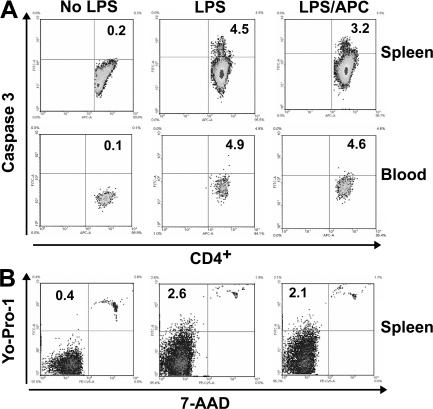
LPS exposure caused activation of the coagulation and fibrinolytic systems, platelet consumption, stimulation of inflammatory cytokine responses, reduced numbers of circulating lymphocytes and monocytes, and increased numbers of granulocytes (Table S1, available at http://www.jem.org/cgi/content/full/jem.20070404/DC1). Circulating immune cells exhibited increased evidence of apoptosis, as judged by caspase-3 activation, and increased membrane permeability (Fig. 3, A and B and Tables I and II). Differential blood cell counts; histopathology of lung, liver, and spleen (Fig. S1, A–I); composition and abundance of immune cell populations in peripheral blood and spleen (Table S1); or the extent of lymphocyte apoptosis (Fig. 3, A and B and Tables I and II) were not significantly affected by treatment with APC or S360A-APC.
Figure 3.
APC effect on lymphocyte apoptosis. Representative example of apoptosis detection in lymphocyte subpopulations as detected by FITC-labeled caspase-3 antibody (A), or double staining with YO-PRO-1 and 7-AAD nuclear stains (B). Analysis was performed 24 h after administration of LPS, PBS, or LPS and APC. Equivalent experiments were used to generate the data summarized in Tables I and II. APC does not reduce lymphocyte apoptosis. Numbers indicate the percentage of positive cells.
Table I.
Lymphocyte apoptosis using FITC-labeled caspase-3 antibody
| No LPS | LPS | LPS/APC | ||
|---|---|---|---|---|
| Spleen tissue | ||||
| T lymphocytes | CD4+ | 0.97 ± 0.21 | 4.98 ± 0.74*** | 3.45 ± 0.54 |
| CD8+ | 1.03 ± 0.21 | 9.18 ± 1.44*** | 7.21 ± 2.3 | |
| B lymphocytes | B220+ | 0.83 ± 0.32 | 4.8 ± 0.82*** | 3.07 ± 1.32 |
| Granulocytes | GR1+ | 0.27 ± 0.21 | 1.61 ± 0.95* | 2.08 ± 0.84 |
| Peripheral blood | ||||
| T lymphocytes | CD4+ | 0.3 ± 0.2 | 8.19 ± 1.67*** | 5.61 ± 1.94 |
| CD8+ | 0.46 ± 0.3 | 9.14 ± 9.42* | 8.97 ± 7.62 | |
| B lymphocytes | B220+ | 0.6 ± 0.33 | 19.4 ± 11.7* | 9.72 ± 9.68 |
| Granulocytes | GR1+ | 0.01 ± 0.01 | 0.24 ± 0.29* | 0.3 ± 0.2 |
All collections were performed 24 h after LPS challenge and APC treatment . *, P ≤ 0.05; and ***, P ≤ 0.001 compared with PBS control mice using the Student's t test.
Table II.
Lymphocyte apoptosis using YO-PRO-1 nuclear stain
| No LPS | LPS | LPS/APC | ||
|---|---|---|---|---|
| Spleen tissue | ||||
| T lymphocytes | CD4+ | 0.61 ± 0.37 | 2.20 ± 0.56** | 4.57 ± 3.48 |
| CD8+ | 1.36 ± 0.72 | 4.44 ± 1.01** | 7.11 ± 4.47 | |
| B lymphocytes | B220+ | 1.24 ± 0.07 | 6.42 ± 0.88*** | 7.43 ± 5.17 |
| Granulocytes | GR1+ | 1.03 ± 0.21 | 5.05 ± 2.37* | 7.61 ± 3.93 |
| Peripheral blood | ||||
| T lymphocytes | CD4+ | 0.23 ± 0.06 | 1.69 ± 0.66* | 2.67 ± 0.68 |
| CD8+ | 1.32 ± 0.28 | 7.04 ± 5.73* | 14.4 ± 10.2 | |
| B lymphocytes | B220+ | 0.66 ± 0.14 | 4.45 ± 2.23* | 5.04 ± 2.71 |
| Granulocytes | GR1+ | 0.07 ± 0.06 | 0.09 ± 0.34 | 1.28 ± 1.07 |
All collections were performed 24 h after LPS challenge and APC treatment . *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001 compared with PBS control mice using the Student's t test.
At 24 h after LPS administration, mean plasma levels of 19 out of 23 assayed cytokines were somewhat lower in the APC-treated group, and the putative effects of APC treatment were found for IL-12p40/IL-12p70, IL-4, IL-5, Il-10, GM-CSF, G-CSF, IFN-γ, and KC (Table S1). However, correction for multivariate testing rendered all differences statistically nonsignificant. The effects of APC on the early cytokine response to LPS were determined in additional cohorts of mice at 3 h after LPS challenge, and data were analyzed with respect to 7-d survival. For these comparisons, 8 out of 10 APC-treated mice and 1 out of 10 PBS-infused mice were 7-d survivors. A tentative correlation between treatment and survival was observed for changes in IL-12p40/IL-12p70, GM-CSF, IFN-γ, IL-17, RANTES, and IL-5 (Table S2, available at http://www.jem.org/cgi/content/full/jem.20070404/DC1), but correcting for multivariate testing rendered the differences nonsignificant.
