Abstract
The initial B cell repertoire contains a considerable proportion of autoreactive specificities. The first major B cell tolerance checkpoint is at the stage of the immature B cell, where receptor editing is the primary mode of eliminating self-reactivity. The cells that emigrate from the bone marrow have a second tolerance checkpoint in the transitional compartment in the spleen. Although it is known that the second checkpoint is defective in lupus, it is not clear whether there is any breakdown in central B cell tolerance in the bone marrow. We demonstrate that receptor editing is less efficient in the lupus-prone strain MRL/lpr. In an in vitro system, when receptor-editing signals are given to bone marrow immature B cells by antiidiotype antibody or after in vivo exposure to membrane-bound self-antigen, MRL/lpr 3-83 transgenic immature B cells undergo less endogenous rearrangement and up-regulate recombination activating gene messenger RNA to a lesser extent than B10 transgenic cells. CD19, along with immunoglobulin M, is down-regulated in the bone marrow upon receptor editing, but the extent of down-regulation is fivefold less in MRL/lpr mice. Less efficient receptor editing could allow some autoreactive cells to escape from the bone marrow in lupus-prone mice, thus predisposing to autoimmunity.
The process of V(D)J rearrangement, by virtue of the relatively random nature of V, D, and J gene selection and junctional diversity, creates a wide variety of autoreactive specificities (1). In normal individuals, this does not pose a problem, because the immune system has established several checkpoints to ensure that autoreactive lymphocytes will be eliminated or controlled (1, 2). During B cell development, reactivity to self-antigens is first assessed at the immature B cell stage in the bone marrow and then again at the transitional stage in the spleen. Autoreactive B cells at these two checkpoints can be either eliminated or rendered nonfunctional by clonal deletion or anergy; for the transitional B cells in the spleen, these are the predominant tolerance mechanisms (3). However, for autoreactive B cells in the bone marrow, the primary mechanism for removal of a self-reactive B cell receptor (BCR) to membrane-bound antigens is receptor editing (4–7). This mechanism provides the cell with a new receptor through continued rearrangement at the light chain loci (or on rare occasions, at the heavy chain locus) and replacement of the former light chain with a new light chain (8). Receptor editing has been shown to be a highly efficient mechanism to replace these autoreactive receptors with a nonself-reactive receptor (4, 9–11). If the new light chain makes a nonautoreactive receptor, the cell can mature and emigrate to the spleen. If receptor editing does not succeed in producing a new receptor that is not autoreactive, anergy or clonal deletion should follow (12). For B cells reactive with soluble antigen, both receptor editing and anergy are used as mechanisms of central tolerance (13). For the few autoreactive cells that do emerge from the bone marrow, contact with self-antigen during the transitional B cell tolerance checkpoint in the spleen normally results in apoptosis or anergy. Which tolerance mechanism (receptor editing, anergy, or clonal deletion) is invoked is dependent not only on the maturation stage of the B cell but also on many other factors, including the nature of the antigen, the avidity of the BCR for the antigen, and the strength of the signal induced by the interaction of the BCR with the self-antigen.
Efficient B cell tolerance is essential to prevent autoimmunity. It has been shown that over half of early immature B cells have self-reactive receptors, but at this first tolerance checkpoint in the bone marrow, a considerable number of these autoreactive specificities are lost by one of these tolerance mechanisms (1). Where the breakdown in tolerance occurs in autoimmune patients and autoimmune-prone mice is not clear. In systemic lupus erythematosus (SLE), somatically mutated IgG autoantibodies against DNA and other nuclear components are often the pathogenic antibodies found in immune complexes in the kidney, suggesting antigen activation and breakdown of tolerance in the periphery. Indeed, an extensive study of the peripheral blood of three SLE patients demonstrated that the second checkpoint, the transition between newly emigrated B cells in the peripheral blood and mature naive B cells, is clearly defective in SLE patients (14, 15). However, much less information is available about potential defects in the bone marrow of SLE patients or lupus-prone strains of mice. Most of the SLE patients in the study of Yurasov et al. (14) also showed increases in the frequency of Hep-2–reactive B cells and polyreactive B cells in newly emigrated B cells as compared with controls, although the increase reached the level of significance (P < 0.2) in only one of the patients for polyreactive antibodies. This new emigrant stage is particularly important, because the higher levels of B cell–activating factor in autoimmune individuals could thwart the second checkpoint and allow many autoreactive B cells that leave the bone marrow to mature into naive B cells. Because the autoreactive profile of the newly emigrated B cells in normal individuals mirrors that of the immature B cells in the bone marrow (1), these data are suggestive that central tolerance may have defects in SLE, although one cannot determine from this study if it is receptor editing or clonal deletion that may cause this potential defect.
In this study, we wished to specifically ask whether receptor editing was impaired in lupus-prone mice. In two mouse models of autoimmunity, including the one we are currently studying, it has been reported that central tolerance to strong self-antigens is not affected in vivo, as demonstrated by the fact that all B cells in the periphery of the mice have edited their receptors (16, 17). However, the number of B cells in the spleens of those BCR transgenic mice is very low, suggesting that clonal deletion was the major mechanism of central tolerance in these mice that are unable to undergo effective receptor editing because of the inability to delete the randomly integrated BCR transgene. We reproduce the published findings in this paper, but a careful inspection of several parameters characteristic of receptor editing in B10 and MRL/lpr BCR transgenic mice lead us to suggest that although clonal deletion to this particular strong, membrane-bound self-antigen is intact in these mice, receptor editing is less efficiently induced in the MRL/lpr mice. Using an in vitro receptor-editing culture system, we demonstrate that immature B cells from the MRL/lpr BCR transgenic mice display less induced endogenous κ rearrangement and less RAG messenger RNA (mRNA) up-regulation upon BCR stimulation compared with immature B cells from the nonautoimmune B10.D2 3-83 transgenic mice. In addition, we also found evidence of receptor-editing impairments in MRL/lpr BCR transgenic mice that are exposed to self-antigen in vivo. Furthermore, the different Igκ/Igλ ratio in the transitional compartment in these mice suggests that some, presumably low affinity, autoreactive B cells exit the bone marrow in MRL/lpr mice. To determine whether the differences in receptor editing between autoimmune and nonautoimmune strains also extended to nontransgenic mice, we assessed the transit time through the pre–B cell compartment (10, 13). The more rapid transit time in nontransgenic MRL/lpr and MRL/+ mice compared with nontransgenic B6 bone marrow suggests that polyclonal nontransgenic MRL/lpr and MRL/+ mice may also undergo less receptor editing in vivo. Finally, we observed that the level of CD19 is greatly reduced on bone marrow cells as a result of receptor-editing signals in B10 transgenic mice in vivo but is reduced to a much lesser extent in MRL/lpr immature B cells, which is consistent with less receptor editing in these lupus-prone mice. Although we show that clonal deletion is effective to a strong, membrane-bound antigen in vivo, B cells with reactivity to soluble or weaker self-antigens may well escape from the bone marrow if not eliminated by receptor editing. Thus, this relative receptor-editing defect in lupus mice, coupled with a less stringent affinity threshold for clonal deletion, is likely to result in the release of some autoreactive cells from the bone marrow, thus providing the potential for subsequent activation by exposure to self-antigen.
