Abstract
The γδ T cell receptor for antigen (TCR) comprises the clonotypic TCRγδ, the CD3 (CD3γε and/or CD3δε), and the ζζ dimers. γδ T cells do not develop in CD3γ-deficient mice, whereas human patients lacking CD3γ have abundant peripheral blood γδ T cells expressing high γδ TCR levels. In an attempt to identify the molecular basis for these discordant phenotypes, we determined the stoichiometries of mouse and human γδ TCRs using blue native polyacrylamide gel electrophoresis and anti-TCR–specific antibodies. The γδ TCR isolated in digitonin from primary and cultured human γδ T cells includes CD3δ, with a TCRγδCD3ε2δγζ2 stoichiometry. In CD3γ-deficient patients, this may allow substitution of CD3γ by the CD3δ chain and thereby support γδ T cell development. In contrast, the mouse γδ TCR does not incorporate CD3δ and has a TCRγδCD3ε2γ2ζ2 stoichiometry. CD3γ-deficient mice exhibit a block in γδ T cell development. A human, but not a mouse, CD3δ transgene rescues γδ T cell development in mice lacking both mouse CD3δ and CD3γ chains. This suggests important structural and/or functional differences between human and mouse CD3δ chains during γδ T cell development. Collectively, our results indicate that the different γδ T cell phenotypes between CD3γ-deficient humans and mice can be explained by differences in their γδ TCR composition.
The γδ TCR is a multimeric complex consisting of a clonotypic TCRγδ heterodimer, the CD3δε and/or CD3γε dimer, and the ζζ dimer. Because γδ TCR signaling regulates the commitment of double-negative (CD4−CD8−) cells to the γδ T cell lineage and is required for their subsequent differentiation into mature γδ T cells, the development of γδ T cells depends on the expression of the γδ TCR. Indeed, neither CD3ε- nor CD3γ-deficient mice have γδ T cells (1–3). Although the overall structure and function of the γδ TCRs in mice and humans are quite similar, ablation of the highly related CD3γ and CD3δ subunits has markedly different effects in these two species. Hence, γδ T cell development is severely impaired in CD3γ-deficient mice but not in their human counterparts (3, 4). Conversely, CD3δ deficiency results in a block in human, but not mouse, γδ T cell development (5, 6).
In contrast to the γδ TCR, the αβ TCR has been studied extensively and its minimal stoichiometry is proposed to be TCRαβCD3ε 2δγζ2 in mice and humans (7, 8). Unlike the mouse αβ TCR, mouse γδ TCR does not incorporate CD3δ even if this subunit is expressed intracellularly (9, 10), explaining why γδ T cells develop normally in CD3δ-deficient mice (6). Interestingly, the composition of the mouse γδ TCR complex changes in activated cells: a differentially glycosylated form of CD3γ becomes incorporated into the receptor (9) and ζ can be substituted by the FcRγ chain (10).
Contradictory findings have been reported concerning human γδ TCR stoichiometry. Primary human γδ T cells were found to incorporate little or no CD3δ into their surface γδ TCRs (10). In contrast, human γδ T cell clones and lines were found to possess both CD3δε and CD3γε dimers (11, 12). In light of the reported activation-induced changes in mouse γδ TCR composition, it is possible that although CD3δ is not incorporated into TCRs of naive human γδ T cells, this chain becomes part of the receptor on γδ T cell clones that have undergone activation and expansion.
In this study, we use blue native PAGE (BN-PAGE) and specific anti-CD3 antibodies to determine the stoichiometries of human and mouse γδ TCRs. These data are complemented by studies on the human CD3γ (hCD3γ) deficiency phenotype, as well as those of CD3γδ-deficient mice supplemented with mouse or hCD3δ transgenes. In conclusion, we show that there are differences in the stoichiometries and, thus, subunit requirements for the assembly of mouse and human γδ TCRs.
RESULTS AND DISCUSSION
γδ T cells with high levels of γδ TCR are present in CD3γ-deficient patients
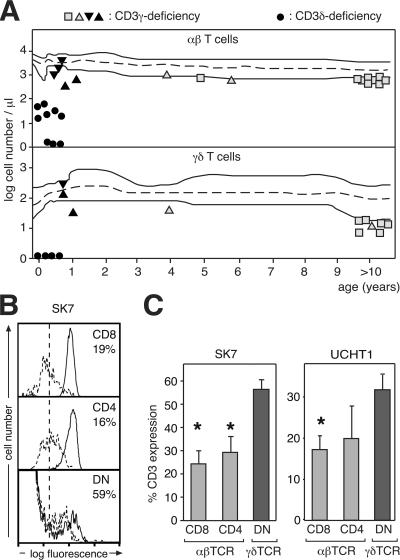
In CD3γ knockout (CD3γ−/−) mice, γδ T cell development is blocked (3); however, this is not the case in CD3γ-deficient humans. We have studied four CD3γ-deficient patients (13, 14), including one >20 yr old, and consistently found that γδ T cells are present in their peripheral blood (Fig. 1 A). As is the case with αβ T cells, the number of γδ T cells in these patients was at or just below the lower limit (P5) of healthy CD3γ-sufficient controls. In the absence of CD3γ, CD3 expression by αβ T cells is reduced to ∼20% of that of healthy controls (4). However, when we analyzed γδ T cells from these patients by flow cytometry using anti-CD3 antibodies, we found that the amount of γδ TCR per T cell was only reduced to 30–55% of healthy individuals, depending on the antibody used (Fig. 1, B and C). These data show that hCD3δ can compensate, at least partially, for the lack of hCD3γ in assembly and surface transport of the human γδ TCR. In fact, in the absence of CD3γ, these processes appear to occur more efficiently in γδ T cells than in αβ T cells. As a consequence, γδ T cells can develop in CD3γ-deficient patients, indicating that hCD3δ can functionally replace hCD3γ to promote γδ T cell development. In conclusion, the human γδ TCR can assemble and signal for selection efficiently without hCD3γ.