In summary, APC reduces mortality in mouse models of lethal endotoxemia, diminishes the abundance of apoptotic cells in organs, and preserves the vascular permeability barrier, and these beneficial effects of APC require its proteolytic activity. On the other hand, we were not able to document a robust effect of APC treatment on the plasma abundance of various inflammatory cytokines, or on the viability or composition and abundance of lymphocytes.
Therapeutic efficacy of APC requires functional EPCR and PAR1
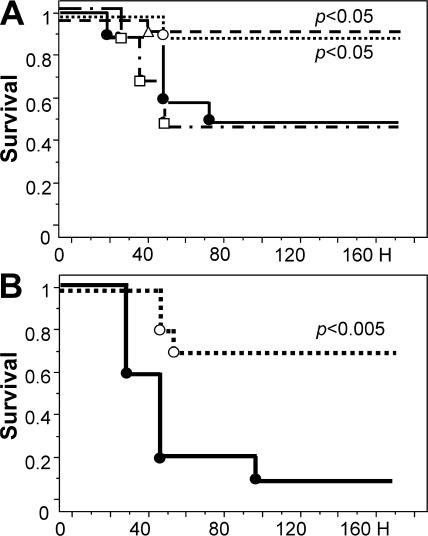
To clarify the importance of the candidate receptors EPCR and PAR1 for the therapeutic efficacy of APC, the ability of APC to reduce LPS-induced mortality was determined in genetically altered mice, designated EPCRδ/δ mice, expressing greatly diminished amounts of EPCR (<10% of cell-surface expression; reference 15) and in knockout mice completely devoid of PAR1 (PAR1−/− mice) (16, 17). Without APC treatment, the 7-d mortality and survival time of PAR1−/− mice after challenge with an LD50 of LPS were indistinguishable from those of WT mice. APC treatment of PAR1−/− mice challenged with an LD50 of LPS reproducibly prolonged the time of survival (P < 0.05) but did not affect 7-d mortality (Fig. 4 A). Replicating earlier observations (18), EPCRδ/δ mice displayed somewhat higher mortality (80%) in response to an LPS dose producing 50% mortality in WT or PAR1−/− mice (Fig. 4 B). Treatment of LPS-challenged EPCRδ/δ mice with APC did not alter the time of survival or 7-d mortality (Fig. 4 B).
Figure 4.
Endotoxemia mortality reduction by APC requires EPCR and PAR1. (A) Survival of PAR1−/− mice (n = 17 per group) infused with 10 μg APC (○) or PBS (•), followed LPS-challenge. APC treatment of PAR1−/− mice prolongs survival time (P < 0.05). (B) Survival of mice (n = 12 per group) with reduced EPCR function (EPCRδ/δ) infused over 20 min with 10 μg APC (○) or PBS (•), followed by LPS challenge. The statistical significance of mortality and survival time was determined by the Kaplan-Meyer log-rank test.
At 24 h after LPS challenge, thrombin–antithrombin complex (TAT) levels and platelet consumption in PAR1−/− and EPCRδ/δ mice were not different from those measured in WT mice (Table S3, available at http://www.jem.org/cgi/content/full/jem.20070404/DC1).d-dimer levels were significantly increased in EPCRδ/δ and PAR1−/− mice compared with WT mice. APC treatment of EPCRδ/δ, but not of PAR1−/−, mice reducedd-dimer levels to a similar extent seen for WT mice (Table S3). The cytokine profile of LPS-challenged EPCRδ/δ mice at 24 h was similar to that of WT mice. Of note, APC treatment of LPS-challenged EPCRδ/δ, but not of PAR1−/−, mice elicited a highly significant 10- and 7-fold augmentation of IL-6 and monocyte chemoattractant protein 1 levels, respectively (Table S3).
These results document that normal expression of EPCR and PAR1 is essential for mortality reduction by APC in mouse models of lethal endotoxemia. On the other hand, the reproducible prolongation of survival time in APC-treated PAR1−/− mice, which is not observed in EPCRδ/δ mice, indicates that some beneficial, EPCR-dependent effects of APC do not require PAR1.
APC variants with reduced anticoagulant, but preserved signaling, function prevent endotoxemia mortality
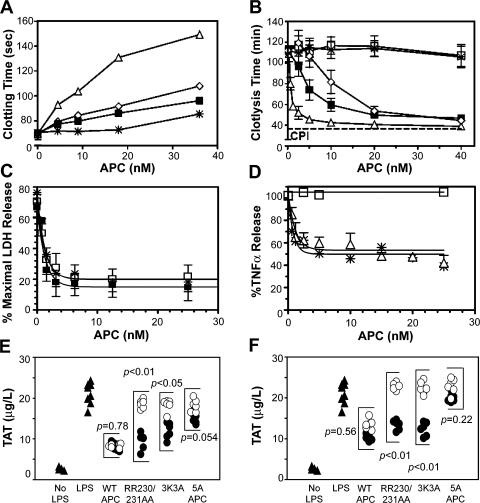
The above observations (see previous section) demonstrate that EPCR and PAR1, and in extension the cell signaling activity of APC mediated by these receptors, are required for the ability of APC to reduce mortality. To determine whether this pathway is not only necessary but also sufficient for APC's therapeutic efficacy, we tested the effect of APC variants with greatly diminished anticoagulant activity but preserved receptor-mediated cytoprotective function on endotoxemia survival. Alanine substitutions in two surface loops of human APC diminish its anticoagulant activity while preserving EPCR- and PAR1-dependent direct effects on cells (19). In this case, corresponding variants of recombinant mouse APC were prepared: (a) 230/231-APC (RR230/231AA-APC), (b) 3K3A-APC (KKK192-194AAA-APC), and (c) 5A-APC (combining the RR230/231AA and KKK192-194AAA substitutions). These variants exhibited anticoagulant activities of 25, 33, and <8% of WT APC, respectively (Fig. 5 A). The relative profibrinolytic activity of each APC molecule was similar to its anticoagulant activity (Fig. 5 B), because the major mechanism for APC-mediated profibrinolytic activity in this assay involves inhibition of thrombin-dependent activation of thrombin-activatable fibrinolysis inhibitor. 3K3A-APC and 5A-APC retained normal antiapoptotic activity in assays of staurosporine-induced apoptosis of endothelial cells (Fig. 5 C), and 5A-APC exhibited normal antiinflammatory activity in assays of LPS-stimulated TNF-α release (Fig. 5 D).