RESULTS
MRL/lpr immature B cells undergo less receptor editing in an in vitro culture system
To directly assess whether there is a relative defect in the ability of MRL/lpr lupus-prone mice to undergo receptor editing, it was important to directly compare MRL/lpr mice with B10 mice in the same experimental system. Because the 3-83 transgenic BCR reacts with H-2Kk but not with H-2Kd, we crossed B10.D2 mice bearing the 3-83 heavy and light chain BCR transgene with MRL/lpr mice and backcrossed to MRL/lpr for seven generations, screening for H-2d at each generation, to obtain MRL/lpr 3-83 BCR transgenic mice on the H-2d background. Hence, these two 3-83 transgenic strains bearing H-2d do not encounter any self-antigen in vivo.
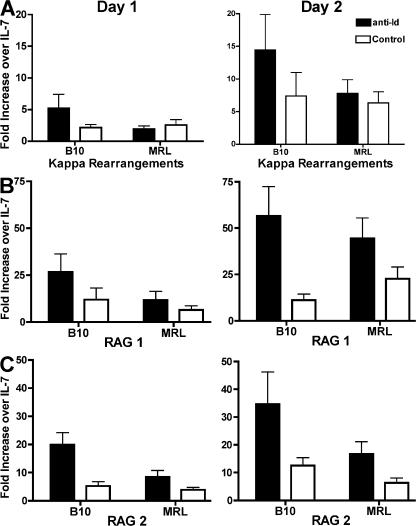
We assessed the ability of both strains to undergo receptor editing in a previously described in vitro culture system (18). Unfractionated bone marrow cell preparations were cultured for 5 d with IL-7, and because these cells were from BCR transgenic mice, the resulting population of cells was >95% immature B cells. IL-7 was then removed from the cultures, and the cells were either cultured with an irrelevant antibody (control culture) or with anti-Id antibody (receptor-editing culture). We observed that removal of IL-7 after 5 d of culture induced some κ rearrangements, as assessed by real-time PCR. BCR ligation with the anti-Id antibody resulted in increased levels of endogenous κ rearrangement in 3-83 transgenic mice on the B10.D2 background, whereas lower levels of rearrangements were induced in the control culture as compared with the IL-7 cultures (Fig. 1). In contrast, the level of induced κ rearrangement in cultures of MRL/lpr H-2d immature B cells was only marginally higher in the cultures stimulated with anti-Id as compared with the control cultures, suggesting that the immature B cells from MRL/lpr mice do not respond as efficiently to BCR signals by receptor editing.
Figure 1.
Lower level of receptor editing in cultures of MRL/lpr (H-2d) versus B10.D2 3-83 transgenic bone marrow. (A) Level of endogenous κ rearrangement induced in receptor-editing bone marrow cultures of B10.D2 and MRL/lpr (H-2d) 3-83 transgenic mice. After 5 d of culture with IL-7, the cells were removed from IL-7 and cultured with anti-Id or with a control antibodies. DNA was obtained from the IL-7 culture and on days 1 and 2 after removal from IL-7. The fold increase ± SEM of κ rearrangements in anti-Id and control cultures over the level in the IL-7 cultures is shown. Data are derived from 7 to 10 experiments. (B and C) Level of RAG 1 and 2 up-regulation, respectively, in receptor-editing cultures. The fold increase ± SEM of RAG 1 and 2 mRNA in anti-Id and control cultures over IL-7 cultures is shown. Data are derived from 10 to 13 experiments.
BCR signaling in immature B cells in this culture system leads to up-regulation of RAG 1 and RAG 2 mRNA (18). Therefore, using RAG up-regulation as an early marker for the induction of receptor editing, we tested whether B10 and MRL/lpr mice differ in the magnitude of their receptor-editing responses. Withdrawal of IL-7 in the control cultures resulted in increased levels of RAG 1 and RAG 2 expression, but exposure to anti-Id antibody greatly increased the levels of RAG mRNA in B10.D2 immature B cells. However, immature B cells from MRL/lpr transgenic mice did not up-regulate their RAG expression as effectively as immature B cells from B10.D2 transgenic mice when stimulated through the BCR (Fig. 1). Therefore, immature B cells from MRL/lpr transgenic mice show an overall lower level of appropriate responsiveness to receptor-editing signaling.
MRL/lpr mice down-regulate surface IgM after receptor-editing signaling
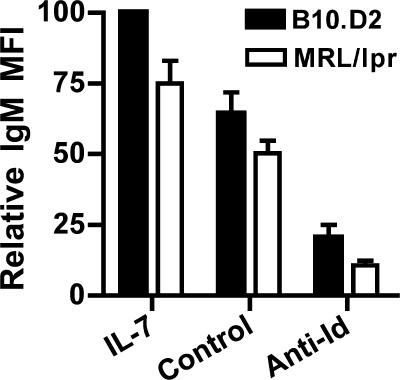
When immature B cells interact with antigen, the surface IgM level is substantially down-regulated. Because MRL/lpr mice are more refractory to BCR signaling with regard to induced light chain rearrangement and RAG up-regulation, we determined whether immature B cells from MRL/lpr mice were defective in the initial IgM down-regulation in response to signaling through the BCR. We analyzed immature B cells from the IL-7 culture, the receptor-editing culture, and the control culture by flow cytometry. As seen in Fig. 2, the mean fluorescence intensity (MFI) of IgM on immature B cells is lower on the MRL/lpr than on the B10.D2 cells in the IL-7 cultures, but both down-regulate IgM in response to anti-Id stimulation to a similar extent. Thus, despite the fact that immature B cells from MRL/lpr mice do not respond to this BCR cross-linking with as robust a receptor-editing response as immature B cells from B10.D2, surface IgM is down-regulated similarly.
Figure 2.
IgM is down-regulated in response to BCR ligation in immature B cells in bone marrow cultures of both B10.D2 and MRL/lpr (H-2d) 3-83 transgenic mice. Immature B cells from receptor-editing and control cultures from B10.D2 and MRL/lpr (H-2d) 3-83 mice were stained for surface expression of IgM after 2 d of culture with the anti-Id or control antibody, and the MFI ± SEM of IgM was compared between IL-7, anti-Id, and control cultures for each strain. Because the experiments were performed on FACSCalibur and LSR II instruments, with greatly varying absolute magnitudes of MFI, we express the data as relative MFI, with the MFI of IgM in the IL-7 culture from the B10.D2 transgenic mice being 100% for each experiment. Results are from three to experiments.
Greater calcium flux in response to BCR ligation in immature B cells from MRL/lpr mice
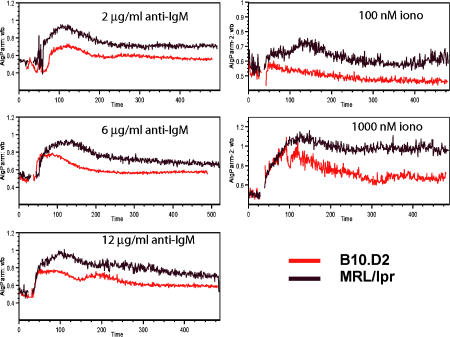
Because the results from the receptor-editing cultures demonstrate that BCR signaling of immature B cells from MRL/lpr transgenic mice does not induce as much endogenous light chain rearrangement and does not result in as much up-regulation of RAG mRNA compared with immature B cells from B10.D2 transgenic mice, we tested whether immature B cells from MRL/lpr transgenic mice were generally hyporesponsive to signals through the BCR. Bone marrow cells from day 5 IL-7 cultures were loaded with Indo-1 and subsequently stimulated with the varying concentrations of F(ab′)2 anti-IgM (Fig. 3). The level of calcium flux was measured by flow cytometry. MRL/lpr transgenic mice showed a greater calcium flux compared with B10.D2 transgenic mice at all concentrations of anti-IgM. Furthermore, MRL/lpr B cells fluxed more calcium in response to low concentrations of ionomycin. Hence, the immature B cells from the MRL/lpr mice are hyperresponsive at a stage downstream of BCR signaling.