Figure 1.
CD3γ-deficient patients show abundant peripheral blood γδ T cells with high levels of γδ TCR. (A) Presence of γδ T cells in hCD3γ deficiency. Peripheral blood cell counts from four CD3γ-deficient individuals are plotted as a function of age in comparison with the normal distribution (dashed line). Three were homozygous for a p.K69X mutation (triangles), and one was compound heterozygous for p.M1V and p.N28V/H29X (squares). CD3δ-deficient patients (circles) are included for comparison. Filled symbols identify SCID patients, who died before 1 yr of age. (B) CD3 expression is higher on CD3γ-deficient γδ than αβ T cells. Flow cytometry histograms of anti-CD3 (SK7)–stained CD3γ-deficient T cells (dashed lines) are compared with healthy controls (continuous lines) either in αβ (CD8 and CD4; top and middle) or γδ (double negative; bottom) T cells. Numbers indicate TCR expression (mean fluorescence intensity) on cells from CD3γ-deficient patients as a percentage of that on cells from healthy donors. The vertical dashed line indicates the background fluorescence using an irrelevant antibody. (C) Quantification of the CD3 expression on the indicated cell types from CD3γ-deficient patients as a percentage of that on the same cell types from healthy donors (percentage of CD3 expression). Data are expressed as the percent mean fluorescence intensity ± SEM from three different patients using the anti-CD3 antibodies SK7 (left) or UCHT1 (right). Similar results were obtained using other anti-CD3 antibodies, as well as other gating criteria (not depicted). *, P < 0.05 compared with γδ T cells.
The human γδ TCR includes CD3δ
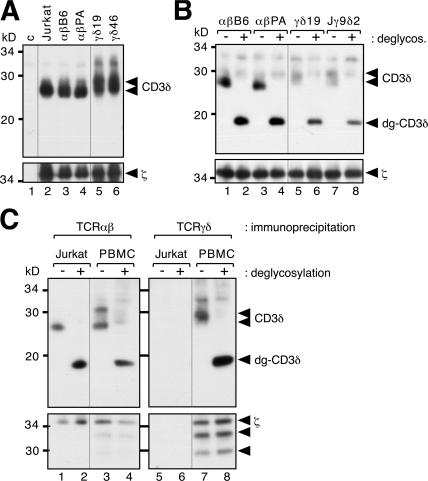
The different subunit requirements for γδ T cell development in mice and humans could reflect distinct γδ TCR subunit composition in these species. To clarify the composition of the human γδ TCR, we used established human γδ T cell clones as well as primary γδ T cells. Because our γδ T cell clones contained ∼5% residual irradiated feeder cells expressing the αβ TCR, we depleted αβ TCRs after cell lysis by immunopurification with anti-TCRβ antibodies (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20070782/DC1). This was done for all experiments in which γδ T cell clones were used. In the first experiment, we lysed human αβ as well as γδ T cell clones and immunopurified the TCRs with anti-ζ antibodies. After nonreducing SDS-PAGE, purified proteins were detected using anti-CD3δ and anti-ζ antibodies (Fig. 2 A). The αβ TCR of the αβ T cell line Jurkat and the αβ clones αβB6 and αβPA (lanes 2–4), as well as the γδ TCR of clones γδ19 and γδ46 (lanes 5 and 6), all contained CD3δ. The reduced electrophoretic mobility of CD3δ associated with the γδ TCR could be caused by more complex glycosylation (11). To test this, we treated purified TCRs with N-glycosidase F, which cleaves N-linked carbohydrate moieties. Indeed, the deglycosylated forms of αβ TCR– and γδ TCR–associated CD3δ are the same size (Fig. 2 B, lanes 2, 4, and 6). This clearly indicates that the γδ TCR expressed on human γδ clones contains CD3δ.
Figure 2.
The human γδ TCR includes CD3δ. (A) Human γδ T cell clones incorporate CD3δ into the γδ TCR. Anti-ζ immunopurified TCRs from Jurkat, αβ (αβB6 and αβPA), and γδ (γδ19 and γδ46) T cell clones were separated on nonreducing SDS-PAGE and analyzed via Western blotting using anti-CD3δ and anti-ζ antibodies. In the control (C), which was loaded on another gel, anti-ζ antibodies and protein G–coupled sepharose were incubated in lysis buffer alone. (B) αβ TCR– and γδ TCR–associated CD3δ chains are differentially glycosylated. Anti-ζ immunopurified TCRs from αβ (αβB6 and αβPA) and γδ (γδ19) T cell clones, as well as the Jurkat variant Jγ9δ2, were left untreated (−) or subject to N-glycosidase F treatment (+) and analyzed as in A. Glycosylated (CD3δ) and deglycosylated (dg-CD3δ) CD3δ chains are indicated by arrowheads. (C) The γδ TCR on primary human γδ T cells contains CD3δ. TCRs from Jurkat and human PBMCs were immunopurified using anti-TCRβ and anti-TCRγδ antibodies and subjected to deglycosylation and analysis as in B.