Figure 5.
Anticoagulant and cytoprotective activities of mouse APC variants. (A) Anticoagulant activity of recombinant mouse WT APC (Δ) and APC variants (230/231-APC [◊], 3K3A-APC [▪], and 5A-APC [*]) determined in vitro in plasma. (B) Profibrinolytic activity of WT APC (Δ) and APC variants (230/231-APC [◊], 3K3A-APC [▪], 5A-APC [*], and S360A-APC [□]). (C) Antiapoptotic activity of WT APC (Δ) and APC variants (3K3A-APC [▪] and 5A-APC [*]) in assays of staurosporin-induced (0.25 μM, 24 h) mouse endothelial cell apoptosis. (D) Antiinflammatory activity of WT APC (Δ), WT protein C zymogen (□), and 5A-APC (*) induced in mouse Raw264.7 macrophage-like cells. Data represent the mean ± SD. (E and F) APC effect on in vivo thrombin generation in endotoxin-challenged mice. Endotoxin-challenged mice (n = 6 per group) were infused with PBS (▴), 10 (•) or 2 (○) μg WT APC, or APC variants, and thrombin generation was determined at 2 h (E) and at 30 min (F) after APC infusion by measuring plasma levels of TAT complex. Administration of 2 μg 5A-APC did not significantly alter in vivo thrombin generation.
The in vivo anticoagulant activity of APC variants was determined by monitoring TAT levels after LPS-induced endotoxemia. A 10-μg bolus of WT APC given simultaneously with LPS suppressed circulating TAT at 2 h; the same dose of 5A-APC had no significant anticoagulant effect, whereas 10 μg of RR230/231AA-APC and 3K3A-APC showed intermediate TAT reductions (Fig. 5 E). A lower WT APC dose (2 μg) markedly reduced TAT, whereas RR230/231AA-APC and 3K3A-APC had intermediate anticoagulant effects, and 5A-APC had no anticoagulant effect (Fig. 5 E). Similar results were obtained when TAT was measured 30 min after administration of LPS and APC (Fig. 5 F), indicating that the relative in vivo anticoagulant potency of APC variants mirrors their relative in vitro anticoagulant potency.
RR230/231AA-APC and the related variant, 5A-APC, were similarly effective as WT APC in reducing LPS-induced mortality (unpublished data). Notably, 5A-APC at 10 or 2 μg significantly reduced 7-d mortality (Fig. 6 A), even when administered as a single bolus 3 h after LPS challenge (Fig. 6 B).
Figure 6.
5A-APC reduces mortality of endotoxemia. (A) Survival of mice (n = 10 per group) that were infused with PBS (•, control), 10 μg 5A-APC (□), or 2 μg (▪) 5A-APC for 20 min, followed by a 40-mg/kg i.p. injection of LPS. (B) Survival of mice (n = 10 per group) that were given a 10-μg i.v. bolus of 5A-APC (○) or PBS (□) 3 h after receiving 40 mg/kg LPS via i.p. injection. 5A-APC reduces mortality. The statistical significance of mortality reduction by APC treatment was determined by the Kaplan-Meyer log-rank test.
This suggests that a >10-fold reduction of APC anticoagulant activity, while retaining its ability to engage EPCR and PAR1, does not eliminate APC's capacity to reduce mortality of endotoxemia; by extension, one may conclude that the interaction of APC with EPCR and PAR1 is not only essential but also sufficient to replicate the treatment benefit conveyed by normal, fully anticoagulant APC.
5A-APC prevents mortality of lethal bacterial sepsis
Experimental endotoxemia induced by LPS evokes some inflammatory responses characteristic for bacterial sepsis, but the associated pathogenesis occurs entirely independent of the presence of the actual pathogen. We therefore examined whether the receptor-mediated effects of 5A-APC are also sufficient to reduce mortality in three models of sepsis triggered by live pathogens, i.e., i.p. infection with gram-positive Staphylococcus aureus, with gram-negative Escherichia coli O55:B5 that was the source of the LPS preparations used in the endotoxemia models, and in a model of focal polymicrobial peritonitis with endogenous, fecal microorganisms triggered by colon ascendens stent implantation.