Figure 3.
Immature B cells from MRL/lpr (H-2d) 3-83 mice show stronger calcium flux than immature B cells from B10.D2 3-83 mice. Immature B cells were obtained from day 6 or 7 IL-7 cultures. Calcium flux was measured by flow cytometry with Indo-loaded cells. Results are representative of three experiments.
RAG mRNA production is stimulated only at very low concentrations of ionomycin in MRL/lpr immature B cells
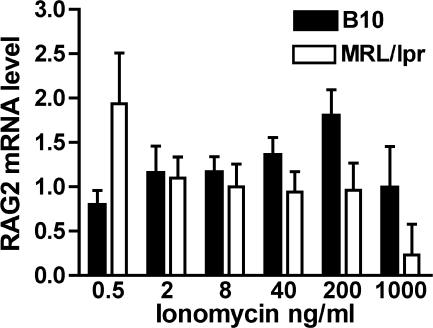
It has been shown that immature B cells only up-regulated RAG mRNA after culture with low concentrations of ionomycin that produced minimal calcium flux (19). Because immature B cells from MRL/lpr mice show increased calcium flux in response to BCR ligation or to low concentrations of ionomycin compared with immature B cells from B10.D2 mice, we hypothesized that RAG mRNA would only be induced at very low concentrations of ionomycin in immature B cells from MRL/lpr mice. To test this hypothesis, we cultured bone marrow cells from both strains of transgenic mice with IL-7 for 5 d to obtain pure populations of immature B cells and then cultured the immature B cells with varying doses of ionomycin for 24 h. Cells were harvested, and the level of RAG2 mRNA was determined. In the B10.D2 transgenic mice, optimal RAG induction occurred at moderate concentrations of ionomycin (200 ng/ml; Fig. 4), whereas for MRL/lpr transgenic mice, the optimal level of RAG induction was far lower (0.5 ng/ml). Thus, we propose that immature B cells from MRL/lpr mice may fail to up-regulate RAG mRNA in the receptor-editing cultures because the signal they receive may already be beyond the maximum stimulation threshold for RAG mRNA induction.
Figure 4.
Level of RAG 2 mRNA expression after culture with ionomycin. Immature B cells from day 6 IL-7 cultures of B10.D2 and MRL/lpr (H-2d) 3-83 bone marrow cells were cultured with varying concentrations of ionomycin for 24 h. RNA was prepared, and the cDNA was analyzed by real-time PCR. Results are ±SEM of six experiments.
Bone marrow cells from MRL/lpr BCR transgenic mice exhibit reduced responses to receptor-editing signals in vivo
Because the BCR light chain transgenes are not integrated into the light chain locus, these mice cannot undergo effective receptor editing even if the transgene is expressed in mice bearing an H-2 haplotype, such as H-2k, with which the BCR strongly reacts. However, the bone marrow cells in these mice can be analyzed for the same parameters of attempted receptor editing (i.e., RAG mRNA up-regulation and induction of endogenous κ rearrangement) that we analyzed in the in vitro receptor-editing culture system.
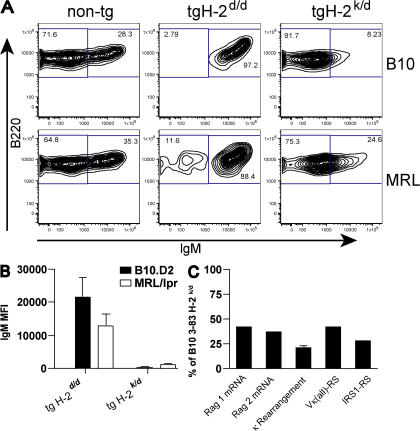
We crossed B10.D2 3-83 transgenic mice with B10.BR mice, and crossed MRL/lpr H-2d transgenic mice with MRL/lpr mice, resulting in F1 mice that were H-2k/d. Thus, all cells in the mouse expressed a strong self-antigen. The transgenic mice on the nonantigenic H-2d background have very few pro– or pre–B cells because of the presence of the rearranged heavy and light chain transgenes (20). Hence, most bone marrow B progenitor cells are homogeneous in composition and are of the immature B cell phenotype (Fig. 5 A). In contrast, the majority of developing B cells in the bone marrow of H-2k/d mice of both the MRL/lpr and B10 backgrounds greatly down-regulate IgM in response to exposure to self-antigen and, thus, resemble the pre–B cell phenotype (Fig. 5 A). However, the down-regulation is less complete in the MRL/lpr transgenic mice. When the IgM+ immature B cell gate established on nontransgenic mice was applied to the transgenic bone marrow CD43−B220+AA4+ (pre–B cell plus immature B cell phenotype) cells, 6.25 ± 0.17% of B10 cells and 17.24 ± 0.14% of MRL/lpr cells fell in the IgM+ gate on the H2d/k background, compared with ∼90-95% of CD43−B220+AA4+ B cells from either strain of transgenic mice on the H-2d background (a total of 10–12 mice of each genotype were examined; Fig. 5 and not depicted). To quantify this difference in the extent of down-regulation of IgM, we calculated the MFI of this population of cells. Fig. 5 B shows that the B220+CD3−AA4+ CD43− cells from B10 3-83 H-2k/d mice displayed a 50-fold reduction in MFI for IgM compared with B10.D2 H-2d transgenic mice, whereas MRL/lpr 3-83 mice only showed a 10-fold reduction in MFI upon exposure to H-2k. Thus, the extent of down-regulation of IgM is fivefold less in the bone marrow of the MRL/lpr transgenic mice upon exposure to self-antigen. Furthermore, in the absence of exposure to self-antigen, the MFI of IgM on the immature B cells from MRL/lpr 3-83 mice is 40% lower than the MFI on immature B cells from B10.D2 transgenic mice.
Figure 5.
Bone marrow cells of MRL/lpr 3-83 transgenic mice on an antigenic H-2k/d background show less down-regulation of IgM and lower levels of receptor editing ex vivo. (A) FACS analysis of bone marrow cells from B10.D2 and MRL/lpr 3-83 mice on the nonantigenic H-2d background and on the antigenic H-2k/d background, as well as nontransgenic littermates. Bone marrow cells were gated on the AA4+B220+CD43− population. The percentage of cells in each gate is shown. (B) IgM MFI ± SEM of AA4+B220+CD43− cells from eight experiments. (C) B220+ MACS-purified bone marrow cells from B10.D2 and MRL/lpr H-2k/d 3-83 transgenic mice were processed into DNA and cDNA. DNA was used for measurement of endogenous κ rearrangements and RS rearrangements, and cDNA was used to quantitate RAG 1 and 2 mRNA expression levels. For each of eight experiments, the level of rearrangements or RAG mRNA was compared between B10 and MRL/lpr H-2 k/d B220+ cells, and the results are expressed as the mean ± SEM of the level in the MRL/lpr B220+ cells as a percentage of the level in the B10 cells.
RNA and DNA were isolated from MACS-purified B220+ cells from transgenic mice of both strains on both antigenic H-2k/d and nonantigenic H-2d backgrounds. Fig. 5 C shows that cells from MRL/lpr H-2k/d transgenic mice displayed less than half as much RAG mRNA up-regulation as a result of in vivo self-antigen exposure than B10 H-2k/d transgenic mice, suggesting that cells from MRL/lpr mice do not efficiently process the receptor-editing signals. Furthermore, MRL/lpr H-2k/d transgenic mice displayed approximately twofold less endogenous κ and recombining sequence (RS) rearrangements than B10 H-2k/d transgenic mice. These findings are very consistent with our in vitro receptor-editing culture results and provide further evidence of inefficient receptor-editing responses to BCR ligation by self-antigen in autoimmune-prone MRL/lpr mice.