To identify the cause of this differential glycosylation, we used an αβ TCR–deficient variant of Jurkat stably expressing transfected TCRVγ9 and Vδ2 chains, named Jγ9δ2 (15). The CD3δ of Jγ9δ2 had a similar mobility to CD3δ in the γδ clones (Fig. 2 B, lanes 5 and 7). Therefore, the complex CD3δ glycosylation is intrinsic to the γδ TCR and not caused by different cellular environments of αβ and γδ T cells. Incorporation of CD3δ into the γδ TCRs of human clones and cell lines was confirmed by anti-CD3δ immunoprecipitation and subsequent anti-ζ Western blotting (Fig. S1 and not depicted). This is in line with earlier reports using γδ T cell clones and lines (11, 12). When primary human γδ T cells were used, CD3δ could not be detected (10); however, the composition of the mouse γδ TCR changed upon cultivation of primary γδ T cells such that ζ was replaced by FcRγ (10). Likewise, the TCR of primary human γδ T cells might not contain CD3δ but may incorporate it during cultivation.
To determine whether CD3δ is present in the γδ TCR of primary human T cells, we lysed PBMCs from a healthy donor and purified αβ and γδ TCRs with anti-TCRβ and anti-TCRγδ antibodies, respectively. Purified proteins were left untreated or deglycosylated and separated by SDS-PAGE (Fig. 2 C). Jurkat cells were used as a control. Indeed, the γδ TCR and αβ TCR roughly contained equal amounts of CD3δ (lanes 4 and 8, when normalized to ζ). The CD3δ double band from γδ T cells exhibited a slower electrophoretic mobility than that from αβ T cells (lanes 3 and 7). In addition, shorter ζ chains, which probably represent differential mRNA splicing, were incorporated into the γδ TCR (lanes 7 and 8). In an earlier study, CD3δ was not found associated with the γδ TCR from primary human T cells (10). It is likely that, because of its different glycosylation, the γδ TCR–associated CD3δ had similar mobility to CD3γ and, therefore, could not be resolved when biotinylated proteins were detected by SDS-PAGE and streptavidin Western blotting (10). In conclusion, our data show that the human γδ TCR contains CD3δ in cultured as well as in primary γδ T cells.
The human γδ TCR has a stoichiometry of TCRγδCD3ε2γδζ2
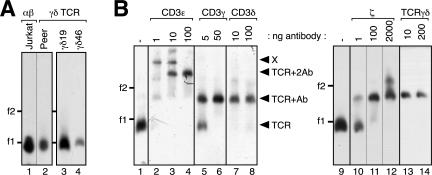
BN-PAGE is a method used to study the native structures of multiprotein complexes (16). In our experiments, we used this technique to analyze the size of the human γδ TCR compared with that of the αβ TCR. After digitonin lysis of Jurkat and the human γδ T cell line Peer, as well as γδ T cell clones γδ19 and γδ46, TCRs were purified, separated by BN-PAGE, and detected by immunoblotting with an anti-ζ antibody (Fig. 3 A). The αβ TCR, with a stoichiometry of TCR αβ CD3ε 2γδζ2 (7, 8), had the same size as the γδ TCR, suggesting a similar stoichiometry for the γδ TCR. Similar results were obtained from nonpurified TCRs (unpublished data).
Figure 3.
The human γδ TCR has a stoichiometry of TCRγδCD3ε2γδζ2. (A) The digitonin-solubilized γδ TCR is the same size as the αβ TCR. TCRs from Jurkat, Peer, and the γδ T cell clones γδ19 and γδ46 were purified, separated by BN-PAGE, and analyzed via Western blotting using the anti-ζ antibody. (B) Digitonin-extracted TCRs from γδ T cell clone γδ19 were incubated with the indicated amounts of antibodies against hCD3ε (UCHT1), hCD3γ (HMT3.2), hCD3δ (APA1/2), ζ (G3), and hTCRγδ (5A6.E9), separated by BN-PAGE and analyzed as in A. The number of shifts correlates with the number of antibody binding sites in the TCR complex, as indicated by arrowheads. The marker protein is ferritin in its 24-meric (f1, 440 kD) and 48-meric (f2) forms.
To determine the stoichiometry of the human γδ TCR, we made use of the native antibody mobility shift (NAMOS) assay that we previously developed to determine the αβ TCR stoichiometry (8, 17). The digitonin-extracted γδ TCR was incubated with different amounts of an anti-CD3ε antibody, UCHT1, and then subjected to BN-PAGE (Fig. 3 B, lanes 2–4). At the highest antibody concentration, the γδ TCR shifted twice (lane 4, arrowhead labeled TCR+2Ab). This indicates that the γδ TCR has two binding sites for UCHT1, because each antibody molecule bound to the complex produces a discrete change in electrophoretic mobility. At nonsaturating antibody concentrations, a partial shift, indicating γδ TCR bound to only one antibody (TCR+Ab) and cross-linked products, in which one antibody bound to two TCRs (marked with X), were observed (lanes 2 and 3). These data show that the human γδ TCR incorporates two CD3 dimers.
To verify the specificity of anti-CD3γ and anti-CD3δ antibodies, we expressed individual mouse and human TCR subunits in Drosophila S2 cells and performed subsequent immunopurifications, verifying antibody specificity for HMT3.2 (anti-hCD3γ) and APA1/2 (anti-hCD3δ; Fig. S2 A, available at http://www.jem.org/cgi/content/full/jem.20070782/DC1). Using these antibodies in the NAMOS assay revealed that one copy each of CD3γ and CD3δ are present in the human γδ TCR (Fig. 3 B, lanes 5–8). This is in agreement with the fact that CD3ε pairs with either CD3γ or CD3δ (18). An anti-TCRγδ antibody produced only one shift, whereas anti-ζ produced two shifts (lanes 9–14). Although ζ is a homodimer, the antibody could not bind twice to most γδ TCRs (lane 12). This is caused by steric hindrance, because ζ is very small (16 kD) (8). The same band patterns were observed for several other human γδ T cell clones analyzed (unpublished data). In conclusion, the digitonin-solubilized human γδ TCR has a stoichiometry of TCRγδCD3ε 2γδζ2.
The mouse γδ TCR has a stoichiometry of TCRγδCD3ε2γ2ζ2
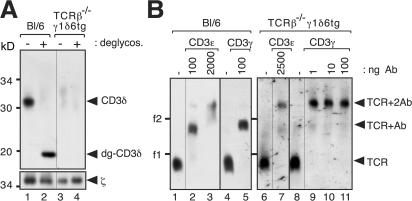
The mouse γδ TCR was reported to lack CD3δ (10). We aimed to study mouse γδ TCR stoichiometry using our reagents and methods. Splenocytes from TCRβ−/− mice (19) carrying transgenes for the TCRVγ1.1 and TCRVδ6 chains (TCRβ−/−γ1δ6tg) (20) served as a source of primary γδ T cells. Initially, we compared the αβ TCR from wild-type Bl/6 mice with the γδ TCR from TCRβ−/−γ1δ6tg mice by anti-ζ immunopurification and anti-CD3δ Western blotting (Fig. 4 A). As expected, the γδ TCR did not contain CD3δ (lanes 3 and 4). BN-PAGE showed that the digitonin-solubilized γδ TCR has a similar mobility to the αβ TCR for which the stoichiometry has been determined to be TCRαβCD3ε 2γδζ2 (Fig. 4 B, lanes 1,4, 6, and 8) (8). To ascertain mouse γδ TCR stoichiometry, the NAMOS assay was applied using antibodies that had been controlled for specificity (Fig. S2 B). Anti-CD3ε, as well as anti-CD3γ, antibodies produced two shifts, indicating that the mouse γδ TCR contains two CD3γε dimers (Fig. 4 B, lanes 6–11). Because CD3ε always pairs with either CD3γ or CDδ and because the mobility of the γδ TCR in BN-PAGE was the same as that of the αβ TCR, we concluded that the digitonin-solubilized mouse γδ TCR has a stoichiometry of TCRγδCD3ε 2γ2ζ2. This stoichiometry is in agreement with the conserved charge distributions in the transmembrane segments of the αβ and γδ TCR subunits and with the 1:2 ratio of TCRγδ/CD3ε in primary mouse γδ T cells (21).
Figure 4.
The mouse γδ TCR has a stoichiometry of TCRγδCD3ε2γ2ζ2. (A) The γδ TCR on primary mouse γδ T cells does not contain CD3δ. TCRs from splenocytes from wild-type (Bl/6) and TCRβ−/−γ1δ6tg mice were immunopurified with an anti-ζ antiserum. They were left untreated or were deglycosylated, separated via SDS-PAGE and analyzed by Western blotting as in Fig. 2 (B and C). (B) The mouse γδ TCR has two CD3εγ dimers. Splenocytes from wild-type (Bl/6) and TCRβ−/−γ1δ6tg mice were lysed in digitonin, and purified TCRs were incubated with the indicated amounts of antibodies against CD3ε (145-2C11) or CD3γ (17A2), separated by BN-PAGE, and analyzed by Western blotting as in Fig. 3. Lanes 1, 4, 6, and 8 show TCRs alone. The number of shifts correlates with the number of antibody binding sites in the TCR complex, as indicated by arrowheads. The marker protein is ferritin in its 24-meric (f1, 440 kD) and 48-meric (f2) forms.
Mouse TCRδ can bind to mouse CD3γε (mCD3γε) but not to mCD3δε (9, 10, 21), whereas human TCRδ binds both hCD3δ&egr; and hCD3γε (15). These results are in agreement with the different stoichiometries determined in our experiments for human and mouse γδ TCRs.
Human, but not mouse, CD3δ can restore γδ T cell development in CD3γ/CD3δ double-deficient (CD3γδ−/−) mice
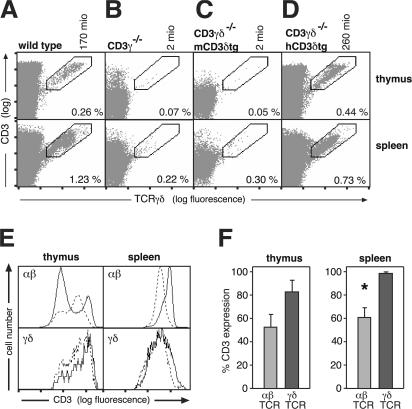
We asked whether the different subunit requirements for mouse versus human γδ TCR formation were caused by sequence differences in their respective CD3δ subunits. As expected, our CD3γ−/− and CD3γδ−/− mice both lack γδ T cells (Fig. 5 B and not depicted) (3, 22). This was not caused by limiting amounts of CD3δ, because a mCD3δ transgene (mCD3δtg) could not rescue γδ T cell development (Fig. 5 C). In contrast, the CD3γδ−/− mouse strain carrying an hCD3δ transgene (CD3γδ−/−hCD3δtg) (23) has as many γδ T cells as wild-type mice (Fig. 5, A and D). These cells could be detected in the thymus, spleen, lymph nodes, and blood (Fig. 5 and not depicted), indicating that hCD3δ can functionally replace mCD3γ in the mouse γδ TCR, whereas mCD3δ cannot. Sequence- and structure-wise, hCD3δ is more related to mCD3δ than to mCD3γ (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20070782/DC1) (24, 25). The property of hCD3δ to replace mCD3γ is probably independent of the signal-transducing immunoreceptor tyrosine-based activation motif sequence of the cytoplasmic tail, because γδ T cell development is unaffected in mice lacking the CD3γ immunoreceptor tyrosine-based activation motif (26). Thus, the functional differences between hCD3δ and mCD3δ might map to the extracellular region, as the transmembrane regions are highly conserved between the different CD3 chains (Fig. S3). Indeed, the ectodomains are critical for TCR assembly, suggesting that hCD3δ can assemble within mouse TCRγδ to form a functional γδ TCR, whereas mCD3δ cannot. This conclusion is in line with the finding that mouse TCRδ can assemble with hCD3δε (15), in contrast to mCD3δε.
Figure 5.
hCD3δ can substitute both mCD3γ and mCD3δ in γδ T cell development. Thymocytes and splenocytes from wild-type (A), CD3γ−/−(B), CD3γδ−/−mCD3δtg (C), and CD3γδ−/−hCD3δtg (D) mice were surface stained with anti-CD3 (145-2C11) and anti-TCRγδ (GL3) antibodies and analyzed by flow cytometry. Percentages of cells in the marked regions and the total number of γδ T cells (in millions) in the thymi are shown within and above the dot plots, respectively. (E) CD3 expression is higher on CD3γ-deficient γδ than αβ T cells. Flow cytometry histograms of anti-CD3 (2C11)–stained CD3γδ−/−hCD3δtg T cells (dashed lines) are compared with wild-type mice (continuous lines) either in αβ (top) or γδ (bottom) T cells from the thymus (left) or spleen (right). (F) Quantification of the CD3 expression on αβ or γδ T cells from CD3γδ−/−hCD3δtg mice as a percentage of that on the same cell types from wild-type mice (percentage of CD3 expression). The CD3 high population was used for the αβ TCR in thymocytes. Data are expressed as the percent mean fluorescence intensity ± SEM from two independent experiments. *, P < 0.05 compared with γδ T cells. mio, millions.
In our mice, αβ TCR expression was reduced to 60%, whereas that of γδ TCR was 80–100% of wild-type TCR levels. As in hCD3γ-deficient patients (Fig. 1), in CD3γδ−/−hCD3δtg mice, the γδ TCR expression level was less affected by the absence of CD3γ than that of the αβ TCR (Fig. 5, E and F) when compared with wild-type mice.
The stoichiometries of human and mouse γδ TCRs correlate well with the phenotypes for human and mCD3 deficiencies. Mice lacking CD3δ exhibit normal γδ T cell development (6), consistent with the finding that mCD3δ is not part of the mouse γδ TCR (9, 10). In contrast, CD3γ−/− mice do not contain γδ T cells (3), because CD3γ is an obligatory subunit of the mouse γδ TCR. In humans, both CD3δ and CD3γ are part of the γδ TCR (Figs. 2 and Figs.3; and Fig. S1). CD3γ-deficient γδ TCRs are still able to support γδ T cell development in humans (Fig. 1), likely because hCD3δ can partially substitute for hCD3γ. Remarkably, hCD3δ is also able to rescue γδ T cell development in CD3γδ−/− mice, indicating its ability to substitute for mCD3γ in the mouse γδ TCR as well. In contrast, CD3δ-deficient patients do not develop γδ T cells (Fig. 1 A) (5). Presumably, hCD3γ cannot substitute for hCD3δ in human γδ TCR formation and function. Along this line, replacement of the mTCRα connecting peptide by the one of mTCRδ promotes the exclusion of mCD3δ from the complex (27), suggesting that the connecting peptide of mTCRδ is involved in the association with CD3γ but does not permit the assembly of CD3δ. A difference in the connecting peptide sequences of human and mouse TCRδ could be responsible for the differential involvement in γδ TCR assembly of CD3δ in both species.
Conclusions
Using both conventional immunopurification followed by Western blotting and our novel NAMOS assay, we have determined the human digitonin-solubilized γδ TCR stoichiometry to be TCRγδCD3ε 2γδζ2. The CD3δ chain is differentially glycosylated depending on its association with the αβ or the γδ TCR (11), likely accounting for contradictory results previously reported (10, 12). We show that the mouse γδ TCR stoichiometry is TCRγδCD3ε 2γ2ζ2, as proposed by Hayes and Love (21). Clarification of both mouse and human γδ TCR stoichiometries finally explains the different phenotypes observed in CD3-deficient humans and mice. We show that, in contrast to mCD3δ, an hCD3δ transgene is able to rescue γδ T cell development in mice lacking both mCD3δ and mCD3γ. This indicates important structural and functional differences between hCD3δ and mCD3δ chains, as already suggested from the analysis of αβ T cells (23). Indeed, the phenotype of CD3γδ−/−hCD3δtg mice (Fig. 5) resembles that of CD3γ-deficient humans (4, 23), as opposed to that of CD3γ−/− mice (3). This is true for αβ (23) as well as for γδ T cells (Fig. 5). Thus, this humanized CD3γ-deficient mouse strain may be a valuable tool to further study the impact of CD3γ-deficiency in αβ as well as γδ T cell pathophysiology in humans.
MATERIALS AND METHODS
Cells, mice, and antibodies.
Human αβ and γδ T cell clones were generated as previously described (28). TCRαβ−/− Jurkat cells transfected with Vγ9δ2 (Jγ9δ2) were previously described (15). Human PBMCs were isolated from a healthy donor using a Ficoll gradient. TCRβ−/− (19) and Vγ1.1Vδ6tg mice (a gift of P. Pereira, Institut Pasteur, Paris, France) (20), both on a C57BL/6 background, were mated, generating the TCRβ−/−γ1δ6tg strain. CD3γ−/− (3), CD3γδ−/−mCD3δtg, and CD3γδ−/−hCD3δtg (23) mice were previously described (D. Kappes [Fox Chase Cancer Center, Philadelphia, PA] and C. Terhorst [Beth Israel Deaconess Medical Center, Boston, MA] provided the mCD3δtg and CD3γδ−/− mice, respectively). Mice were killed between 6 and 12 wk of age, and lymphocytes were isolated from tissues indicated in the figures using standard protocols. Animal research was approved by the Regierungspräsidium-Freiburg (G.02/84) and the local animal care commission. Antibodies are described in Supplemental materials and methods (available at http://www.jem.org/cgi/content/full/jem.20070782/DC1).
Flow cytometry.
Normal distributions for αβ and γδ T cell numbers were obtained from the literature (29, 30). The normal ranges (which include 90% of the data) were depicted in a logarithmic scale as median values (dashed line) between the 5th and 95th percentiles (P5 and P95). In CD3-deficient patients, αβ T cells were defined as CD4+ and CD8+ or CD8bright, thus excluding most γδ T cells (<8%). γδ T cells were defined as surface TCRδ+ using the antibodies 11F2 or Immu510. In patients, γδ T cell counts may be underestimated because of the γδ TCR expression defect.
Mouse cells were stained with PE-conjugated GL3, FITC-conjugated H57-597, and biotinylated 145-2C11 antibodies. Streptavidin–PE-Cy5 was used as a second-step reagent. Stained cells were analyzed in a flow cytometer (FACSCalibur) using CellQuest software (both purchased from Becton Dickinson).
Cell lysis, TCR purification, and deglycosylation.
Cells were lysed using 1% digitonin or 0.5% Brij96V, and immunoprecipitations were performed using the antibodies 448, Jovi1, and 5A6.E9, as previously described (17). TCRs bound to the beads were treated with 1 U N-glycosidase F (Roche Diagnostics).
For TCR immunopurifications used in BN-PAGE, 107 cells were incubated with 200 μM pervanadate and lysed, and phosphorylated proteins were purified with 2 μg 4G10 and 5 μl protein G–coupled sepharose (GE Healthcare). Native elution was done in BN buffer including 50 mM phenylphosphate, the detergent indicated in the figures, and phosphatase to dephosphorylate the TCR (16, 17). In experiments in which γδ T cell clones were used, αβ TCRs were depleted by two sequential immunodepletions using Jovi1 and βF1 bound to protein G–coupled sepharose (Fig. S1).
Gel electrophoresis and Western blotting.
SDS- and BN-PAGE were performed using standard protocols (16). Ferritin in its 24- and 48-meric forms was used as the marker protein (f1 and f2, 440 and 880 kD, respectively). In brief, for the NAMOS assay (unpublished data), antibodies were added to 10 μl of eluted purified TCR before separation by BN-PAGE (4–9%). Western blotting was performed according to standard protocols using 448 (1:5,000), M20, and M20δ (both 1:1,000) antisera.
Online supplemental material.
Supplemental materials and methods provides the specificities and sources of the antibodies. Fig. S1 A shows flow cytometric analysis of a human γδ T cell clone stained for αβ and γδ TCR. Fig. S1 B shows Western blotting of anti–αβ TCR depletion of a human γδ T cell clone lysate. Fig. S1 C shows immunopurification of the TCR from a human γδ T cell clone using an anti-CD3δ antibody. Fig. S2 (A and B) shows that anti–human and anti–mouse CD3γ and CD3δ antibodies are specific for their respective chains by immunoprecipitation of individually expressed CD3 chains in Drosophila S2 cells. In Fig. S3, a sequence alignment of hCD3δ and mCD3δ is discussed. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070782/DC1.
Supplemental Material
Acknowledgments
We would like to thank M. Reth for his scientific support; G. Turchinovich and S. Glatzel for technical assistance; P. Pereira, D. Kappes, and C. Terhorst for mice; and S. Kilic, O. Sanal, and L. Allende for patients' samples.
This work was supported by an Emmy Noether Fellowship to W.W. Schamel from the Deutsche Forschungsgemeinschaft (SCHA 976/1), Ministerio de Educacion y Cultura (MEC) grant BMC2002-01431 to E. Fernández-Malavé, MEC grant BFU2005-01738/BMC to J.R. Regueiro, the European Union–funded grant EPI-PEP-VAC to S. Minguet, a University of Freiburg Wiedereinstiegsstipendium to G.M. Siegers, grant R05/01 from the Deutsche Jose Carreras Leukämie-Stiftung to P. Fisch, and grants SFB620 B6 and Z2 from the Deutsche Forschungsgemeinschaft to W.W. Schamel and P. Fisch. The support of the Fundacion Ramon Areces to the Centro de Biologia Molecular is acknowledged.
The authors have no conflicting financial interests.
G.M. Siegers and M. Swamy contributed equally to this article.
References
- 1.Malissen, M., A. Gillet, L. Ardouin, G. Bouvier, J. Trucy, P. Ferrier, E. Vivier, and B. Malissen. 1995. Altered T cell development in mice with a targeted mutation of the CD3ε gene. EMBO J. 14:4641–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeJarnette, J.B., C.L. Sommers, K. Huang, K.J. Woodside, R. Emmons, K. Katz, E.W. Shores, and P.E. Love. 1998. Specific requirement for CD3ε in T cell development. Proc. Natl. Acad. Sci. USA. 95:14909–14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haks, M.C., P. Krimpenfort, J. Borst, and A.M. Kruisbeek. 1998. The CD3γ chain is essential for development of both the TCRαβ and TCRγδ lineages. EMBO J. 17:1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recio, M.J., M.A. Moreno-Pelayo, S.S. Kilic, A.C. Guardo, O. Sanal, L.M. Allende, V. Perez-Flores, A. Mencia, S. Modamio-Hoybjor, E. Seoane, and J.R. Regueiro. 2007. Differential biological role of CD3 chains revealed by human immunodeficiencies. J. Immunol. 178:2556–2564. [DOI] [PubMed] [Google Scholar]
- 5.Dadi, H.K., A.J. Simon, and C.M. Roifman. 2003. Effect of CD3δ deficiency on maturation of αβ and γδ T-cell lineages in severe combined immunodeficieny. N. Engl. J. Med. 349:1821–1828. [DOI] [PubMed] [Google Scholar]
- 6.Dave, V.P., Z. Cao, C. Browne, B. Alarcon, G. Fernandez-Miguel, J. Lafaille, A. de la Hera, S. Tonegawa, and D.J. Kappes. 1997. CD3δ deficiency arrests development of the αβ but not the γδ T cell lineage. EMBO J. 16:1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alarcon, B., M. Swamy, H.M. van Santen, and W.W. Schamel. 2006. T-cell antigen-receptor stoichiometry: pre-clustering for sensitivity. EMBO Rep. 7:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swamy, M., S. Minguet, G.M. Siegers, B. Alarcon, and W.W. Schamel. 2007. A native antibody-based mobility-shift technique (NAMOS-assay) to determine the stoichiometry of multiprotein complexes. J. Immunol. Methods. 324:74–83. [DOI] [PubMed] [Google Scholar]
- 9.Hayes, S.M., K. Laky, D. El-Khoury, D.J. Kappes, B.J. Fowlkes, and P.E. Love. 2002. Activation-induced modification in the CD3 complex of the γδ T cell receptor. J. Exp. Med. 196:1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes, S.M., and P.E. Love. 2002. Distinct structure and signaling potential of the gamma delta TCR complex. Immunity. 16:827–838. [DOI] [PubMed] [Google Scholar]
- 11.Krangel, M.S., B.E. Bierer, P. Devlin, M. Clabby, J.L. Strominger, J. McLean, and M.B. Brenner. 1987. T3 glycoprotein is functional although structurally distinct on human T-cell receptor γ T lymphocytes. Proc. Natl. Acad. Sci. USA. 84:3817–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Neerven, J., J.E. Coligan, and F. Koning. 1990. Structural comparison of αβ and γδ T cell receptor-CD3 complexes reveals identical subunit interactions but distinct cross-linking patterns of T cell receptor chains. Eur. J. Immunol. 20:2105–2111. [DOI] [PubMed] [Google Scholar]
- 13.Arnaiz-Villena, A., M. Timon, A. Corell, P. Perez-Aciego, J.M. Martin-Villa, and J.R. Regueiro. 1992. Brief report: primary immunodeficiency caused by mutations in the gene encoding the CD3γ subunit of the T-lymphocyte receptor. N. Engl. J. Med. 327:529–533. [DOI] [PubMed] [Google Scholar]
- 14.van Tol, M.J.D., O. Sanal, R. Langlois van den Bergh, Y. van de Wal, M.T.L. Roos, A.I. Berkel, J.M. Vossen, and F. Koning. 1997. CD3γ chain deficiency leads to a cellular immunodeficiency with mild clinical presentation. The Immunologist. Suppl. 1:41–42. [Google Scholar]
- 15.Alibaud, L., J. Arnaud, R. Llobera, and B. Rubin. 2001. On the role of CD3δ chains in TCRγδ/CD3 complexes during assembly and membrane expression. Scand. J. Immunol. 54:155–162. [DOI] [PubMed] [Google Scholar]
- 16.Swamy, M., G.M. Siegers, S. Minguet, B. Wollscheid, and W.W. Schamel. 2006. Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci. STKE. 2006:pl4. [DOI] [PubMed] [Google Scholar]
- 17.Schamel, W.W., I. Arechaga, R.M. Risueno, H.M. van Santen, P. Cabezas, C. Risco, J.M. Valpuesta, and B. Alarcon. 2005. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 202:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koning, F., W.L. Maloy, and J.E. Coligan. 1990. The implications of subunit interactions for the structure of the T cell receptor-CD3 complex. Eur. J. Immunol. 20:299–305. [DOI] [PubMed] [Google Scholar]
- 19.Mombaerts, P., A.R. Clarke, M.L. Hooper, and S. Tonegawa. 1991. Creation of a large genomic deletion at the T-cell antigen receptor β-subunit locus in mouse embryonic stem cells by gene targeting. Proc. Natl. Acad. Sci. USA. 88:3084–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malissen, M., P. Pereira, D.J. Gerber, B. Malissen, and J.P. DiSanto. 1997. The common cytokine receptor γ chain controls survival of γδ T cells. J. Exp. Med. 186:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes, S.M., and P.E. Love. 2006. Stoichiometry of the murine γδ T cell receptor. J. Exp. Med. 203:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, B., N. Wang, M. Salio, A. Sharpe, D. Allen, J. She, and C. Terhorst. 1998. Essential and partially overlapping role of CD3γ and CD3δ for development of αβ and γδ T lymphocytes. J. Exp. Med. 188:1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez-Malave, E., N. Wang, M. Pulgar, W. Schamel, B. Alarcon, and C. Terhorst. 2006. Overlapping functions of human CD3 and mouse CD3 in T cell development revealed in a humanized CD3-deficient mouse. Blood. 108:3420–3426. [DOI] [PubMed] [Google Scholar]
- 24.Sun, Z.Y., S.T. Kim, I.C. Kim, A. Fahmy, E.L. Reinherz, and G. Wagner. 2004. Solution structure of the CD3εδ ectodomain and comparison with CD3εγ as a basis for modeling T cell receptor topology and signaling. Proc. Natl. Acad. Sci. USA. 101:16867–16872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnett, K.L., S.C. Harrison, and D.C. Wiley. 2004. Crystal structure of a human CD3εδ dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl. Acad. Sci. USA. 101:16268–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haks, M.C., T.A. Cordaro, J.H. van den Brakel, J.B. Haanen, E.F. de Vries, J. Borst, P. Krimpenfort, and A.M. Kruisbeek. 2001. A redundant role of the CD3 γ-immunoreceptor tyrosine-based activation motif in mature T cell function. J. Immunol. 166:2576–2588. [DOI] [PubMed] [Google Scholar]
- 27.Werlen, G., B. Hausmann, and E. Palmer. 2000. A motif in the alphabeta T-cell receptor controls positive selection by moldulating ERK activity. Nature. 406:422–426. [DOI] [PubMed] [Google Scholar]
- 28.Fisch, P., M. Malkovsky, E. Braakman, E. Sturm, R.L. Bolhuis, A. Prieve, J.A. Sosman, V.A. Lam, and P.M. Sondel. 1990. γδ T cell clones and natural killer cell clones mediate distinct patterns of non–major histocompatibility complex–restricted cytolysis. J. Exp. Med. 171:1567–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comans-Bitter, W.M., R. de Groot, R. van den Beemd, H.J. Neijens, W.C. Hop, K. Groeneveld, H. Hooijkaas, and J.J. van Dongen. 1997. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J. Pediatr. 130:388–393. [DOI] [PubMed] [Google Scholar]
- 30.Ikinciogullari, A., T. Kendirli, F. Dogu, Y. Egin, I. Reisli, S. Cin, and E. Babacan. 2004. Peripheral blood lymphocyte subsets in healthy Turkish children. Turk. J. Pediatr. 46:125–130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.