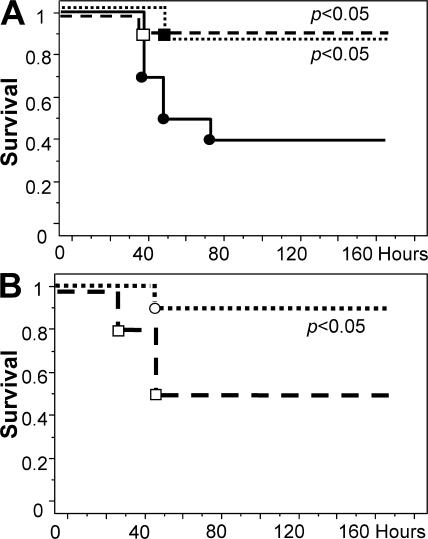
Administration of a single dose of either 10 or 2 μg 5A-APC immediately after infection with S. aureus did not alter survival time or overall mortality (Fig. 7 A). In contrast, two consecutive doses of 5A-APC administered 3 and 10 h after infection significantly reduced mortality (Fig. 7 B). A comparable reduction of mortality was achieved when the first of two doses was administered concomitant with bacterial inoculation (unpublished data). Corresponding results were obtained after infection with E. coli, as two consecutive 2-μg doses of 5A-APC administered at the time of infection and 10 h thereafter significantly improved survival (Fig. 7 C). Likewise, administration of two consecutive 2-μg doses of 5A-APC 3 and 10 h after colon ascendens stent peritonitis (CASP) induction significantly improved survival (S360A-APC, 9 survivors; 5A-APC, 17 survivors; n = 22 per group; Fig. 7 D).
Figure 7.
5A-APC reduces mortality in bacterial sepsis. (A) Survival of mice (n = 10 per group) given either a 10-μg (□) or 2-μg (▪) i.v. bolus of 5A-APC or PBS (•, control), followed immediately by i.p. infection with 108 S. aureus. (B) Survival of mice (n = 20 per group) given a 2-μg i.v. bolus of either 5A-APC (▪) or PBS (•, control) 3 and 10 h after i.p. infection with 108 S. aureus. (C) Survival of mice (n = 20 per group) given a 2-μg i.v. bolus of either 5A-APC (▪) or PBS (•, control) at 3 and 10 h after i.p. infection with 2.5 × 108 E. coli (O55:B5). (D) Survival of mice (n = 22 per group) after CASP. Mice were given a 2-μg i.v. bolus of either 5A-APC (▪) or S360A-APC (•) at 3 and 10 h after CASP. The statistical significance of mortality reduction by APC treatment was determined by the Kaplan-Meyer log-rank test.
These data document that APC variants, such as 5A-APC, with selectively reduced anticoagulant but near-normal cell signaling potency reduce mortality associated with bacterial sepsis to a similar extent as in mouse models of LPS-induced endotoxemia.
DISCUSSION
Our studies imply that the cell signaling activities of APC, mediated by the receptors EPCR and PAR1, are much more important for APC's ability to reduce sepsis mortality than its anticoagulant activities. These findings may help resolve the debate about the controversial role of APC-initiated cell signaling mechanisms in sepsis (20–23). Based on mechanistic insights gained from the analysis of mouse endotoxemia, we provide proof of principle that a selective reduction of APC's anticoagulant activity by site-directed mutagenesis retains APC's efficacy in mortality reduction in endotoxemia, as well as in three different models of bacterial sepsis. The latter observation is consistent with the notion that the EPCR and PAR1 dependence of APC efficacy demonstrated for endotoxemia also plays a dominant role in mortality reduction in models of bacterial infection.
A first important implication of our findings is that variants such as 5A-APC may provide a safer alternative for the treatment of patients with severe sepsis by reducing the risk of severe bleeding associated with currently practiced APC therapy. Although our data strongly imply that the EPCR- and PAR1-mediated effects of APC are both necessary and sufficient for mortality reduction in mouse models of endotoxemia and bacterial sepsis, human patients with severe sepsis and multiorgan failure may nevertheless benefit from anticoagulation. In particular, in patients with sepsis complicated by disseminated intravascular coagulation, anticoagulation may reduce mortality risk and reduce morbidity secondary to thrombotic organ damage. The availability of variants such as 5A-APC, in which APC-mediated cell signaling activity required for mortality reduction and anticoagulant potency are dissociated, should enable the managing of APC infusion and anticoagulation as separate therapeutic entities, tailoring each to the specific needs of a given patient. Second, compared with normal APC, the efficacy of APC variants with selectively reduced anticoagulant activity likely can be explored in different modes of administration (i.e., continuous infusion vs. bolus) over a much greater dose range without a concomitant increase in associated bleeding risk. Indeed, although it was found that APC failed to protect septic children and adult patients with less than severe sepsis, there was no attempt of dose optimization in these groups, providing a potential explanation for the lack of APC efficacy. The peak plasma concentrations of APC achieved by bolus injections in our experiments exceed by one to two orders of magnitude the level of APC maintained in septic patients by 96 h of continuous infusion (∼45 ng/ml) (24), approaching the plasma concentration of endogenous protein C. Because protein C zymogen and APC bind EPCR with comparable affinity (25), exogenously administered APC must outcompete endogenous protein C to engage EPCR. This may explain, on the one hand, why patients with low levels of endogenous protein C are most likely to benefit from APC therapy (25); on the other hand, this suggests that engagement of EPCR is at best suboptimal under conditions currently established for APC therapy. Collectively, these considerations provide a strong rationale to reevaluate the optimal dose and mode of administration of APC and variants thereof in septic patients.
The primary, successfully achieved objective of this study was to gain initial insight into the role of receptor-mediated APC effects on sepsis survival. Accordingly, we chose 7-d mortality as the endpoint of our experimental analyses. A pressing, as yet unanswered question remains about the nature of the survival-enhancing downstream effects triggered by APC through EPCR and PAR1. Although we document APC-dependent alterations of coagulation, inflammation, apoptosis, and microvascular permeability in endotoxemia through premortem and survival analyses, it is unclear whether the observed changes can indeed account for the improved survival of APC-treated mice. For example, it appears doubtful whether the rather minor reduction of apoptotic cell abundance in tissue parenchyma suffices to improve survival. In contrast to observations of reduced lymphocyte apoptosis in human patients undergoing APC therapy (26), which could in theory explain the survival-promoting effect of APC (27), we were unable to correlate apoptosis or abundance of lymphocyte subpopulations with APC treatment or survival in mouse models. It remains to be determined whether this discrepancy is caused by species-specific APC effects, differences between mouse endotoxemia and human sepsis, or dosing differences. Likewise, with the exception of APC-treated EPCR-deficient mice, we only observed minor changes in the plasma cytokine profile and only moderate reductions of lung inflammation in APC-treated mice. The drastic APC-induced increase of IL-6 and MCP-1 in EPCR-deficient mice might instead constitute a nonphysiological, experimental artifact reflecting direct engagement of PAR1 by high bolus doses of APC. We note that endothelial-associated EPCR has, at least in mice, the ability to sequester a stable pool of APC that is excluded from the circulation and accounts for approximately one third of the total APC pool (28). Near absence of EPCR from endothelial cells may therefore lead (but only in EPCR-deficient mice) to a further increase of APC available for PAR1 interaction. APC modulation ofd-dimer follows a similar mouse strain–specific pattern, with unknown underlying mechanism and relevance for survival.
The identification of EPCR and PAR1 as the necessary and sufficient mediators of APC's therapeutic benefit, as established by this study, constitutes an essential first step toward the identification of the as yet elusive downstream effects controlled by this signaling pathway that are critical for survival. For example, the importance of the documented endothelial permeability barrier protection by APC for survival of endotoxemia can be examined by testing 5A-APC efficacy in mice with endothelial cell–restricted loss of EPCR or PAR1 function. Identification of the critical cell populations targeted by APC, characterization of APC effects on this cell population, validation of findings in models of bacterial sepsis, and hypothesis-based analysis of human patients will provide further insight into the survival-enhancing function of APC.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were obtained from Charles River Laboratories. Transgenic EPCRδ/δ and PAR1−/− male mice have been previously described (15–17). Animals used for this study had been backcrossed onto an inbred C57BL/6 background. All experiments involving animals were performed in adherence to the National Institutes of Health guidelines on the use of laboratory animals and were approved by the Medical College of Wisconsin's Institutional Animal Care and Use Committee.
Chemicals and reagents.
Recombinant mouse APC, active site–mutant APC (S360A-APC), double-mutant APC (RR230/231AA-APC), and fivepoint-mutant APC (5A-APC) were produced as previously described for human APC (19). Heparin sodium salt and LPS (E. coli O55:B5) were purchased from Sigma-Aldrich.
Infusion of APC.
8–12-wk-old C57BL/6 male mice were weighed to determine the amount of LPS required to achieve a dose of 40 mg/kg. Mice were initially gas sedated in a plastic-enclosed chamber with isoflurane-soaked gauze. The mice were then placed supine with a nose cone and received inhalation anesthesia
The femoral vein was aseptically exposed under inhalation anesthesia with 2% isoflurane/oxygen through a 5-mm incision of the skin. A 0.6-mm (outer diameter) silicone catheter connected to a syringe injector was inserted into the vein and ligated in place. APC, APC variants, or vehicle (PBS) were infused at a rate of 9 μl per minute for 20 min (180 μl total). After infusion, the catheter was removed, the vein was closed by permanent ligation, and the skin wound was closed by suture. LPS was administered immediately thereafter via an i.p. injection. Alternatively, bolus doses of APC, APC variants, or PBS were administered through i.v. injection into the retroorbital venous plexus.
Analysis of hemostatic parameters and plasma cytokine levels.
Blood was obtained through a puncture of the vena cava 10 min after administration of 500 U heparin. Differential blood analysis and platelet counts were determined in a counter (HEMAVET; Drew Scientific). Plasma was prepared and stored at −80°C until use. TAT andd-dimer were measured by ELISA (Enzygnost TAT Micro, Dade Behring; d-di Asserachrome, Diagnostica Stago). Fibrinogen levels were measured by ELISA as previously described (29). Cytokines were measured using a 23-plex Bio-Plex system (Bio-Rad Laboratories) for mouse cytokines, with a reader (Illuminex 200; Bio-Rad Laboratories) and the Bio-Plex manager program (Bio-Rad Laboratories) for analysis.
Flow cytometry.
Flow cytometric analysis of spleen and peripheral blood cells for CD45, CD3, CD4, CD8, Mac-1, B220, Gr1, and NK1.1 was performed on a five-color flow cytometer (LSRII; BD Biosciences) with fluorophor-conjugated antibodies (BD Biosciences). Before analysis, the cells were counted, and samples were adjusted to have the same cell number (2.5 × 107 cells). Analysis was performed using FACScan Diva software (BD Biosciences).
Histology.
Tissue was removed from the mouse without perfusion, placed in 10% neutral-buffered formalin solution, and embedded in paraffin. Microphotographs of hematoxylin and eosin–stained sections were captured using a microscope (Eclipse 600; Nikon) equipped with a digital camera (Spot Insight; Diagnostic Instruments) and analyzed with Spot advanced software (Diagnostic Instruments).
TUNEL assay.
Slides were deparaffinized by incubation overnight at 60°C, followed by xylene treatment. Tissue was hydrolyzed by nanopure water, followed by digestion with 40 μg proteinase K. TUNEL assay was performed using an in situ cell death detection kit (Roche), according to the manufacturer's directions. Tissue sections were counterstained with nuclear HOECHST stain. Images were captured with a fluorescent microscope (Axioskop; Carl Zeiss MicroImaging, Inc.) coupled to a SenSys camera (Roper Scientific) and analyzed using Metamorph software (Molecular Devices). Analysis was performed on 10 randomly chosen fields per section, with five sections per mouse and five mice each in the treatment and control groups.
Caspase-3 activity.
Caspase-3 protease activity in the liver was measured using a caspase-3 colorimetric assay kit (R&D Systems), according to the manufacture's instructions. In brief, whole liver was weighed and homogenized in 1 ml of cell lysis buffer. Homogenates were then centrifuged for 1 min at 10,000 g. The supernatant was incubated with Asp-Glu-Val-Asp-p-nitroanilide (pNA) and reaction buffer for 2 h at 37°C. Levels of the chromophore pNA released by caspase-3 activity were spectrophotometrically quantified. The data were normalized for protein concentration.
Microvascular permeability.
Dextran infusion studies were performed as previously described (30). In brief, FITC-labeled high MW dextran (MW = 200 × 104, 100 mg/kg; Sigma-Aldrich) and rhodamine-labeled low MW dextran (MW = 4 × 104, 100 mg/kg; Sigma-Aldrich) were retroorbitally injected under 2.5% avertin anesthesia 8 h after LPS challenge. 15 min after dextran injection, the animals were killed, and heart, lungs, kidney, and liver were removed and immediately embedded in optimum cutting temperature tissue freezing medium (Fisher Scientific), frozen in liquid nitrogen, and stored at −80°C until further use. 10-μm tissue sections were cut and analyzed on a Nikon microscope equipped with a Spot Insight digital camera, an ultraviolet light source, and filters to visualize FITC and rhodamine fluorescence. All images were acquired with identical camera settings and exposure times. Measurements were made using ImageJ software (National Institutes of Health).
Measurement of apoptosis by flow cytometry.
Flow cytometric analysis of apoptosis on spleen and peripheral blood cells for CD4, CD8, CD25, B220, and Gr1 was performed as previous described (31). In brief, samples were adjusted for cell number (5 × 105 cells) and stained with 250 nmol/liter YO-PRO-1 nuclear stain (Invitrogen) for 20 min in the dark, and 250 nM 7-amino-actinomycin D (7-AAD; BD Biosciences) nuclear stain was added 10 min before acquiring events. Together, these two stains distinguish early and late apoptotic cells from dead and live cells (31). Cells stained with YO-PRO-1 only were considered apoptotic. Cells stained by both YO-PRO-1 and 7-AAD were considered dead. Intracellular caspase-3 was detected by flow cytometry using FITC–anti–active caspase-3 mAb (BD Biosciences), as previously described (31).
MPO activity assay.
MPO activity was measured as a marker of neutrophil activity within lung tissue, as previously described (32). In brief, lungs were washed with sterile PBS, weighed, and homogenized in 2 ml of ice-cold lysis buffer (100 mM NaCl, 20 mM NaPO4, 15 mM EDTA, pH 7.4), and cells were collected by centrifugation and lysed through four freeze–thaw cycles in potassium phosphate buffer, pH 6, containing 0.5% hexadecyltrimethyl ammoniumbromide and 10 mM EDTA. Protein concentrations of the lysate were measured using a bicinchoninic assay and equal amounts of protein lysate were added to each well. MPO activity was determined by measuring the H2O2-dependent oxidation of 3,3′5,5 teramethylbensidine. The reaction was stopped with 2 M sulfuric acid, followed by reading the absorbance at 450 nm. MPO activity was expressed as the change of OD450 per minute. All reagents were purchased from Sigma-Aldrich.
Characterization of recombinant APC variants.
Recombinant mouse APC variants were prepared as previously described for human APC (19). Anticoagulant activity of the APC mutants was measured using the activated partial thromboplastin time. Profibrinolytic activity was measured in in vitro plasma clot lysis assays (33). The clot lysis time of a 10-nM thrombin-induced clot that incorporates 30 U/ml tissue plasminogen activator was defined as the time to reach a half-maximal decrease in turbidity. Antiapoptotic activity of WT APC and APC variants was measured in assays of staurosporin-induced (0.25 μM, 24 h) mouse endothelial cell apoptosis (MS1 cells; American Type Culture Collection). Cytotoxicity was determined by lactate dehydrogenase release (Roche) and expressed as the percentage of maximum release recorded for permeabilized cells. Antiinflammatory activity was determined as the inhibition of TNF-α release (mouse TNF-α ELISA; Invitrogen) by APC of LPS-stimulated (10 ng/ml LPS [E. coli 055:B5] for 4 h) mouse Raw264.7 macrophage-like cells.
Bacterial sepsis models.
S. aureus (American Type Culture Collection) was grown from single-colony isolates on tryptic soy agar (TSA). Isolates were expanded in 5 ml of tryptic soy broth (TSB) overnight at 37°C. 1 ml of overnight culture was diluted into 20 ml of fresh TSB. Logarithmic growth phase cultures were harvested by centrifugation (6,000 rpm for 10min), washed in 10 ml PBS, and resuspended in 1 ml PBS. Cell numbers were determined by dilution plating on TSA. 108 bacteria were used for infection based on previous LD50 experiments.
E. coli (American Type Culture Collection) was prepared in a similar manner as described in the previous paragraph using Luria-Bertani (LB) broth and agar. Cell numbers were determined by dilution plating on LB agar. 2.5 × 108 bacteria were used for infection based on previous LD50 experiments.
The CASP procedure was performed as previously described (34, 35). An 18-gauge venous catheter was prepared by circumferentially incising with a scalpel, sparing only a slim bar ∼2 mm from the orifice. The abdominal wall was opened under general anesthesia through a 1-cm midline incision, and the cecum and ascending colon were exteriorized. The prepared catheter was inserted into the antimesenteric wall of the colon (∼10 mm from the ileocecal valve) via a puncture hole (18-gauge needle) and affixed to the wall with an 8-0 nylon suture. To ensure proper intraluminal positioning of the stent and initiate bacteremia, stool was milked from the cecum into the ascending colon and the stent until a small drop of stool appeared. The abdominal cavity was flushed with 0.5 ml of sterile saline, and the wound was closed by suture.
Data analysis.
All values are expressed as the mean ± SD of mice per experiment. The differences between all groups were analyzed by the Student's t test. Survival curves were analyzed by the Kaplan-Meyer log-rank test. All statistics were performed using the StatView program for Windows (version 5.0; SAS Institute, Inc.).
Online supplemental material.
Tables S1–3 show cytokine data measured at 3 and 24 h after LPS challenge, and hematoxylin and eosin–stained sections of mouse tissue taken 24 h after LPS challenge. Fig. S1 depicts the histopathology of LPS-challenged mice with and without APC treatment. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070404/DC1.
Supplemental Material
Acknowledgments
We thank Dr. S. Coughlin for discussions for this study.
This work was supported by National Institutes of Health grants HL60655 (to H. Weiler); HL31950 and HL52246 (to J.H. Griffin); HL48772 (to N. Mackman); and HL073750 (to F.J. Castellino), as well as the Ziegler Family Chair for Research (H. Weiler), the Kleiderer-Pezold Endowed professorship (F.J. Castellino), and a Basic Research Scholar Award from the American Society of Hematology (to L.O. Mosnier).
J.H. Griffin, L.O. Mosnier, and H. Weiler have pending patent applications related to reagents and technologies described in this study. The authors have no additional conflicting financial interests.
Abbreviations used: 7-AAD, 7-amino-actinomycin D; APC, activated protein C; CASP, colon ascendens stent peritonitis; EPCR, endothelial cell protein C receptor; MPO, myeloperoxidase; MW, molecular weight; PAR1, protease-activated receptor 1; TAT, thrombin–antithrombin complex; TUNEL, Tdt-mediated dUTP-biotin nick-end labeling.
J.H. Griffin and H. Weiler contributed equally to this work.
N. Mackman's present address is Dept. of Medicine, University of North Carolina, Chapel Hill, NC 27599.
References
- 1.Esmon, C.T. 2006. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost. 32(Suppl. 1):49–60. [DOI] [PubMed] [Google Scholar]
- 2.Mosnier, L.O., B.V. Zlokovic, and J.H. Griffin. 2007. The cytoprotective protein C pathway. Blood. 109:3161–3172. [DOI] [PubMed] [Google Scholar]
- 3.Dahlback, B., and B.O. Villoutreix. 2005. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler. Thromb. Vasc. Biol. 25:1311–1320. [DOI] [PubMed] [Google Scholar]
- 4.Joyce, D.E., L. Gelbert, A. Ciaccia, B. DeHoff, and B.W. Grinnell. 2001. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 276:11199–11203. [DOI] [PubMed] [Google Scholar]
- 5.Riewald, M., R.J. Petrovan, A. Donner, B.M. Mueller, and W. Ruf. 2002. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 296:1880–1882. [DOI] [PubMed] [Google Scholar]
- 6.Mosnier, L.O., and J.H. Griffin. 2003. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 373:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo, H., D. Liu, H. Gelbard, T. Cheng, R. Insalaco, J.A. Fernandez, J.H. Griffin, and B.V. Zlokovic. 2004. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 41:563–572. [DOI] [PubMed] [Google Scholar]
- 8.Feistritzer, C., and M. Riewald. 2005. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 105:3178–3184. [DOI] [PubMed] [Google Scholar]
- 9.Finigan, J.H., S.M. Dudek, P.A. Singleton, E.T. Chiang, J.R. Jacobson, S.M. Camp, S.Q. Ye, and J.G. Garcia. 2005. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 280:17286–17293. [DOI] [PubMed] [Google Scholar]
- 10.Bernard, G.R., J.L. Vincent, P.F. Laterre, S.P. LaRosa, J.F. Dhainaut, A. Lopez-Rodriguez, J.S. Steingrub, G.E. Garber, J.D. Helterbrand, E.W. Ely, and C.J. Fisher Jr. 2001. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 344:699–709. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein, B., S. Nadel, M. Peters, R. Barton, F. Machado, H. Levy, D.J. Haney, B. Utterback, M.D. Williams, and B.P. Giroir. 2006. ENHANCE: results of a global open-label trial of drotrecogin alfa (activated) in children with severe sepsis. Pediatr. Crit. Care Med. 7:200–211. [DOI] [PubMed] [Google Scholar]
- 12.Abraham, E., K. Reinhart, S. Opal, I. Demeyer, C. Doig, A.L. Rodriguez, R. Beale, P. Svoboda, P.F. Laterre, S. Simon, et al. 2003. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. J. Am. Med. Assoc. 290:238–247. [DOI] [PubMed] [Google Scholar]
- 13.Warren, B.L., A. Eid, P. Singer, S.S. Pillay, P. Carl, I. Novak, P. Chalupa, A. Atherstone, I. Penzes, A. Kubler, et al. 2001. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. J. Am. Med. Assoc. 286:1869–1878. [DOI] [PubMed] [Google Scholar]
- 14.Gale, A.J., X. Sun, M.J. Heeb, and J.H. Griffin. 1997. Nonenzymatic anticoagulant activity of the mutant serine protease Ser360Ala-activated protein C mediated by factor Va. Protein Sci. 6:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellino, F.J., Z. Liang, S.P. Volkir, E. Haalboom, J.A. Martin, M.J. Sandoval-Cooper, and E.D. Rosen. 2002. Mice with a severe deficiency of the endothelial protein C receptor gene develop, survive, and reproduce normally, and do not present with enhanced arterial thrombosis after challenge. Thromb. Haemost. 88:462–472. [PubMed] [Google Scholar]
- 16.Darrow, A.L., W.P. Fung-Leung, R.D. Ye, R.J. Santulli, W.M. Cheung, C.K. Derian, C.L. Burns, B.P. Damiano, L. Zhou, C.M. Keenan, et al. 1996. Biological consequences of thrombin receptor deficiency in mice. Thromb. Haemost. 76:860–866. [PubMed] [Google Scholar]
- 17.Connolly, A.J., H. Ishihara, M.L. Kahn, R.V. Farese Jr., and S.R. Coughlin. 1996. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 381:516–519. [DOI] [PubMed] [Google Scholar]
- 18.Iwaki, T., D.T. Cruz, J.A. Martin, and F.J. Castellino. 2005. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood. 105:2364–2371. [DOI] [PubMed] [Google Scholar]
- 19.Mosnier, L.O., A.J. Gale, S. Yegneswaran, and J.H. Griffin. 2004. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood. 104:1740–1744. [DOI] [PubMed] [Google Scholar]
- 20.Ruf, W. 2005. Is APC activation of endothelial cell PAR1 important in severe sepsis?: Yes. J. Thromb. Haemost. 3:1912–1914. [DOI] [PubMed] [Google Scholar]
- 21.Esmon, C.T. 2005. Is APC activation of endothelial cell PAR1 important in severe sepsis?: No. J. Thromb. Haemost. 3:1910–1911. [DOI] [PubMed] [Google Scholar]
- 22.Ludeman, M.J., H. Kataoka, Y. Srinivasan, N.L. Esmon, C.T. Esmon, and S.R. Coughlin. 2005. PAR1 cleavage and signaling in response to activated protein C and thrombin. J. Biol. Chem. 280:13122–13128. [DOI] [PubMed] [Google Scholar]
- 23.Slofstra, S.H., H. ten Cate, and C.A. Spek. 2006. Signal transduction induced by activated protein C: no role in protection against sepsis? Trends Mol. Med. 12:374–381. [DOI] [PubMed] [Google Scholar]
- 24.Bernard, G.R., E.W. Ely, T.J. Wright, J. Fraiz, J.E. Stasek Jr., J.A. Russell, I. Mayers, B.A. Rosenfeld, P.E. Morris, S.B. Yan, and J.D. Helterbrand. 2001. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit. Care Med. 29:2051–2059. [DOI] [PubMed] [Google Scholar]
- 25.Liaw, P.C., C.T. Esmon, K. Kahnamoui, S. Schmidt, S. Kahnamoui, G. Ferrell, S. Beaudin, J.A. Julian, J.I. Weitz, M. Crowther, et al. 2004. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 104:3958–3964. [DOI] [PubMed] [Google Scholar]
- 26.Bilbault, P., T. Lavaux, A. Launoy, M.P. Gaub, N. Meyer, P. Oudet, T. Pottecher, A. Jaeger, and F. Schneider. 2007. Influence of drotrecogin alpha (activated) infusion on the variation of Bax/Bcl-2 and Bax/Bcl-xl ratios in circulating mononuclear cells: a cohort study in septic shock patients. Crit. Care Med. 35:69–75. [DOI] [PubMed] [Google Scholar]
- 27.Hotchkiss, R.S., and D.W. Nicholson. 2006. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 6:813–822. [DOI] [PubMed] [Google Scholar]
- 28.Zheng, X., W. Li, J.M. Gu, D. Qu, G.L. Ferrell, N.L. Esmon, and C.T. Esmon. 2007. Effects of membrane and soluble EPCR on the hemostatic balance and endotoxemia in mice. Blood. 109:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerlin, B., B.C. Cooley, B.H. Isermann, I. Hernandez, R. Sood, M. Zogg, S.B. Hendrickson, M.W. Mosesson, S. Lord, and H. Weiler. 2004. Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood. 103:1728–1734. [DOI] [PubMed] [Google Scholar]
- 30.Maas, M., M. Stapleton, C. Bergom, D.L. Mattson, D.K. Newman, and P.J. Newman. 2005. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am. J. Physiol. Heart Circ. Physiol. 288:H159–H164. [DOI] [PubMed] [Google Scholar]
- 31.Glisic-Milosavljevic, S., J. Waukau, S. Jana, P. Jailwala, J. Rovensky, and S. Ghosh. 2005. Comparison of apoptosis and mortality measurements in peripheral blood mononuclear cells (PBMCs) using multiple methods. Cell Prolif. 38:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatia, M., A.K. Saluja, B. Hofbauer, J.L. Frossard, H.S. Lee, I. Castagliuolo, C.C. Wang, N. Gerard, C. Pothoulakis, and M.L. Steer. 1998. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. USA. 95:4760–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosnier, L.O., P.A. von dem Borne, J.C. Meijers, and B.N. Bouma. 1998. Plasma TAFI levels influence the clot lysis time in healthy individuals in the presence of an intact intrinsic pathway of coagulation. Thromb. Haemost. 80:829–835. [PubMed] [Google Scholar]
- 34.Maier, S., T. Traeger, M. Entleutner, A. Westerholt, B. Kleist, N. Huser, B. Holzmann, A. Stier, K. Pfeffer, and C.D. Heidecke. 2004. Cecal ligation and puncture versus colon ascendens stent peritonitis: two distinct animal models for polymicrobial sepsis. Shock. 21:505–511. [DOI] [PubMed] [Google Scholar]
- 35.Zantl, N., A. Uebe, B. Neumann, H. Wagner, J.R. Siewert, B. Holzmann, C.D. Heidecke, and K. Pfeffer. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 66:2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.