MRL/lpr 3-83 BCR transgenic mice undergo proper deletional central tolerance checkpoint controls in vivo
Although the transgenic mice cannot undergo much receptor editing (i.e., replacing the 3-83 BCR) even in the presence of strong self-antigen, it has been shown that a small percentage of cells do successfully inactivate the 3-83 transgenic light chain and emerge into the periphery as Id− IgM+ B cells (6). In addition, that study demonstrated that about half of the splenic B cells expressed λ light chains. Thus, most transgenic B cells undergo deletion in the bone marrow, but the rare ones that successfully edit will emigrate to the spleen. In a subsequent study, it was shown that this low frequency of successful receptor editing is true for transgenic mice on the MRL/lpr background (16).
Here, we directly compared the spleen populations of the F1 H-2k/d 3-83 transgenic mice on the MRL/lpr and B10 background. Spleen cells from both strains of H-2k/d mice were analyzed by flow cytometry for the presence of Igκ+ and Igλ+ as well as for Id+ cells. Consistent with the previous studies, we did not observe any Id+ cells in the spleens of either strain of mice (unpublished data). Furthermore, we confirmed that there is only a small number of B cells that have successfully receptor edited and emigrated from the bone marrow into the spleens of both strains of mice (Table I).
Table I.
Percentage of B cells and light chain usage in the spleens of 3-83 transgenic mice
| B220+ AA4+ transitional cells
|
B220+ AA4− mature B cells
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Straina | MHC | % B cellsb | % κ+λ− | % κ+λ+ | % κ−λ+ | % κ+λ− | % κ+λ+ | % κ−λ+ |
| B10 | d/d | 41.23 | 99.6 | 0.05 | 0.01 | 99.06 | 0.28 | 0.52 |
| B10 | k/d | 3.98 | 64.53 | 10.85 | 23.29 | 36.83 | 18.67 | 44.23 |
| MRL/lpr | d/d | 26.86 | 99.48 | 0.02 | 0.02 | 99.72 | 0.02 | 0.06 |
| MRL/lpr | k/d | 3.08 | 76.41 | 5.59 | 16.95 | 46.07 | 5.56 | 48.06 |
3-83 transgenic mice.
B cells were identified as B220+CD3− Ig+ cells. Data are the mean of five mice of each strain on the H-2dd background, and nine mice of each strain on the H-2k/d background.
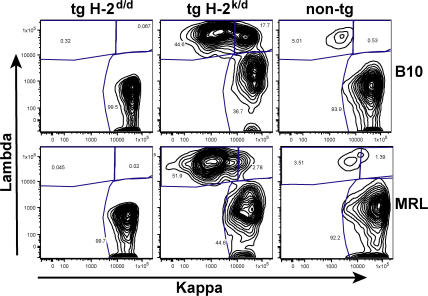
We further investigated the spleens to determine if the MRL/lpr transgenic mice were specifically deficient in mature B cells or if the proportion of transitional cells was higher in these mice. The immature B cell stage in the bone marrow is the first tolerance checkpoint, whereas the stage of emergence from the transitional compartment in the spleen to mature AA4− B cells is the second tolerance checkpoint (1). Nontransgenic MRL/lpr mice have a much smaller proportion of AA4+ transitional cells than C57BL/6 mice, and this is also the case in the 3-83 transgenic mice on the H-2d background (Table I). When the transgene is expressed on the antigenic H-2k/d background, the percentage of transitional cells drops somewhat in both strains, although the decrease is proportionally larger in the B10 mice (Table I). Interestingly, the AA4+ transitional B cells, although completely Id− in both strains, have a much higher percentage of Igκ+ Igλ− B cells (64–76%) than the mature AA4− B cells (37–46%) in both strains of mice. Because the spleen cells do not express RAG mRNA, the decrease in the proportion of Igκ+ Id− cells between the transitional and the mature B cell compartment must occur by deletion at this second tolerance checkpoint. The transitional cells in the MRL/lpr transgenic mice have a lower percentage of Igλ+ κ− cells, but the percentage of Igλ+ κ− cells in the mature B cells is similar in both strains. The predominant difference between the B10 and MRL/lpr splenic B cells is that B10 mice have a higher percentage of Igκ+ Igλ+ double-expressing cells in both the AA4+ transitional cells and, even more strikingly, in the AA4− mature B cells (Fig. 6 and Table I).
Figure 6.
κ and λ light chain expression in spleen cells of 3-83 transgenic mice on the H-2k/d and H-2d background. Spleen cells from B10 and MRL/lpr transgenic mice were surface stained with antibodies reactive with B220, AA4, CD3, κ, and λ. B220+CD3− cells were divided into AA4+ and AA4− gates and then analyzed for κ and λ staining. B220+Ig− cells were gated out. The percentage of cells in each gate is shown. FACS profiles shown of the AA4− spleen cells are representative of five experiments with several mice per experiment. The results from all of the mice in all experiments are tabulated in Table I.
Because we observed a decrease in the percentage of Igκ+ cells as the cells matured from the transitional compartment into the naive splenic compartment, we also analyzed the few IgM+ bone marrow immature B cells in the H-2k/d F1 mice. In both strains, we found that the majority of IgM+ cells expressed Igκ yet were Id− (unpublished data). As with the transitional splenic compartment, the percentage of Igκ+ cells was higher in the MRL/lpr immature B cells than in the rare B10 immature B cells.
These findings demonstrate that the deletion mechanism of tolerance is intact for the MRL/lpr H-2k/d transgenic mice at both the first tolerance checkpoint (immature bone marrow B cells) and at the second checkpoint (transitional to naive B cell in the spleen), in that all B cells that emerge in the periphery in both strains of mice are Id−. However, the proportion of Igκ+ Igλ+ double-expressing cells and of Igκ− Igλ+ cells is higher in the B10 background, suggesting that more robust receptor editing is taking place in the transgenic mice on the B10 background.
CD19 expression is decreased in H-2k/d mice
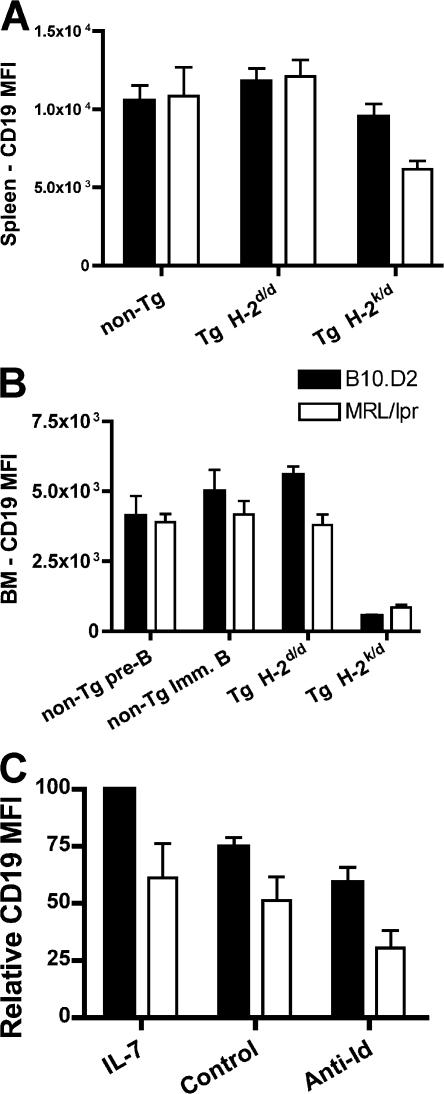
In the FACS analysis of spleen cells from H-2k/d mice, we initially used CD19 to identify the B cells. Surprisingly, the MFI of CD19 was reduced twofold in the few B cells present in the MRL/lpr H-2k/d mice compared with splenic B cells from MRL/lpr 3-83 mice on the nonantigenic H-2d background (Fig. 7 A). The MFI of CD19 was also reduced in the splenic B cells of B10 H-2k/d transgenic mice compared with B10.D2 transgenic mice, but only by 20%.
Figure 7.
Modulation of CD19 levels after exposure to self-antigen. (A) Spleen cells from H-2k/d and H-2d transgenic mice were gated on B220+CD3−CD19+ cells, and the MFI ± SEM of CD19 from five experiments is shown. (B) Bone marrow cells from the same mice were gated on B220+CD43−AA4+ cells, and the MFI ± SEM of CD19 is shown. (C) The MFI of CD19 on immature B cells from bone marrow cultures with IL-7 or from day 2 after the transfer into the anti-Id (receptor-editing culture) or control culture conditions. Results are ±SEM.
We also analyzed the level of CD19 in the bone marrow cells of the receptor-editing H-2k/d mice. We found a dramatic reduction of CD19, but in a reciprocal pattern. B10 mice displayed a 10.5-fold reduction in the MFI of CD19, whereas MRL/lpr mice only showed a 4.5-fold reduction (Fig. 7 B). Because B10 mice undergo more receptor editing in the bone marrow, the latter observation suggests that CD19 is down-regulated during receptor editing in proportion to the extent of receptor editing.
In addition, we found that MRL/lpr immature B cells have consistently lower levels of CD19 in the bone marrow compared with B10 mice both for transgenic (on the nonantigenic H-2d background) and nontransgenic mice (Fig. 7 B). Thus, we find that receptor-editing results in down-regulation of CD19 as well as IgM and, in agreement with our observation that B10 mice undergo more receptor editing than MRL/lpr mice, the extent of down-regulation of both CD19 and IgM is greater in B10 transgenic bone marrow than in MRL/lpr bone marrow. Surprisingly, the MRL/lpr B cells maintain a suppressed level of CD19 in mature splenic cells after the cessation of receptor editing, whereas the B10 mice revert to almost normal levels.
Surface CD19 levels decrease in receptor-editing cultures
In the in vitro receptor-editing cultures, we also observed a decrease in surface expression of CD19 in the cells cultured with anti-Id compared with the control cultures, which was consistent with the hypothesis that this decrease is a reflection of receptor editing (Fig. 7 C). However, the extent of this decrease was much less than that observed in vivo. As with the decrease in IgM in the culture system, the relative decrease in CD19 was similar between the two strains of mice, even though the MRL/lpr transgenic mice underwent less receptor editing in vitro. However, the level of IgM and CD19 in the day 5 IL-7 cultures is 25 and 40% less, respectively, in the MRL/lpr immature B cells than in those from the B10 transgenic cultures (Figs. 2 and 7 C).
Transit time through the pre–B cell compartment is faster for MRL/lpr mice
The data presented so far is limited to BCR transgenic mice. Determining whether nontransgenic lupus-prone mice have a defect in receptor editing in vivo is less straightforward to analyze. These mice have a vast array of different antigen receptors with a wide range of affinities for a host of soluble and membrane-bound self-antigens.
One assay to indicate if MRL/lpr mice undergo less receptor editing in vivo is to measure the transit time through the pre–B cell compartment. It has been shown that cells undergoing receptor editing are delayed in their transit time through the pre–B cell compartment (10, 13). A single BrdU injection will label the large pre–B cells that are undergoing extensive proliferation as a result of pre-BCR signaling. Hence, one can follow those labeled cells and determine the time course of accumulation of BrdU-labeled cells in the immature B cell compartment. If receptor editing is taking place at a lower frequency in autoimmune-prone mice, we would predict that the cells from MRL/lpr mice would transit more rapidly through the pre–B cell stage because not as many of the cells would be returned there to undergo receptor editing.
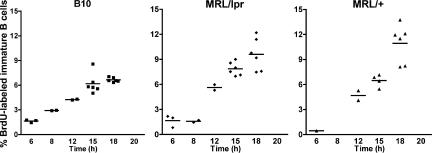
To test this, MRL/lpr and C57BL/6 mice were injected i.p. with BrdU and analyzed at various time points by flow cytometry. It can be seen that the proportion of BrdU-labeled immature B cells rises much faster in MRL/lpr than in B6 mice (Fig. 8). This shorter transit time through the pre–B cell compartment is consistent with the hypothesis that MRL/lpr mice undergo less receptor editing in vivo.
Figure 8.
Transit time through the pre–B cell compartment is faster in the bone marrow of MRL/lpr and MRL/+ mice as compared with B6 mice. Nontransgenic B6, MRL/lpr, and MRL/+ mice were injected once i.p. with BrdU and analyzed at various time points. Cells were surface stained, permeabilized, and stained with FITC-BrdU to determine transit time through the pre–B cell compartment. The percentage of BrdU-labeled immature B cells from each mouse is plotted. The horizontal bar represents the mean of each time point.
Defect in receptor editing is not caused by the lpr mutation
To determine if the defect in receptor editing is caused by the lpr mutation in the Fas gene, we also used BrdU to determine the kinetics of B cell development in the bone marrow of MRL/+ mice and compared the results with those of MRL/lpr mice. Fig. 8 shows that the kinetics of appearance of BrdU-labeled immature B cells is similar in both of these strains, suggesting that the lower level of receptor editing in MRL/lpr mice is not caused by the defect in the Fas gene.
DISCUSSION
Although it is generally agreed that the breakdown of tolerance and activation of autoreactive B cells in lupus primarily occurs in the periphery, we present evidence that there is also a defect in central tolerance in lupus-prone mice. The bone marrow central tolerance checkpoint can be accomplished by either receptor editing or by clonal deletion. In nonautoimmune strains of mice, receptor editing has been shown to be the major mechanism of B cell tolerance in the bone marrow (4, 13), but there are little data to distinguish whether autoimmune-prone mice undergo less receptor editing in situations where clonal deletion may compensate for inefficient receptor editing and, thus, mask this potentially important defect.
In this study, we use a well-established in vitro system to measure defined classic parameters of receptor editing (i.e., up-regulation of RAG mRNA and induction of endogenous light chain rearrangement) to demonstrate that MRL/lpr lupus-prone mice have a less robust receptor-editing response to BCR ligation at the immature B cell stage (18). Because the BCR is encoded by heavy and light chain transgenes that are not targeted into the heavy and light chain loci in the mice we used in this study, effective receptor editing per se cannot occur, but these parameters give an excellent readout of the attempted response of immature B cells to receptor-editing signals. Furthermore, we used these same parameters of receptor editing to demonstrate that receptor editing is also less efficient even in response to strong stimulation by ubiquitously expressed membrane-bound self-antigen in vivo. In the latter case, however, clonal deletion is very effective, and thus, no Id+ cells can be found in the periphery.
There are data in the literature consistent with our hypothesis that autoimmune individuals have less efficient central tolerance mechanisms. Older studies have proposed that humans with autoimmune diseases have either a decreased or an increased incidence of receptor editing, using parameters such as the increased usage of downstream Jκ or Jλ genes (21–25). More recently, Yurasov et al. analyzed the repertoire of newly emigrated and mature naive cells from the peripheral blood of SLE patients, demonstrating that this second tolerance checkpoint is much less effective in SLE patients (14). The first tolerance checkpoint occurs in the bone marrow and, thus, was not directly investigated in the lupus patients. However, in previous work, these investigators showed that the autoimmune and polyreactive profiles of the late immature B cells from the bone marrow resembled the peripheral blood new emigrants (1). The new emigrant B cells in the SLE patients had a slightly higher proportion of polyreactive and autoreactive cells than controls, which is suggestive of a defect in an early tolerance checkpoint. Whether this potential defect is caused by less efficient receptor editing or less efficient clonal deletion was not investigated in that study.
In agreement with previously published studies, we observed that the few B cells that were found in the spleen of either MRL/lpr or B10 3-83 transgenic mice on the antigenic H-2k/d background had all undergone receptor editing, as demonstrated by the complete absence of Id+ cells in the spleen and also by the presence of a large proportion of λ+ cells (6, 16). Surprisingly, however, the transitional B cells expressed a much higher proportion of κ+ cells than the mature AA4− cells, and importantly, the MRL/lpr transitional cells expressed an even higher percentage of κ+ cells than the B10 transitional cells. In the bone marrow, the majority of immature B cells, which are all Id−, are κ+. Because it is known that κ alleles rearrange before λ alleles, we hypothesize that the receptor-editing process in response to in vivo exposure to H-2Kk will first create endogenous κ rearrangements, and that some of the κ+ cells that have undergone a first round of receptor editing may still have some reactivity to self-antigen with their new receptors, stimulating continued receptor editing. The subset of cells with lower self-reactivity would then emigrate to the spleen but should be deleted in the transitional stage, resulting in a mature splenic population that is depleted of all self-reactive cells. Our data clearly show that the population of receptors in the immature transitional compartment is very different from that of the naive AA4− splenic B cell compartment; hence, it must be the case that clonal deletion is taking place between the transitional and the naive B cell compartments, because there is no RAG expression at this stage.
The fact that MRL/lpr H-2k/d transgenic mice have a higher proportion of κ+ cells in both the bone marrow immature B cell compartment and in the splenic transitional compartment than the B10 H-2k/d mice suggests that receptor editing is not occurring as frequently in the MRL/lpr mice; therefore, we propose that more of the lower affinity self-reactive immature B cells from MRL/lpr mice are leaving the bone marrow. This suggests that the threshold for permitting immature B cells to leave the bone marrow in MRL/lpr mice is less stringent than the threshold in B10 mice, and these self-reactive B cells that emigrate to the spleen in MRL/lpr mice could be a reservoir of potentially pathogenic autoreactive B cells.
Another major difference between the peripheral B cells in the two strains of mice is that the B10 transgenic H-2k/d mice have a higher proportion of κ and λ double-expressing cells than the MRL mice. This is reminiscent of the studies of Li et al. with the 3H9/56R anti-DNA heavy chain knock-in mice (26, 27). The 3H9/56R H chain creates an anti-DNA receptor with most light chains, except for those encoded by a few Vκ genes termed “Vκ editors,” and Vλx. When the anti-DNA heavy chain was present on the BALB background, all peripheral B cells either expressed a light chain using a Vκ editor, or coexpressed both a Vκ editor and a λ receptor on their surface, each at half the normal surface density of IgM (27). In the latter case, the λ receptor would generate an anti-DNA specificity, but because the cells coexpressed a Vκ editor, the innocuous BCR expressing the Vκ editor light chain may have allowed the exit of these potentially autoreactive B cells from the bone marrow. The lower level of λ+ BCR on the surface may prevent the double expressers, which account for ∼30% of the splenic B cell population in these mice, from becoming activated by self-antigen. In contrast, when the same 3H9/56R heavy chain was present on the MRL/lpr background, no κ/λ double expressers were observed. Similarly, we also observed a higher proportion of κ/λ double expressers in the nonautoimmune B10 3-83 H-2k/d mouse strain than in the MRL/lpr transgenic mice. Furthermore, some 3H9/56R cells were found that express only λ1, and thus bound DNA, in the MRL/lpr transgenic mice (26). The fact that these cells were found in the periphery suggests a defect in central tolerance in the MRL/lpr, but not BALB/c, 3H9/56R transgenic mice, reinforcing our hypothesis that the threshold for exit of low affinity self-reactive B cells from the bone marrow may be lower for MRL/lpr than for nonautoimmune mice. It may be that the hyperactivity of the immature B cells in MRL/lpr mice (Fig. 3) results in their exit from the bone marrow before appropriate receptor editing has been completed.
CD19 is an important coreceptor in the BCR signaling complex, and a reduction in the expression of CD19 can result in less robust BCR signaling in mature B cells (28–30). CD19 also functions in immature B cells. In the CD19−/− 3-83 transgenic mice on the nonantigen B10.D2 background, receptor editing was greatly stimulated (31). Signals generated through CD19 are critical for activation of phosphoinositide 3-kinase, which generates PIP3 and suppresses RAG mRNA expression (32). In immature B cells receiving a receptor-editing signal, the level of PIP3 falls and RAG mRNA levels remain high. We analyzed the level of CD19 in the bone marrow cells of the transgenic MRL/lpr and B10 H-2k/d mice that receive strong receptor-editing signals in vivo. We observed substantial down-regulation of both CD19 and IgM after BCR cross-linking. Importantly, however, the extent of down-regulation of both CD19 and IgM was far greater in the B10 mice than in the MRL/lpr mice, which was consistent with our hypothesis that MRL/lpr mice undergo less receptor editing. Thus, the extent of down-regulation of CD19, which likely affects intracellular PIP3 levels, appears to be proportional to the extent of receptor editing.
We observed much more modest down-regulation of CD19 in the in vitro IL-7 culture system, where BCR cross-linking was induced by anti-Id antibody, mimicking soluble antigen. We attribute this modest CD19 down-regulation to the weaker signal presumably given by soluble versus membrane-bound antigen. In agreement with this, the extent of IgM down-regulation is also less in the cultured immature B cells than in the bone marrow in vivo. This suggests that the dramatic down-regulation of IgM and CD19 in the bone marrow of mice exposed to membrane-bound self-antigen is indicative of the increased strength of the interaction with the ubiquitously expressed cell-surface MHC class I molecule.
In the spleens of transgenic or nontransgenic MRL/lpr and B10 mice, we observed similar levels of CD19 expression between the two strains. This is in contrast to one study of SLE patients in which it was shown that naive peripheral blood B cells from SLE patients have a 20% lower MFI of CD19 than healthy controls (33), though another study did not show much difference in CD19 expression (34). In contrast, nontransgenic MRL/lpr mice have lower levels of CD19 and IgM than B10 mice in immature B cells. The lower MFI of both IgM and CD19 on bone marrow immature B cells from MRL/lpr mice might suggest that they receive a weaker signal through engagement of their BCR with self-antigen than immature B cells from B10 mice. This weaker signal could contribute to the less effective receptor-editing response. However, our data showing increased calcium flux in immature B cells from MRL/lpr mice demonstrate that the explanation is more complex. To our knowledge, this is the first demonstration of increased calcium flux in bone marrow immature B cells in lupus mice. These data are consistent with previous observations in peripheral blood B cells from SLE patients in which IgM cross-linking results in increased calcium flux and increased tyrosine phosphorylation of signaling proteins (35, 36). Peripheral T cells from MRL/lpr mice have also been demonstrated to be hyperresponsive to stimulation through their TCR (37). Because we observed that immature B cells from IL-7–driven cultures of MRL/lpr mice are hyperresponsive with regard to the extent of calcium mobilization after either ionomycin or anti-IgM stimulation, this suggests that the hyperresponsiveness is downstream of IgM.
Hyperresponsiveness to BCR stimulation may have contrasting effects for peripheral versus immature B cells. It is known that there are many differences in the way that immature and mature splenic B cells respond to BCR ligation, which could be caused by differences in the level of individual proteins involved in signaling cascades, or whether the BCR signals from inside or outside lipid rafts (19, 38, 39). In the periphery, hyperresponsiveness may lead to inappropriate activation of low affinity self-reactive cells. However, hyperresponsiveness may be a detriment in immature bone marrow cells. It is possible that the immature B cells from MRL/lpr mice are receiving too strong a signal to up-regulate RAG mRNA. It has been shown that immature 3-83 transgenic B cells from IL-7 cultures only up-regulate RAG mRNA when they are stimulated with concentrations of ionomycin that are so low that they barely elicit a calcium flux response (19). To test the hypothesis that MRL/lpr may have too strong a response to IgM or ionomycin stimulation, we cultured immature B cells from both strains of mice with varying concentrations of ionomycin. Consistent with our hypothesis, immature B cells from MRL/lpr mice up-regulated RAG mRNA at much lower concentrations of ionomycin than immature B cells from B10 mice. It may be the case that the receptor-editing and apoptosis/clonal deletion signals may go through two different pathways, and that the clonal deletion/apoptosis signaling pathway is functioning very well in MRL/lpr mice, at least with regard to strong membrane-bound antigens.
Many lupus susceptibility loci have been identified, but it is unlikely that any of the ones described thus far control the receptor-editing defect that we describe in this paper (40, 41). The only genetic interval that may affect receptor editing is the Sle1b locus of the NZM2410 strain. Recently, it has been shown that one key polymorphic gene within this region is Ly108, a signaling lymphocyte activation molecule family member (42, 43). Transfection of the Ly108.1 isoform of this gene into an immature B cell line, WEHI 231, results in less RAG up-regulation after BCR cross-linking than transfection of the Ly108.2 isoform. B6 mice express more of the Ly108.2 isoform, whereas B6.Sle1bz mice express more of the Ly108.1 isoform. This suggests that this Sle1b lupus susceptibility gene may affect receptor editing, although the effect of the Ly108 polymorphism in controlling RAG mRNA levels in vivo in B6 versus B6.Sle1bz congenic mice was not assayed. However, expression of the Ly108.1 allele results in less calcium flux in immature B6.Sle1bz B cells after BCR cross-linking, which is the opposite of what we observed in the immature B cells from MRL/lpr mice. Thus, there must be different susceptibility genes controlling the poor RAG up-regulation in the WEHI Ly108.1 transfectants and in the MRL/lpr mice.
In conclusion, we suggest that immature B cells from MRL/lpr mice make less effective responses to engagement of their receptors with self-antigen. They are less efficient at up-regulating RAG mRNA, and they down-regulate surface IgM and its coreceptor CD19 less efficiently than B10 transgenic cells. The faster transit time of B cell precursors through the pre–B cell compartment from nontransgenic MRL/lpr and MRL/+ mice is consistent with the hypothesis that polyclonal, nontransgenic MRL mice undergo less receptor editing in vivo to a wide variety of normal self-antigens. The MRL background must contribute genes that allow these less appropriate responses to self-antigen at this critical tolerance checkpoint, resulting in a higher proportion of B cells that may leave the bone marrow and emigrate to the periphery, where they can be activated by exposure to antigen, eventually resulting in autoimmune disease.
MATERIALS AND METHODS
Mice.
Mice were maintained in The Scripps Research Institute (TSRI) animal resources facility according to TSRI's Institutional Animal Care and Use guidelines. All experimental mice were between 6–9 wk of age. The generation of the MRL/lpr 3-83 transgenic mice on the H-2d/d background was previously described (8). Mice were used at the seventh and eighth generations. To produce the MRL/+ H-2d/d 3-83 transgenic mice, MRL/lpr (H-2d/d) 3-83 transgenic mice were backcrossed onto MRL/+ (H-2k) mice and screened to obtain transgenic mice homozygous for the wild-type Fas allele and for H-2d.
To study the extent of receptor editing in vivo, B10.D2 and MRL/lpr H-2d/d 3-83 transgenic mice were crossed with either B10.BR (H-2k) or MRL/lpr (H-2k) mice, respectively, and H-2k/d F1 offspring were screened for the presence of the transgene. Animals that were transgene positive were used for analysis.
Receptor-editing culture system.
We use a receptor-editing culture system, as previously described (8, 18). In brief, bone marrow cells are removed from mice and cultured in IMDM media supplemented with IL-7. On day 5, the IL-7 is removed, and cells are washed and recultured in IMDM media plus either an anti-Id antibody (S23), which is specific for the 3-83 BCR, or nonreactive control antibody (Y3) for 48 h. Cells are removed at 24 and 48 h and processed for DNA and RNA. In addition, an aliquot of the cells is left in IL-7–supplemented IMDM media. Data are expressed as the fold increase in cultures with anti-Id compared with the IL-7 cultures, or fold increase in control cultures compared with the IL-7 cultures.
DNA/RNA/complementary DNA (cDNA).
DNA and RNA were extracted from cells as per the manufacturer's instructions (QIAGEN). cDNA was made from RNA extractions as per manufacturer's instructions using the Super Script II kit (Invitrogen).
Quantitative real-time PCR.
κ rearrangements (5′-GGCTGCAGSTTCA-GTGGCAGTGGAT-3′ and 5′-GCCACAGACATAGACAACGGAAGAA-3′), levels of RAG mRNA (Rag 1, 5′-CATTCTAGCACTCTGGCCGG-3′ and 5′-TCATCGGGTGCAGAACYGAA-3′; Rag 2, 5′-TTAATTCCTGGCTTGGCCG-3′ and 5′-TTCCTGCTTGTGGATGTGAAAT-3′), and RS rearrangements (IRS1, 5′-CATTTGGCGTTCACCCTGCC-3′; RS-3′, 5′-TGTTTTCCTGGAGCTCCTCAGG-3′) were assessed by real-time PCR using a sequence detection system (ABI 7900; Applied Biosytems) and a Quantitec SYBR PCR kit (QIAGEN). PCR conditions were as follows: 95° for 15 min, cycled 45 times at 94° for 15 sec, 60° for 30 sec, and 72° for 30 sec. All samples were normalized to actin, either DNA or cDNA, by real-time PCR analysis using the ΔCT calculation. The following actin primers were used: DNA actin (5′-AGGCATGGAGTCCTGTGGTATC-3′ and 5′-AGCCACAGGTCCTAAGGCCAG-3′) and cDNA actin (5′-TCTGGCTCCTAGCACCATGAAGA-3′ and 5′-GGGACTCATCGTACTCCTGCTTG-3′). Data are expressed as ΔCT or as fold increase using the formula 2ΔCT.
Ionomycin cultures.
Cells from day 5 IL-7 cultures were washed and cultured with varying concentrations of ionomycin (Sigma-Aldrich) for 24 h. Cells were then harvested and RNA was prepared.
Calcium flux.
Cultured bone marrow cells were incubated with 20 μM Indo-1 AM (Invitrogen) in loading solution for 45 min at 37°C. Cells were then washed and subsequently stimulated with varying concentrations of a F(ab′)2 fragment of donkey anti–mouse IgM (Jackson ImmunoResearch Laboratories) or ionomycin. Measurement of the amount of intracellular calcium flux after stimulation was performed for 8 min on a digital flow cytometer (LSR II; BD Biosciences).
Flow cytometric analysis and cell sorting.
Cells from the bone marrow and spleen were stained for various cell-surface markers. Bone marrow cells were stained for anti–mouse CD43-FITC (BD Biosciences), IgM-PE (eBioscience), AA4-allophycocyanin (APC; eBioscience), and CD19–Pacific blue (Biolegend) or B220-PeCy7 (Biolegend). Spleen cells were stained using varying combinations of anti–mouse CD19-APC or Pacific blue, or B220-PeCy7, IgM-PE, IgD-FITC (Biolegend), κ-PE (BD Biosciences), λ-biotin (BD Biosciences), AA4-APC, CD21-FITC (BD Biosciences), CD23-PE (Biolegend), or biotin-conjugated anti-Id. Biotinylated antibodies were detected using streptavidin-conjugated Pacific blue or APC (Biolegend). Cells were analyzed using an LSR II or a FACSCalibur instrument (BD Biosciences) and FlowJo software (Tree Star, Inc). Cell sorting was performed on either a FACSVantage DiVa or FACSAria (both from BD Biosciences).
MACS purification.
B220+ cells were purified from the bone marrow by staining with anti-B220 conjugated with PE, followed by positive purification of the cells on an anti-PE column according to the manufacturer's instructions (Miltenyi Biotec).
BrdU labeling.
Mice were injected once i.p. with 100 μl (10 mg/ml) of BrdU (BD Biosciences) and killed at the time points indicated in the figures. Bone marrow was isolated, stained for the surface markers IgM (PE conjugated; eBioscience) and AA4 (APC conjugated; eBioscience), and fixed. Cells were then permeabilized and stained with FITC-BrdU antibody according to the manufacturer's protocol (FITC-BrdU flow kit; BD Biosciences). Analysis of BrdU/FITC staining was performed on an LSR II.
Acknowledgments
We gratefully acknowledge expert technical help from Sara Salerno, Trigere Popoff, and Siddarth Menon. We also thank Drs. Annica Martensson, Amanda Gavin, and Laurent Verkoczy for helpful advice and discussions on the receptor-editing culture system, and thank Drs. John Carey, Martin Weigert, and Argyrios Theofilopoulos for very helpful discussions on the manuscript. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID) or the National Institutes of Health (NIH).
This work was supported by NIAID grant R01 AI61167 to A.J. Feeney. J. Lamoureux was supported by NIH training grant T32 HL07195.
The authors have no conflicting financial interests.
Abbreviations used: APC, allophycocyanin; BCR, B cell receptor; cDNA, complementary DNA; MFI, mean fluorescence intensity; mRNA, messenger RNA; RS, recombining sequence; SLE, systemic lupus erythematosus.
References
- 1.Wardemann, H., S. Yurasov, A. Schaefer, J.W. Young, E. Meffre, and M.C. Nussenzweig. 2003. Predominant autoantibody production by early human B cell precursors. Science. 301:1374–1377. [DOI] [PubMed] [Google Scholar]
- 2.Goodnow, C.C., J. Sprent, B. Fazekas de St Groth, and C.G. Vinuesa. 2005. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 435:590–597. [DOI] [PubMed] [Google Scholar]
- 3.Carsetti, R., G. Kohler, and M.C. Lamers. 1995. Transitional B cells are the target of negative selection in the B cell compartment. J. Exp. Med. 181:2129–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halverson, R., R.M. Torres, and R. Pelanda. 2004. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat. Immunol. 5:645–650. [DOI] [PubMed] [Google Scholar]
- 5.Gay, D., T. Saunders, S. Camper, and M. Weigert. 1993. Receptor editing: an approach by autoreactive B cells to escape tolerance. J. Exp. Med. 177:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiegs, S.L., D.M. Russell, and D. Nemazee. 1993. Receptor editing in self-reactive bone marrow B cells. J. Exp. Med. 177:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su, T.T., and D.J. Rawlings. 2002. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J. Immunol. 168:2101–2110. [DOI] [PubMed] [Google Scholar]
- 8.Watson, L.C., C.S. Moffatt-Blue, R.Z. McDonald, E. Kompfner, D. Ait-Azzouzene, D. Nemazee, A.N. Theofilopoulos, D.H. Kono, and A.J. Feeney. 2006. Paucity of V-D-D-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT−/− and TdT+/+ mice. J. Immunol. 177:1120–1128. [DOI] [PubMed] [Google Scholar]
- 9.Retter, M.W., and D. Nemazee. 1998. Receptor editing occurs frequently during normal B cell development. J. Exp. Med. 188:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casellas, R., T.A. Shih, M. Kleinewietfeld, J. Rakonjac, D. Nemazee, K. Rajewsky, and M.C. Nussenzweig. 2001. Contribution of receptor editing to the antibody repertoire. Science. 291:1541–1544. [DOI] [PubMed] [Google Scholar]
- 11.Ait-Azzouzene, D., L. Verkoczy, J. Peters, A. Gavin, P. Skog, J.L. Vela, and D. Nemazee. 2005. An immunoglobulin Cκ-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J. Exp. Med. 201:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melamed, D., R.J. Benschop, J.C. Cambier, and D. Nemazee. 1998. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 92:173–182. [DOI] [PubMed] [Google Scholar]
- 13.Hippen, K.L., B.R. Schram, L.E. Tze, K.A. Pape, M.K. Jenkins, and T.W. Behrens. 2005. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J. Immunol. 175:909–916. [DOI] [PubMed] [Google Scholar]
- 14.Yurasov, S., H. Wardemann, J. Hammersen, M. Tsuiji, E. Meffre, V. Pascual, and M.C. Nussenzweig. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J. Exp. Med. 201:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yurasov, S., T. Tiller, M. Tsuiji, K. Velinzon, V. Pascual, H. Wardemann, and M.C. Nussenzweig. 2006. Persistent expression of autoantibodies in SLE patients in remission. J. Exp. Med. 203:2255–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubio, C.F., J. Kench, D.M. Russell, R. Yawger, and D. Nemazee. 1996. Analysis of central B cell tolerance in autoimmune-prone MRL/lpr mice bearing autoantibody transgenes. J. Immunol. 157:65–71. [PubMed] [Google Scholar]
- 17.Rathmell, J.C., and C.C. Goodnow. 1994. Effects of the lpr mutation on elimination and inactivation of self-reactive B cells. J. Immunol. 153:2831–2842. [PubMed] [Google Scholar]
- 18.Melamed, D., and D. Nemazee. 1997. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc. Natl. Acad. Sci. USA. 94:9267–9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benschop, R.J., D. Melamed, D. Nemazee, and J.C. Cambier. 1999. Distinct signal thresholds for the unique antigen receptor-linked gene expression programs in mature and immature B cells. J. Exp. Med. 190:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelanda, R., S. Schwers, E. Sonoda, R.M. Torres, D. Nemazee, and K. Rajewsky. 1997. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 7:765–775. [DOI] [PubMed] [Google Scholar]
- 21.Dorner, T., N.L. Farner, and P.E. Lipsky. 1999. Ig lambda and heavy chain gene usage in early untreated systemic lupus erythematosus suggests intensive B cell stimulation. J. Immunol. 163:1027–1036. [PubMed] [Google Scholar]
- 22.Dorner, T., S.J. Foster, N.L. Farner, and P.E. Lipsky. 1998. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J. Clin. Invest. 102:688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meffre, E., E. Davis, C. Schiff, C. