Abstract
To investigate if transporter associated with antigen processing (TAP)–1 is required for CD8+ T cell–mediated control of Toxoplasma gondii in vivo, we compared the resistance of TAP-1−/−, CD8−/−, and wild-type (WT) mice to infection with the parasite. Unexpectedly, TAP-1−/− mice displayed greater susceptibility than CD8−/−, β2-microglobulin−/− (β2m−/−), or WT mice to infection with an avirulent parasite strain. The decreased resistance of the TAP-1−/− mice correlated with a reduction in the frequency of activated (CD62Llow CD44hi) and interferon (IFN)-γ–producing CD4+ T cells. Interestingly, infected TAP-1−/− mice also showed reduced numbers of IFN-γ–producing natural killer (NK) cells relative to WT, CD8−/−, or β2m−/− mice, and after NK cell depletion both CD8−/− and WT mice succumbed to infection with the same kinetics as TAP-1−/− animals and displayed impaired CD4+ T cell IFN-γ responses. Moreover, adoptive transfer of NK cells obtained from IFN-γ+/+, but not IFN-γ−/−, animals restored the CD4+ T cell response of infected TAP-1−/− mice to normal levels. These results reveal a role for TAP-1 in the induction of IFN-γ–producing NK cells and demonstrate that NK cell licensing can influence host resistance to infection through its effect on cytokine production in addition to its role in cytotoxicity.
Toxoplasma gondii is an obligate intracellular pathogen capable of infecting a variety of phagocytic and nonphagocytic host cells by an active penetration process leading to the formation of a parasitophorous vacuole that remains segregated from host endocytic/lysosomal compartments (1–4). Although T. gondii produces an asymptomatic infection in most immunocompetent hosts, encysted parasites may reactivate in immunocompromised individuals, leading to uncontrolled parasite growth and severe tissue damage (5–7). During the acute phase, T. gondii triggers a strong innate response mediated by macrophages, DCs, neutrophils, and NK cells (8–13). The latter cell population is thought to contribute to early resistance to the parasite through its production of the effector cytokine IFN-γ (10, 14–16). Adaptive immunity to T. gondii depends on IFN-γ–producing CD4+ and CD8+ T cells, with the latter subset playing a more dominant role during the chronic phase of infection (17–20).
CD8+ T cell priming through the classical MHC class I processing pathway is typically associated with presentation of endogenous antigens (Ags) such as viral or tumor proteins. In this mechanism, cytosolic Ags undergo proteolytic degradation by the proteasome, followed by transfer of the resulting peptides to the endoplasmic reticulum mediated by the transporter associated with Ag processing (TAP) (21–24). DCs, which are regarded as the most potent APCs (25), also have the ability to process phagocytosed exogenous Ags and present them on MHC class I molecules by cross-presentation (26–30).
We and others have previously shown that presentation of OVA by DCs infected with transgenic T. gondii tachyzoites expressing this model Ag is TAP-1 dependent (31, 32) and also requires intact host cell proteasome function (32). Thus, although secluded in the parasitophorous vacuole, T. gondii–derived Ag use what appears to be a cytosolic pathway for MHC class I processing and presentation.
These conclusions on the mechanism of CD8+ T cell priming by T. gondii were based entirely on in vitro observations in a model system. In the present study, we addressed the role of the TAP-1–dependent pathway of Ag presentation in host resistance to T. gondii infection in vivo. Unexpectedly, we found that TAP-1–deficient mice are more susceptible to the parasite than either CD8−/− or β2-microglobulin−/− (β2m−/−) animals. Further analysis demonstrated that this early mortality is associated with defective NK cell, as well as CD4+ T cell, IFN-γ production. Thus, our observations reveal a previously unappreciated influence of TAP-1 in the regulation of NK cell–dependent cytokine responses to a pathogen and provide further in vivo evidence for the role of NK cells in the activation of IFN-γ–producing CD4+ T lymphocytes during infection.
RESULTS
TAP-1 is required for CD8+ T cell–mediated control of T. gondii in vivo
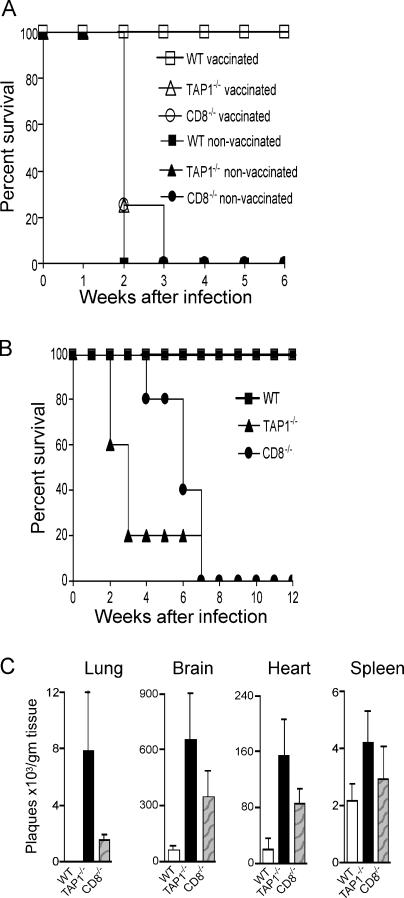
Using OVA expressing transgenic parasites, we have previously shown that priming of naive CD8+ OT-I T cells by T. gondii–infected DCs is strictly TAP-1 dependent (32). To determine whether TAP-1 processing is required for CD8+ T cell–mediated resistance to T. gondii in vivo, we used two different experimental models. In the first model, WT, TAP-1−/−, and CD8−/− mice were vaccinated with an attenuated strain of T. gondii (ts-4) and then challenged with a normally lethal virulent parasite strain (RH). Vaccine protection in this model has previously been shown to be ablated by CD8+ T cell depletion (18), and as predicted, both TAP-1−/− and CD8−/− animals failed to develop protective immunity and succumbed to challenge infection with similar kinetics to those observed in nonvaccinated mice (Fig. 1 A).
Figure 1.
TAP-1−/− mice fail to mount protective CD8 T cell responses and display enhanced susceptibility to infection with an avirulent strain of T. gondii. (A) WT (n = 5), TAP-1−/− (n = 5), and CD8−/− (n = 5) mice were vaccinated with 2 × 105 tachyzoites of the attenuated ts-4 parasite strain and challenged 2 wk later with 100 virulent RH tachyzoites. Survival was then monitored. (B) The same mouse strains (n = 5 per group) were infected i.p. with 20 cysts of the avirulent ME-49 strain, and survival was monitored for 12 wk after infection. One representative experiment of at least three performed is shown. (C) Parasite burden in the lung, brain, heart, and spleen tissues (mean ± SD values shown) at 18 d after infection in WT, TAP-1−/−, and CD8−/− mice (n = 4) infected as in B.
To further test the role of TAP-1 in host resistance to T. gondii, we used a second model in which the same mouse strains were infected with an avirulent strain (ME49) and monitored their survival and parasite load. Surprisingly, in this setting TAP-1−/− mice displayed greater susceptibility than either CD8−/− or WT animals, with >70% succumbing within 3 wk after infection, whereas no mortality was observed in the CD8−/− group until 6 wk after infection (Fig. 1 B). The early death of TAP-1−/− mice correlated with increased parasite burden in the lung, brain, and heart at 18 d after infection but was less evident in the spleen (Fig. 1 C). Although accurate measurement of brain cyst counts was complicated by the early death of the animals, the surviving TAP-1−/− mice clearly displayed a markedly elevated cyst burden comparable to that of the infected CD8−/− host (unpublished data). Collectively, these results indicated that although TAP-1–dependent processing is required for CD8+ T cell–dependent host protection against T. gondii, in the context of an avirulent infection other components of the immune response may be additionally compromised.
TAP-1–deficient mice exhibit impaired CD4+ T cell responses during acute infection
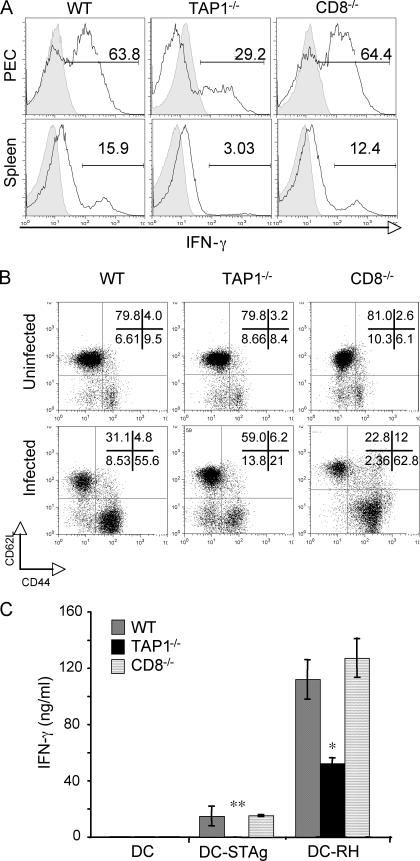
Immune control of T. gondii has been shown to be dependent on IFN-γ production by both CD4+ and CD8+ T cells. Although CD8+ T lymphocytes play a major role in host resistance during the chronic phase, CD4+ T cells are essential during both acute and chronic stages of infection (17–20, 33). Therefore, we asked whether the CD4+ T cell response is impaired in T. gondii–infected TAP-1−/− animals. To address this question, we assayed IFN-γ production by CD4+ T lymphocytes from acutely infected WT, TAP-1−/−, and CD8−/− mice ex vivo by intracellular staining (ICS). As indicated in Fig. 2 A, TAP-1−/− mice exhibited a markedly reduced percentage of IFN-γ–producing CD4+ T cells in both the peritoneal infection site (peritoneal exudate cells [PECs]) and spleen at day 7 after infection, with no significant differences in the total number of these cells in the same tissue sites of the three mouse strains (not depicted). This impaired CD4+ T cell response was also reflected in a lower frequency of activated CD44highCD62Llow CD4+ lymphocytes (Fig. 2 B).
Figure 2.
Acutely infected TAP-1−/− mice exhibit defective CD4+ T cell responses. WT, TAP-1−/−, and CD8−/− mice were infected i.p. with 20 ME-49 cysts, and PECs and spleens were harvested on day 7 after infection (peak of CD4+ T cell response). (A) IFN-γ production by CD4+ T cells from PECs and splenocytes was measured by ICS 5 h after restimulation with plate-bound anti-CD3 mAb. The background staining of CD4+ T cells from uninfected animals is shown in the shaded portions of the plots. Numbers represent the percentage of IFN-γ–producing CD4+ T cells. The histograms shown are from one representative mouse per group (n = 3 per group). (B) Frequency of activated (CD44highCD62Llow) CD4+ T cells directly isolated from PECs of day 7–infected versus uninfected mice. Numbers represent the percentage of CD4+ T cells with high or low expression of the indicated surface marker. The histograms and dot plots shown in A and B are gated on CD4+ T cells and are representative of at least four experiments performed. (C) IFN-γ production by MACS-sorted CD4+ T cells from spleens of the animals described. The CD4+ T cells were restimulated with DCs that were exposed to soluble parasite extract (DC-STAg) or T. gondii infected (DC-RH), and the cytokine in 48-h supernatants was measured by ELISA. Values shown are the mean ± SD of the ELISA readings from three mice per group and are representative of three experiments performed. *, P < 0.05; **, P < 0.02.
To further confirm that TAP-1−/− mice display impaired CD4+ T cell responses against the parasite, we measured IFN-γ production by purified CD4+ lymphocytes after in vitro restimulation with live tachyzoites or their products. To do so, purified splenic CD4+ T cells from day 7–infected mice were incubated with T. gondii–infected DCs (DC-RH) or DCs loaded with soluble T. gondii Ag (DC–soluble tachyzoite Ag [STAg]), and secreted IFN-γ was measured in culture supernatants at 48 h. In agreement with the ex vivo data, CD4+ T cells from infected TAP-1−/− animals produced markedly reduced levels of IFN-γ in response to both DC-RH and DC-STAg when compared with the equivalent cell populations from WT or CD8−/− mice (Fig. 2 C).
DCs are known to play a major role in the initiation of IL-12–dependent cell-mediated immunity against T. gondii and in governing the strongly Th1-polarized adaptive immune response triggered by the parasite (12, 34–38). Therefore, it was possible that the defective CD4+ T lymphocyte response seen in TAP-1−/− mice was caused by impaired DC function. To test this possible explanation of the data, splenocytes from WT or TAP-1−/− mice were stimulated in vitro with STAg, and IL-12p40 production was measured by ELISA. As shown in Fig. S1 A (available at http://www.jem.org/cgi/content/full/jem.20070634/DC1), TAP-1−/− and WT DCs secreted equivalent amounts of IL-12. Because of the key role of DCs in T cell priming, we next evaluated whether TAP-1−/− DCs normally process and present T. gondii Ag to CD4+ T lymphocytes. To do so, we FACS sorted CD11c+ cells from spleens of T. gondii–infected WT or TAP-1−/− animals and used them as APCs to stimulate T. gondii–specific CD4+ T cell clones. Both TAP-1−/−– and WT-derived CD11c+ cells efficiently induced CD4+ T cell proliferation (Fig. S1 B). Interestingly, TAP-1−/− CD11c+ cells stimulated higher T cell proliferation than WT DCs, a finding that may reflect the higher parasite load (Fig. 1 C) in these animals. As predicted, the TAP-1−/− DCs from mice equivalently infected with OVA expressing tachyzoites failed to stimulate transgenic OT-I CD8+ T cells, confirming their defect in CD8+ T cell presentation (unpublished data).
To further confirm that the impaired CD4+ T cell response observed in TAP-1−/− mice is not caused by inefficient Ag presentation by TAP-1−/− DCs, we tested the ability of T. gondii–infected TAP-1−/− BM-derived DCs (BMDCs) to restimulate CD4+ T cells from acutely infected WT mice rather than the T cell clones used in the previous experimental setting. As shown in Fig. S1 (C and D), CD4+ T cell proliferation and IFN-γ production induced by T. gondii–infected TAP-1−/− BMDCs were comparable to that stimulated by WT BMDCs. Collectively, these findings argued that although defective in their ability to prime CD8+ T cell responses, TAP-1−/− DCs produce similar amounts of IL-12 in response to T. gondii compared with WT DCs and efficiently process and present parasite-derived Ag to CD4+ T cells.
T. gondii–infected TAP-1−/− mice display defective NK cell responses
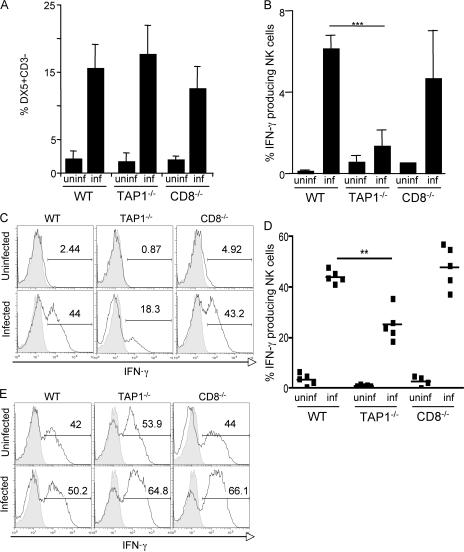
NK cells are known to play a major role in the innate response to T. gondii and to be an early source of IFN-γ (10, 14–16). Although total numbers of NK cells in TAP-1−/− animals are normal, their repertoire and functional maturation may be altered as a consequence of developing in a low MHC class I environment caused by the TAP-1 requirement for normal cell-surface expression of MHC class I molecules (39). For this reason, we next asked if the increased susceptibility of TAP-1−/− mice to T. gondii infection is a consequence of impaired NK cell function. To do so, we examined by ICS spontaneous ex vivo IFN-γ production by NK cells from day 3–infected WT, TAP-1−/−, and CD8−/− mice. Interestingly, although the percentage of NK cells recruited to the peritoneal infection site in infected TAP-1−/− mice was comparable to that in WT and CD8−/− animals (Fig. 3 A), the former mice displayed a significantly reduced frequency of IFN-γ–producing NK cells (Fig. 3 B). Similar results were observed after in vitro restimulation of the same cell populations by cross-linking the activating receptor NK1.1 (Fig. 3, C and D).
Figure 3.
NK cells from infected TAP-1−/− mice display impaired cytokine production. WT, TAP-1−/−, and CD8−/− mice were infected i.p. with 20 cysts, and PECs were harvested 3 d later (peak of NK cell–IFN-γ response). (A) Percentage of NK cells (DX5+CD3−) recruited to the peritoneal infection site as compared with uninfected animals. Values shown are the mean ± SD of assays on individual animals (n = 5 per group). (B) Spontaneous ex vivo IFN-γ production by NK cells from PECs of infected versus uninfected mice, as measured by ICS. Values shown are the mean ± SD of assays on individual animals (n = 3 per group). (C and D) IFN-γ production by NK cells from PECs of infected versus uninfected mice 5 h after restimulation with anti-NK1.1 mAb, as measured by ICS. The histograms shown in C are from one representative mouse per group (n = 5 per group) and are gated on DX5+CD3− cells. The pooled data for that experiment are summarized in D (horizontal bars represent the mean). The data shown in A–D are representative of at least four experiments performed. (E) Splenocytes from the same groups of animals were stimulated with PMA/ionomycin, and IFN-γ production by NK cells was measured by ICS 5 h later. The histograms shown are gated on DX5+CD3− cells and are representative of three experiments performed. (C and E) Isotype controls are shown in the shaded portion of the histograms. Numbers represent the percentage of IFN-γ–producing NK cells. **, P < 0.02; ***, P < 0.001.
After NK1.1 cross-linking, the frequency of IFN-γ–producing NK cells obtained from infected mice was markedly increased as compared with that of uninfected controls in all three animal groups. Nevertheless, IFN-γ production by NK cells derived from infected TAP-1−/− mice was still significantly impaired (Fig. 3, C and D). This finding could not be explained by differential NK1.1 expression, as no differences were observed in the mean fluorescence intensity of NK1.1 staining in cells from the different animal groups (unpublished data). Furthermore, when anti–IL-12p40 mAb was added to the cultures, the frequency of IFN-γ–positive NK cells was not altered, indicating that the differences observed in IFN-γ production by the NK cell populations from the animal groups is not caused by differences in IL-12 secretion during culture (unpublished data). Importantly, after in vitro stimulation with PMA and ionomycin, the frequencies of IFN-γ–producing NK cells from infected TAP-1−/−, CD8−/−, and WT mice were indistinguishable (Fig. 3 E). The latter observation indicated that although defective in their response to T. gondii infection, TAP-1−/− NK cells are nevertheless capable of normal IFN-γ production when appropriately stimulated. Indeed, as has been described previously for unlicensed NK cells obtained from MHC class I–deficient animals (40), extended culture of purified NK cells from TAP-1−/− mice in the presence of recombinant IL-2 restored their ability to produce IFN-γ in response to NK1.1 cross-linking (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070634/DC1).
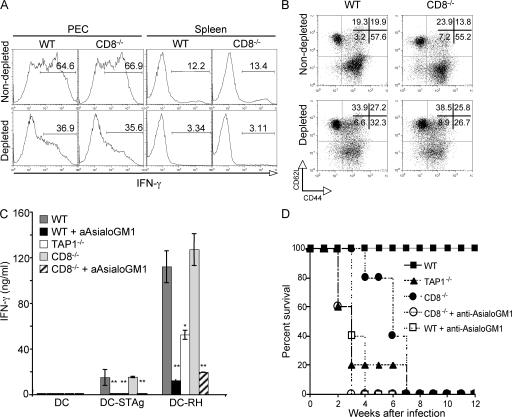
Depletion of NK cells in T. gondii–infected mice leads to impaired CD4+ T lymphocyte responses and enhanced susceptibility
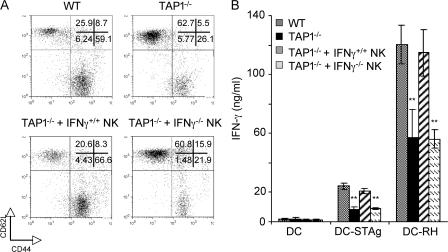
Previous studies have shown that through their ability to produce IFN-γ, NK cells are also important for DC activation and Th1 polarization (41–47). Because of this, we asked whether the diminished CD4+ T lymphocyte response observed in T. gondii–infected TAP-1−/− mice stems from the defective NK cell activity observed in the latter animals. To do so, we tested the effects of in vivo NK cell depletion on IFN-γ production by CD4+ T cells from acutely infected CD8−/− mice that, as noted above, show normal CD4+ as well as NK cell activity. In this experiment, to ensure long-term NK cell depletion the anti-AsialoGM1 antibodies were repeatedly administered on a biweekly basis for 3 wk. As shown in Fig. 4 A, the frequency of IFN-γ–producing CD4+ T lymphocytes in the NK cell–depleted animals at 7 d after infection was markedly reduced both in the peritoneal infection site (PECs) and spleen. The effect of NK cell depletion was also demonstrated in the reduced frequency of activated CD44highCD62Llow CD4+ lymphocytes and in the ability of purified splenic CD4+ T cells to produce IFN-γ in response to restimulation with T. gondii–infected DCs (Fig. 4, B and C). As would be predicted from their loss in both NK and CD4+ cytokine production, these NK cell–depleted CD8−/− mice were more susceptible to infection, succumbing ∼3 wk earlier than the nondepleted CD8−/− control animals and simultaneously with the TAP-1−/− mice included in the experiment (Fig. 4 D). Interestingly, NK cell depletion also led to a profound reduction in both CD4+ IFN-γ production and the frequency of activated CD44highCD62Llow lymphocytes in infected WT animals, and these mice also displayed increased mortality relative to nondepleted control animals (Fig. 4). As expected, anti-AsialoGM1 treatment had no effect on either the survival or CD4+ T cell response of infected TAP-1−/− mice (unpublished data). Because anti-AsialoGM1 can target other cell populations (e.g., CD8+ T lymphocytes) in addition to NK cells (48), independent confirmation of the role of NK cell–IFN-γ production was obtained by demonstrating that injection of TAP-1−/− animals with purified IFN-γ+/+, but not IFN-γ−/−, NK cells before infection results in restored parasite-specific CD4+ T cell responses (Fig. 5).
Figure 4.
NK cell–depleted T. gondii–infected mice exhibit impaired CD4+ T cell responses and enhanced susceptibility. WT, TAP-1−/−, and CD8−/− mice were infected i.p. with 20 ME-49 cysts and, where indicated, injected with anti-AsialoGM1 antibodies on days −1 and +3 relative to infection, followed by biweekly injections as described in Materials and methods. (A–C) PECs and spleens were harvested on day 7 after infection. (A) IFN-γ production by CD4+ T cells from PECs and splenocytes of infected anti-AsialoGM1–treated versus untreated mice was measured by ICS 5 h after restimulation with plate-bound anti-CD3 mAb. Numbers represent the percentage of IFN-γ–producing CD4+ T cells. The histograms and dot plots shown in A and B are gated on CD4+ T cells and are from one representative mouse per group (n = 3 per group). (B) Frequency of activated (CD44highCD62Llow) CD4+ T cells isolated directly from PECs of the same animals. Numbers represent the percentage of CD4+ T cells with high or low expression of the indicated surface marker. (C) MACS-sorted splenic CD4+ T cells from these animals were restimulated with DC-STAg or DC-RH, and IFN-γ production was measured by ELISA in 48-h supernatants. Values shown are the mean ± SD of the ELISA readings from three mice per group. (D) Survival of mice treated as described (n = 5 per group) was monitored for 12 wk after infection. The data shown in A–D are representative of three experiments performed. *, P < 0.05; **, P < 0.02.
Figure 5.
Adoptive transfer of IFN-γ+/+, but not IFN-γ−/−, NK cells restores CD4+ T cell responses in TAP-1−/− mice. WT and TAP-1−/− animals were infected i.p. with 20 ME-49 cysts, and where indicated, TAP-1−/− mice were injected on day −1 with 5 × 106 purified NK cells obtained from the spleens of either IFN-γ+/+ or IFN-γ−/− animals, as described in Materials and methods. (A) Frequency of activated (CD44highCD62Llow) CD4+ T cells isolated directly from PECs of infected animals at day 7 after infection. Numbers represent the percentage of CD4+ T cells with high or low expression of the indicated surface marker. The dot plots shown are gated on CD4+ T cells and are from one representative mouse per group. (B) Purified splenic CD4+ T cells from the same animals were restimulated with DC-STAg or DC-RH, and IFN-γ production was measured by ELISA in 48-h supernatants. Values shown are the mean ± SD of the ELISA readings from two mice per group. The experiments shown are representative of two performed. **, P < 0.02.
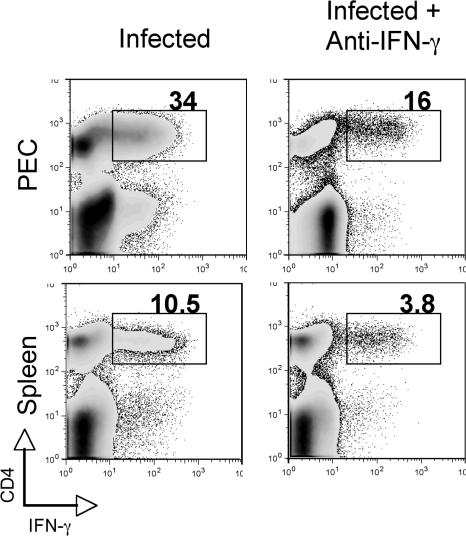
To address whether the role of NK cells in the regulation of CD4+ T cell responses in WT animals also depends on their production of IFN-γ, WT mice were treated with neutralizing anti–IFN-γ mAb at day −1 and day +3 after infection, and levels of IFN-γ–producing CD4+ T cells were determined on day +7 in the PECs and spleens. As shown in Fig. 6, IFN-γ neutralization resulted in decreased frequencies of IFN-γ–producing CD4+ T cells in both sites similar to the reductions previously observed in NK cell–depleted animals (Fig. 4 A).
Figure 6.
Acutely infected WT mice treated with anti–IFN-γ–neutralizing antibodies display decreased frequencies of IFN-γ–producing CD4+ T cells. WT mice were infected i.p. with 20 ME-49 cysts and, where indicated, were treated with anti–IFN-γ–neutralizing mAb on days −1 and +3 relative to infection. PECs and spleens were harvested on day 7 after infection, and IFN-γ production by CD4+ T cells was measured by ICS after restimulation with anti-CD3 mAb. Numbers represent the percentage of IFN-γ–producing CD4+ T cells. The plots shown are representative of one mouse per group (n = 3) from two experiments performed.
In contrast to TAP-1−/− animals, β2m−/− mice display unimpaired NK cell function and succumb with similar kinetics as CD8−/− animals
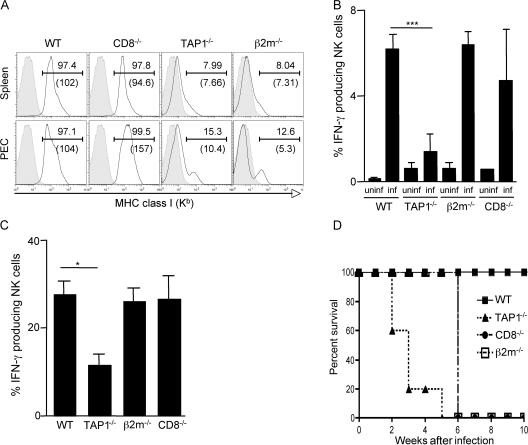
Because MHC class I molecules are known to play a major role in NK cell functional maturation and in target recognition (40, 49–52), we asked whether the defect in NK cell activity observed in T. gondii–infected TAP-1−/− mice is solely the result of their impaired MHC class I expression. To address this issue, MHC class I–deficient β2m−/− as well as WT control mice were infected with ME49 T. gondii cysts, and their NK cell IFN-γ production and survival were monitored. As expected, FACS analysis confirmed the low levels of cell-surface MHC class I molecules on both PECs and splenocytes from infected β2m−/− as well as TAP-1−/− mice (Fig. 7 A). Nevertheless, when assayed at 3 d after infection, the frequency of IFN-γ–producing NK cells from β2m−/− mice measured directly ex vivo or after NK1.1 cross-linking was not reduced relative to that observed in WT control animals (Fig. 7, B and C). These MHC class I–deficient mice survived the acute phase of infection, succumbing with similar kinetics as CD8−/− animals (Fig. 7 D). Thus, although both failing to express normal levels of MHC class I molecules, β2m−/− and TAP-1−/− mice differ in their NK cell responsiveness and host resistance to T. gondii infection.
Figure 7.
T. gondii–infected b2m−/− mice display normal NK cell IFN-g production and succumb at the same time as CD8−/− animals. WT, TAP-1−/−, CD8−/−, and β2m−/− mice (n = 5 per group) were infected i.p. with 20 ME-49 cysts. (A) PECs and spleens were harvested on day 3 after infection, and MHC class I expression was measure by FACS. The data shown are from animals within each group. The numbers indicate the percentage of positive cells (top) and mean fluorescence intensity (bottom). Filled histograms indicate background isotype control staining. (B and C) IFN-γ ICS of NK cells from 3-d infected mice ex vivo (B) or after restimulation with anti-NK1.1 mAb (C), as in Fig. 3. Values shown are the mean ± SD of assays on individual animals. (D) Survival of infected animals (n = 5 per group). The data shown in A–D are representative of three experiments performed. *, P < 0.05; ***, P < 0.001.
To address the possibility that the discrepancy in NK cell response between TAP-1−/− versus β2m−/− mice might reflect differences in the NK cell receptor repertoire of these two MHC class I–deficient strains, we examined their expression of a large battery of inhibitory as well as stimulating NK cell receptors on NK1.1+CD3− gated cells before and 3 d after infection. Significant differences in receptor expression between NK cells from infected TAP-1−/− and β2m−/− mice were noted with five out of the nine antibody reagents tested (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070634/DC1). Of note, no significant differences were detected between the mouse strains in the levels of NKG2D, a receptor recently implicated in the regulation of DC function by NK cells in response to T. gondii (47), nor were differences observed in the expression of the NKG2D ligands RAE1 and MULT1 on STAg-stimulated DCs (unpublished data).
DISCUSSION
TAP-1–deficient mice display enhanced susceptibility to infection with a variety of different pathogens (53, 54). In essentially all of these experiments, the observed loss in host resistance has been attributed to impaired class I presentation and CD8+ T cell function. Consistent with this observation, TAP-1–deficient mice vaccinated with an attenuated strain of T. gondii failed to develop protective immunity to challenge infection, a response characterized by us as highly dependent on IFN-γ–producing CD8+ T cells (18). Unexpectedly, when TAP-1−/− and CD8−/− mice were examined in an avirulent infection model of T. gondii, the former animals proved to be more susceptible than the latter, an observation that correlated with impaired NK cell cytokine production and Th1 cell activation in the TAP-1–deficient animals.
The possible role of TAP-1 in regulating NK cytokine production has, to the best of our knowledge, never been systematically addressed. Although T. gondii–infected mice showed a 50% reduction in the frequency of IFN-γ–producing NK cells as compared with WT animals, no impairment in NK cytokine production was observed in similarly infected MHC class I β2m−/− mice. At first glance, this finding suggests the involvement of distinct receptors regulating IFN-γ production by NK cells from TAP-1−/− versus β2m−/− animals. In this regard, it is important to note that although both express very low levels of MHC class I molecules, NK cells from TAP-1−/− versus β2m−/− mice have been shown in previous reports to display differences in several inhibitory receptors from the Ly49 family (55, 56). As shown in this paper, NK cells from T. gondii–infected TAP-1−/− and β2m−/− animals exhibit significant differences in several different inhibitory as well as stimulating receptors (Fig. S3); however, these differences in terms both of the proportion of cells expressing the receptors and the cell-surface receptor density were too modest to allow us to explain on their basis the functional discrepancy observed between TAP-1−/− and β2m−/− NK cells. It is also possible that TAP-1−/− NK cells possess unique defects in intracellular signaling or lack an as yet unidentified activating receptor.
NK cytolytic function is known to be regulated through recognition of MHC class I on target cells such that, in the absence of TAP-1 and the resulting impairment of MHC class I transport to the cell surface, NK cells become self-tolerant (39, 40, 49, 50, 52). Thus, TAP-1–deficient, similar to MHC class I–deficient, mice have been shown to display NK cell–defective cytolytic activity (39, 52, 55, 57). A plausible explanation for the latter observation is given by the “licensing” hypothesis, proposed by Kim et al. (40), in which specific interactions between NK cell inhibitory receptors and their self–MHC class I ligands render NK cells functionally competent. Therefore, in the absence of the appropriate MHC class I molecules, unlicensed NK cells become self-tolerant. Until now, TAP-1−/− and β2m−/− mice were considered equivalent in lack of licensing or induction of self-tolerance in NK cells. However, the possibility that the different forms of MHC class I molecules expressed on the cell surface in these mice might lead to different modulation of NK cell functions was proposed in an earlier report (39). It is also possible that differential requirements for TAP-1 or β2m for the expression of various MHC class 1b molecules that are ligands for NK cell receptors, such as NKG2D, may modulate NK cell function. In this respect, it is of interest that the reduced NK cell activity in NOD females has been attributed both to polymorphisms in TAP-1 and to the enhanced expression of NKG2D ligands on NK cells (58, 59). Although we observed no differences in the levels of NKG2D on NK cells from these two mouse strains, the possibility of a difference in expression of one or more of the multiple ligands for this receptor on NK cells themselves remains to be ruled out.
An additional consideration is that the licensing requirement initially described by Kim et al. was found to be less pronounced in cytokine-activated NK cells and could be bypassed by PMA and ionomycin stimulation (40). Similarly, activation of NK cells in vivo during viral infection in β2m−/− mice was shown to revert the unlicensed or self-tolerant NK cell phenotype and result in NK cell–mediated protection against infection (60). Interestingly, in the present study we found that the state of NK cell self-tolerance in TAP-1−/− mice, in contrast to β2m−/− mice, cannot be reversed by in vivo activation during T. gondii infection and results in decreased NK cell production of IFN-γ and Th1 cell priming.
Apart from their low expression of MHC class I molecules and decreased number of CD8+ T cells, β2m−/− differ from TAP-1−/− mice in that the former animals are also deficient in CD1d expression (61). This secondary defect could lead to impaired regulatory cell function and increased IFN-γ levels, as demonstrated in studies on T. gondii–infected CD1d−/− mice (62). Moreover, as previously shown by us, β2m−/− mice vaccinated with an attenuated strain of T. gondii unexpectedly develop increased numbers of IFN-γ–producing NK cells, leading to partial protection against lethal parasite challenge (10). Thus, infected β2m−/− mice may display immune response alterations that compensate for the impaired NK and CD4+ T cell function observed in TAP-1−/− mice exposed to the pathogen.
Regardless of the mechanism underlying their defective cytokine production, the deficiency in NK cell IFN-γ synthesis observed in infected TAP-1−/− mice was closely associated with a defect in Th1 cell development that was corrected by adoptive transfer of purified NK cells from IFN-γ+/+, but not IFN-γ−/−, animals. Moreover, NK cell depletion was found to recapitulate these effects on CD4+ T cell responsiveness and host survival in CD8−/− as well as WT animals. The increased susceptibility observed in the NK cell–depleted WT mice was unexpected, because this effect was not seen in a previous study by our group on the influence of treatment with anti-AsialoGM1 on host resistance to T. gondii in WT animals (63). This discrepancy is likely to be caused by differences in the depletion regimens used, which in the present study involved long-term as opposed to merely previous injection of anti-AsialoGM1 antibodies. Indeed, our present findings are consistent with those of a recent study by Khan et al., who described a major effect of NK cell depletion on host resistance to peroral infection with T. gondii using a similar antibody injection protocol as that used in this study (64).
A role for NK cell IFN-γ production in the development of Th1 responses to intracellular pathogens was first proposed in a seminal study by Scharton and Scott in mice infected with Leishmania major (44). The findings presented in this study extend this concept to another important host–parasite model and directly confirm that the effects of NK cell depletion on IFN-γ production by CD4+ T lymphocytes occur at the single-cell level. Unlike T. gondii, L. major induces both Th1 and Th2 cytokines (44), and NK cell depletion in resistant mice infected with the latter pathogen resulted in conversion to a Th2 phenotype. Such a reversion was not detected in our NK cell depletion experiments on T. gondii–infected mice (unpublished data). Thus, our data confirm that NK cell–mediated amplification of Th1 responses also occurs in the setting of an in vivo infection model in which concurrent cross regulation by Th2 cytokines is unlikely.
Using a transgenic adoptive transfer system and antibody-mediated NK depletion, Martin-Fontecha et al. have recently described a major role for NK cell IFN-γ production in Th1 polarization in the response to OVA (46). Although our findings in T. gondii–infected mice are consistent with the pathway proposed in that study, we did not observe a complete ablation of the Th1 response after NK cell depletion. Thus, we favor the view that NK cell IFN-γ production represents a mechanism for enhancing APC-dependent Th1 cell induction rather than a stimulus required for its induction, as proposed by these authors.
Previous studies have noted a tripart interplay between NK cells, CD4+ T lymphocytes, and CD8+ T lymphocytes as sources of IFN-γ in host resistance to intracellular pathogens (10, 18, 20, 44). However, it has been unclear whether these cells cooperate by simply contributing multiple sources of the cytokine or are functionally interconnected. The results presented in this paper emphasize that NK cells and CD4+ and CD8+ T cells are not merely redundant sources of IFN-γ but play important roles in regulating each other's function. Thus, TAP-1, in addition to governing CD8+ T cell effector activity, was found to influence NK cell function and, through IFN-γ, the induction of Th1 lymphocyte responses. Studies are now in progress to further address the role of TAP-1 in the mechanism of this IFN-γ–dependent NK–T cell interplay in T. gondii infection and to determine the function of DCs in this pathway.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from Taconic Farms. TAP-1−/−, β2m−/−, and IFN-γ−/− mice backcrossed to C57BL/6 for 8, 22, and 12 generations, respectively; B6.SJL-RAG1−/− mice; and C57BL/6 OT-I/RAG1 transgenic mice were provided by Taconic Farms from the NIAID animal supply contract. CD8−/− mice backcrossed for 13 generations to C57BL/6 were purchased from the Jackson Laboratory. B6.129F2 mice were bred at the NIAID animal care facility. The latter animals were used as controls to confirm that the phenotype of the TAP-1−/− mice was not the result of contaminating 129 genes in this backcrossed strain. All mice were maintained in an NIAID, NIH animal care facility under specific pathogen-free conditions and treated in accordance with the regulations and guidelines of the Animal Care and Use Committee of the NIH. Age- (6–8 wk) and sex-matched animals were used in all experiments. Although the Taconic Farms TAP-1−/− mice used in most of the experiments were backcrossed for 8 generations, essentially identical survival and in vitro responses were observed when TAP-1−/− mice backcrossed for 10 generations (obtained from the Jackson Laboratory) were used in the same assays (unpublished data). Furthermore, phenotypic analysis demonstrated that the Taconic TAP-1−/− mice displayed B6 rather than 129 allelic markers (65) within the Ly49 complex. Finally, complete genome scans (at 20-megabase resolution, including 150 single nucleotide polymorphisms [SNPs]) performed on multiple Taconic TAP-1−/− mice (by the JMS Allele Typing Lab at the Jackson Laboratory) revealed only five contaminating 129 strain SNPs, all of which were outside of the Ly49 complex. Moreover, only one contaminating 129 SNP was detected in the Jackson TAP-1−/− mice, and this was associated with the TAP-1 gene and unlinked to the Ly49 locus (unpublished data).
Parasites and infection protocol.
Cysts of the avirulent ME-49 strain of T. gondii were obtained from the brains of chronically infected C57BL/6 mice. For experimental infection, animals received 20 ME-49 cysts in a volume of 0.5 ml via the i.p. route. The outcome of infection was determined by survival and, in several cases, by measuring parasite burdens by cyst enumeration in brain tissue or by plaque assay in lung, brain, heart, and spleen tissues (66). In brief, single-cell suspensions were prepared from organs harvested from day 18–infected animals (n = 4 per group). 10-fold serial dilutions were plated on primary human foreskin fibroblasts (HFF) and cultured at 37°C for 2–3 wk. Plaques were enumerated, and total numbers were expressed per tissue weight. The attenuated temperature-sensitive mutant ts-4 and virulent RH strains of T. gondii were maintained by serial passage on HFF cultured at 35°C and 37°C, respectively, in DMEM (Invitrogen) supplemented with 10% heat-inactivated FBS. Parasites were harvested from 80% lysed HFF monolayers. Vaccination and challenge were performed by i.p. injection of 2 × 105 ts-4 tachyzoites, followed by i.p. challenge with 2 × 103 RH tachyzoites 2 wk later. STAg was prepared as previously described (67).
Preparation of APCs.
Mouse BMDCs from C57BL/6 or TAP-1−/− animals were obtained as previously described (68) and infected with irradiated (15,000 rads) T. gondii tachyzoites (RH) at one to five parasites per DC (DC-RH) in 15-ml conical tubes (Sarstedt, Inc.). After incubation for 12 h, free-floating parasites were removed by centrifugation at 900 rpm for 10 min. Alternatively, DCs were incubated with 10 μg/ml STAg. Splenic CD11c+ cells from C57BL/6 or TAP-1−/− animals infected with OVA-expressing RH (32) were purified (>98%) by FACS sorting (FACStar; Beckton Dickinson).
Measurement of IFN-γ production by CD4+ T lymphocytes.
Single-cell suspensions (107 cells per milliliter) were prepared from spleens from day 7 T. gondii–infected mice (n = 3 per group). Spleens were mechanically disrupted, and red blood cells were removed by lysis with ACK lysing buffer (Cambrex Bio Science). CD4+ T lymphocytes were purified by negative selection using MACS microbeads (Miltenyi Biotec) according to the manufacturer's protocol. 105 purified CD4+ T cells were subsequently co-cultured with 2 × 104 DCs, DC-STAg, or DC-RH in 96-well round-bottom microtiter plates, and the culture supernatants were harvested at 48 h for cytokine measurement by ELISA. In a different set of experiments, T. gondii–specific CD4+ T cell clones (69) were co-cultured with FACS-sorted splenic CD11c+ cells from infected C57BL/6 or TAP-1−/− mice in 96-well round-bottom microtiter plates at different T cell/DC ratios, and the culture supernatants were harvested at 48 h. The limit of detection of the ELISA assay was 125 pg/ml.
ICS for IFN-γ.
IFN-γ production by CD4+ T or NK cells at a single-cell level was evaluated on freshly isolated PECs and splenocytes by ICS. PECs were obtained by peritoneal lavage with complete medium, and splenocytes were prepared from mice infected for 3 d and stimulated for 5 h with plate-bound anti-NK1.1 mAb (BD Biosciences) in the presence or absence of anti–IL-12 mAb (clone C17.8). Similarly, cells obtained from mice infected for 7 d were stimulated with plate-bound anti-CD3. In both culture conditions, 10 μg/ml Brefeldin A (Sigma-Aldrich) was added for the last 2 h, and the cells were surface stained with CyChrome–anti-CD4, FITC–anti-CD8, PE–anti-DX5, or allophycocyanin–anti-CD3, PerCP–anti-NK1.1, and PE–anti-DX5, followed by permeabilization and allophycocyanin– or FITC–anti–IFN-γ staining. In some cases, the cells were incubated in the presence of Brefeldin A for 5 h and stained as described above. Fluorescence was measured using a FACSCalibur (Beckton Dickinson), and data were analyzed with FlowJo software (Tree Star, Inc.). No differences in the frequency of IFN-γ–producing cells were observed when NK cells were obtained from infected B6.129 animals as compared with C57BL/6 mice.
Phenotypic analysis of CD4+ T cell populations.
To determine the state of CD4+ T lymphocyte activation, cell-surface staining was performed on freshly isolated PECs and spleens of day 7–infected mice using PE–anti-CD62L, FITC–anti-CD44, CyChrome–anti-CD4, and PerCP–anti-CD8 (BD Biosciences) and analyzed by FACS, as mentioned in the previous section.
In vivo mAb treatment.
To deplete NK cells, WT or CD8−/− mice were injected with 50 μl anti-AsialoGM1 antibody (Wako Pure Chemical) i.v. starting 1 d before infection, followed by biweekly injections until day 15 and weekly thereafter. NK cell depletion was evaluated by FACS analysis on splenocytes, blood, and PECs from mAb-treated mice using anti-DX5–PE, NK1.1-PerCP, and CD3-allophycocyanin (BD Biosciences). >90% depletion of DX5+NK1.1+CD3− in blood and PECs and >75% depletion in the spleen was observed. Control mice received similar amounts of normal rabbit serum. In a different set of experiments, WT mice were injected i.v. with 1 mg anti–IFN-γ mAb (clone XMG-6) on days −1 and +3 relative to infection.
NK cell purification and adoptive transfer.
Single-cell suspensions were prepared from spleens of uninfected IFN-γ+/+ or IFN-γ−/− mice, as described above. NK cells were purified by negative selection using the NK cell isolation kit followed by positive selection with anti-DX5 MACS microbeads (Miltenyi Biotec), according to the manufacturer's instructions. The percentage of NK cells (>95%) was evaluated by FACS as NK1.1+CD3− cells. For adoptive transfer experiments, 5 × 106 NK cells were administered i.p. into TAP-1−/− mice on day −1 before infection. To avoid any possible contamination with IFN-γ+/+ CD4+ T cells, T cell–deficient B6.SJL-RAG1−/− mice were used as IFN-γ+/+ cell donors. Transferred NK cells were detected in PECs of day 7 TAP-1−/−–infected mice by FACS as CD45.1+CD45.2−NK1.1+CD3− cells.
Statistics.
The statistical significance of differences in the means of experimental groups was determined using an unpaired, two-tailed Student's t test.
Online supplemental material.
Fig. S1 demonstrates that the ability of TAP-1−/− DCs to produce IL-12p40 in response to STAg and to process and present T. gondii–derived Ag to CD4+ T cells is not impaired. Fig. S2 shows that preactivation of TAP-1−/− NK cells with IL-2 restores their ability to produce IFN-γ in response to NK1.1 cross-linking. Fig. S3 analyzes differences in the NK cell receptor repertoire in NK1.1+CD3− gated cells from T. gondii–infected TAP-1−/− versus β2m−/− mice. Online supplemental is available at http://www.jem.org/cgi/content/full/jem.20070634/DC1.
Supplemental Material
Acknowledgments
We thank Sara Hieny and Sandy White for excellent technical help throughout this study. We are also grateful to Drs. David Sacks and John Ortaldo for critical reading of the manuscript, Dr. Michael Grigg for advice on the plaque assay, and Drs. Caetano Reis e Sousa and Eleanor Riley for helpful discussions. We also thank Dr. Donna Walls from the Jackson Laboratory for valuable help with the genome scan analysis.
This research was supported by the Intramural Research Program of the NIAID.
The authors have no conflicting financial interests.
Abbreviations used: Ag, antigen; β2m, β2-microglobulin; BMDC, BM-derived DC; HFF, human foreskin fibroblast; ICS, intracellular staining; PEC, peritoneal exudate cell; SNP, single nucleotide polymorphism; STAg, soluble tachyzoite Ag; TAP, transporter associated with Ag processing.
References
- 1.Joiner, K.A., S.A. Fuhrman, H.M. Miettinen, L.H. Kasper, and I. Mellman. 1990. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 249:641–646. [DOI] [PubMed] [Google Scholar]
- 2.Jones, T.C., and J.G. Hirsch. 1972. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J. Exp. Med. 136:1173–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mordue, D.G., and L.D. Sibley. 1997. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 159:4452–4459. [PubMed] [Google Scholar]
- 4.Schwab, J.C., C.J. Beckers, and K.A. Joiner. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. USA. 91:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luft, B.J., R.G. Brooks, F.K. Conley, R.E. McCabe, and J.S. Remington. 1984. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 252:913–917. [PubMed] [Google Scholar]
- 6.Mariuz, P., E.M. Bosler, and B.J. Luft. 1994. Toxoplasmosis in individuals with AIDS. Infect. Dis. Clin. North Am. 8:365–381. [PubMed] [Google Scholar]
- 7.Navia, B.A., C.K. Petito, J.W. Gold, E.S. Cho, B.D. Jordan, and R.W. Price. 1986. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann. Neurol. 19:224–238. [DOI] [PubMed] [Google Scholar]
- 8.Denkers, E.Y., B.A. Butcher, L. Del Rio, and S. Bennouna. 2004. Neutrophils, dendritic cells and Toxoplasma. Int. J. Parasitol. 34:411–421. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio, L., S. Bennouna, J. Salinas, and E.Y. Denkers. 2001. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167:6503–6509. [DOI] [PubMed] [Google Scholar]
- 10.Denkers, E.Y., R.T. Gazzinelli, D. Martin, and A. Sher. 1993. Emergence of NK1.1+ cells as effectors of IFN-γ–dependent immunity to Toxoplasma gondii in MHC class I–deficient mice. J. Exp. Med. 178:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliss, S.K., B.A. Butcher, and E.Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515–4521. [DOI] [PubMed] [Google Scholar]
- 12.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R.N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sher, A., S. Hieny, H. Charest, T. Scharton-Kersten, C. Collazo, R.N. Germain, and C. Reis e Sousa. 1998. The role of dendritic cells in the initiation of host resistance to Toxoplasma gondii. Adv. Exp. Med. Biol. 452:103–110. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, C.A., C.S. Subauste, V.H. Van Cleave, and J.S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter, C.A., L. Ellis-Neyer, K.E. Gabriel, M.K. Kennedy, K.H. Grabstein, P.S. Linsley, and J.S. Remington. 1997. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J. Immunol. 158:2285–2293. [PubMed] [Google Scholar]
- 16.Johnson, L.L., F.P. VanderVegt, and E.A. Havell. 1993. Gamma interferon-dependent temporary resistance to acute Toxoplasma gondii infection independent of CD4+ or CD8+ lymphocytes. Infect. Immun. 61:5174–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175–180. [PubMed] [Google Scholar]
- 18.Gazzinelli, R.T., F.T. Hakim, S. Hieny, G.M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286–292. [PubMed] [Google Scholar]
- 19.Parker, S.J., C.W. Roberts, and J. Alexander. 1991. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin. Exp. Immunol. 84:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki, Y., and J.S. Remington. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J. Immunol. 140:3943–3946. [PubMed] [Google Scholar]
- 21.Cox, J.H., J.W. Yewdell, L.C. Eisenlohr, P.R. Johnson, and J.R. Bennink. 1990. Antigen presentation requires transport of MHC class I molecules from the endoplasmic reticulum. Science. 247:715–718. [DOI] [PubMed] [Google Scholar]
- 22.Lehner, P.J., and P. Cresswell. 1996. Processing and delivery of peptides presented by MHC class I molecules. Curr. Opin. Immunol. 8:59–67. [DOI] [PubMed] [Google Scholar]
- 23.Yewdell, J.W., and J.R. Bennink. 2001. Cut and trim: generating MHC class I peptide ligands. Curr. Opin. Immunol. 13:13–18. [DOI] [PubMed] [Google Scholar]
- 24.Cresswell, P. 2000. Intracellular surveillance: controlling the assembly of MHC class I-peptide complexes. Traffic. 1:301–305. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 26.Cresswell, P., A.L. Ackerman, A. Giodini, D.R. Peaper, and P.A. Wearsch. 2005. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 207:145–157. [DOI] [PubMed] [Google Scholar]
- 27.Bevan, M.J. 1976. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143:1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Haan, J.M., and M.J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437–441. [DOI] [PubMed] [Google Scholar]
- 29.Albert, M.L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 392:86–89. [DOI] [PubMed] [Google Scholar]
- 30.Norbury, C.C., S. Basta, K.B. Donohue, D.C. Tscharke, M.F. Princiotta, P. Berglund, J. Gibbs, J.R. Bennink, and J.W. Yewdell. 2004. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 304:1318–1321. [DOI] [PubMed] [Google Scholar]
- 31.Gubbels, M.J., B. Striepen, N. Shastri, M. Turkoz, and E.A. Robey. 2005. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect. Immun. 73:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertholet, S., R. Goldszmid, A. Morrot, A. Debrabant, F. Afrin, C. Collazo-Custodio, M. Houde, M. Desjardins, A. Sher, and D. Sacks. 2006. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J. Immunol. 177:3525–3533. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, Y., M.A. Orellana, R.D. Schreiber, and J.S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 240:516–518. [DOI] [PubMed] [Google Scholar]
- 34.Sher, A., C. Collazzo, C. Scanga, D. Jankovic, G. Yap, and J. Aliberti. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27:521–528. [DOI] [PubMed] [Google Scholar]
- 35.Aliberti, J., D. Jankovic, and A. Sher. 2004. Turning it on and off: regulation of dendritic cell function in Toxoplasma gondii infection. Immunol. Rev. 201:26–34. [DOI] [PubMed] [Google Scholar]
- 36.Jankovic, D., M.C. Kullberg, S. Hieny, P. Caspar, C.M. Collazo, and A. Sher. 2002. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity. 16:429–439. [DOI] [PubMed] [Google Scholar]
- 37.Macatonia, S.E., N.A. Hosken, M. Litton, P. Vieira, C.S. Hsieh, J.A. Culpepper, M. Wysocka, G. Trinchieri, K.M. Murphy, and A. O'Garra. 1995. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 154:5071–5079. [PubMed] [Google Scholar]
- 38.Liu, C.H., Y.T. Fan, A. Dias, L. Esper, R.A. Corn, A. Bafica, F.S. Machado, and J. Aliberti. 2006. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J. Immunol. 177:31–35. [DOI] [PubMed] [Google Scholar]
- 39.Ljunggren, H.G., L. Van Kaer, H.L. Ploegh, and S. Tonegawa. 1994. Altered natural killer cell repertoire in Tap-1 mutant mice. Proc. Natl. Acad. Sci. USA. 91:6520–6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim, S., J. Poursine-Laurent, S.M. Truscott, L. Lybarger, Y.J. Song, L. Yang, A.R. French, J.B. Sunwoo, S. Lemieux, T.H. Hansen, and W.M. Yokoyama. 2005. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 436:709–713. [DOI] [PubMed] [Google Scholar]
- 41.Moretta, A. 2002. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat. Rev. Immunol. 2:957–964. [DOI] [PubMed] [Google Scholar]
- 42.Della Chiesa, M., S. Sivori, R. Castriconi, E. Marcenaro, and A. Moretta. 2005. Pathogen-induced private conversations between natural killer and dendritic cells. Trends Microbiol. 13:128–136. [DOI] [PubMed] [Google Scholar]
- 43.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharton, T.M., and P. Scott. 1993. Natural killer cells are a source of interferon γ that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mailliard, R.B., Y.I. Son, R. Redlinger, P.T. Coates, A. Giermasz, P.A. Morel, W.J. Storkus, and P. Kalinski. 2003. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol. 171:2366–2373. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Fontecha, A., L.L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260–1265. [DOI] [PubMed] [Google Scholar]
- 47.Guan, H., M. Moretto, D.J. Bzik, J. Gigley, and I.A. Khan. 2007. NK cells enhance dendritic cell response against parasite antigens via NKG2D pathway. J. Immunol. 179:590–596. [DOI] [PubMed] [Google Scholar]
- 48.Kosaka, A., D. Wakita, N. Matsubara, Y. Togashi, S. Nishimura, H. Kitamura, and T. Nishimura. 2007. AsialoGM1+CD8+ central memory-type T cells in unimmunized mice as novel immunomodulator of IFN-gamma-dependent type 1 immunity. Int. Immunol. 19:249–256. [DOI] [PubMed] [Google Scholar]
- 49.Bix, M., N.S. Liao, M. Zijlstra, J. Loring, R. Jaenisch, and D. Raulet. 1991. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 349:329–331. [DOI] [PubMed] [Google Scholar]
- 50.Dorfman, J.R., and D.H. Raulet. 1998. Acquisition of Ly49 receptor expression by developing natural killer cells. J. Exp. Med. 187:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansson, S., M. Johansson, E. Rosmaraki, G. Vahlne, R. Mehr, M. Salmon-Divon, F. Lemonnier, K. Karre, and P. Hoglund. 2005. Natural killer cell education in mice with single or multiple major histocompatibility complex class I molecules. J. Exp. Med. 201:1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao, N.S., M. Bix, M. Zijlstra, R. Jaenisch, and D. Raulet. 1991. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 253:199–202. [DOI] [PubMed] [Google Scholar]
- 53.Behar, S.M., C.C. Dascher, M.J. Grusby, C.R. Wang, and M.B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar, S., and R.L. Tarleton. 1998. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 20:207–216. [DOI] [PubMed] [Google Scholar]
- 55.Dorfman, J.R., J. Zerrahn, M.C. Coles, and D.H. Raulet. 1997. The basis for self-tolerance of natural killer cells in beta2-microglobulin- and TAP-1- mice. J. Immunol. 159:5219–5225. [PubMed] [Google Scholar]
- 56.Salcedo, M., A.D. Diehl, M.Y. Olsson-Alheim, J. Sundback, L. Van Kaer, K. Karre, and H.G. Ljunggren. 1997. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J. Immunol. 158:3174–3180. [PubMed] [Google Scholar]
- 57.Hoglund, P., C. Ohlen, E. Carbone, L. Franksson, H.G. Ljunggren, A. Latour, B. Koller, and K. Karre. 1991. Recognition of beta 2-microglobulin-negative (beta 2m−) T-cell blasts by natural killer cells from normal but not from beta 2m− mice: nonresponsiveness controlled by beta 2m− bone marrow in chimeric mice. Proc. Natl. Acad. Sci. USA. 88:10332−10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce, R.B., L. Trigler, E.K. Svaasand, and C.M. Peterson. 1993. Polymorphism in the mouse Tap-1 gene. Association with abnormal CD8+ T cell development in the nonobese nondiabetic mouse. J. Immunol. 151:5338–5347. [PubMed] [Google Scholar]
- 59.Ogasawara, K., J.A. Hamerman, H. Hsin, S. Chikuma, H. Bour-Jordan, T. Chen, T. Pertel, C. Carnaud, J.A. Bluestone, and L.L. Lanier. 2003. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 18:41–51. [DOI] [PubMed] [Google Scholar]
- 60.Tay, C.H., R.M. Welsh, and R.R. Brutkiewicz. 1995. NK cell response to viral infections in beta 2-microglobulin-deficient mice. J. Immunol. 154:780–789. [PubMed] [Google Scholar]
- 61.Ohteki, T., and H.R. MacDonald. 1994. Major histocompatibility complex class I–related molecules control the development of CD4+8− and CD4−8− subsets of natural killer 1.1+ T cell receptor-α/β+ cells in the liver of mice. J. Exp. Med. 180:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smiley, S.T., P.A. Lanthier, K.N. Couper, F.M. Szaba, J.E. Boyson, W. Chen, and L.L. Johnson. 2005. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J. Immunol. 174:7904–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scharton-Kersten, T., H. Nakajima, G. Yap, A. Sher, and W.J. Leonard. 1998. Infection of mice lacking the common cytokine receptor gamma-chain (gamma(c)) reveals an unexpected role for CD4+ T lymphocytes in early IFN-gamma-dependent resistance to Toxoplasma gondii. J. Immunol. 160:2565–2569. [PubMed] [Google Scholar]
- 64.Khan, I.A., S.Y. Thomas, M.M. Moretto, F.S. Lee, S.A. Islam, C. Combe, J.D. Schwartzman, and A.D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makrigiannis, A.P., A.T. Pau, A. Saleh, R. Winkler-Pickett, J.R. Ortaldo, and S.K. Anderson. 2001. Class I MHC-binding characteristics of the 129/J Ly49 repertoire. J. Immunol. 166:5034–5043. [DOI] [PubMed] [Google Scholar]
- 66.Roos, D.S., R.G. Donald, N.S. Morrissette, and A.L. Moulton. 1994. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 45:27–63. [DOI] [PubMed] [Google Scholar]
- 67.Grunvald, E., M. Chiaramonte, S. Hieny, M. Wysocka, G. Trinchieri, S.N. Vogel, R.T. Gazzinelli, and A. Sher. 1996. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect. Immun. 64:2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldszmid, R.S., J. Idoyaga, A.I. Bravo, R. Steinman, J. Mordoh, and R. Wainstok. 2003. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J. Immunol. 171:5940–5947. [DOI] [PubMed] [Google Scholar]
- 69.Jankovic, D., M.C. Kullberg, C.G. Feng, R.S. Goldszmid, C.M. Collazo, M. Wilson, T.A. Wynn, M. Kamanaka, R.A. Flavell, and A. Sher. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J. Exp. Med. 204:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.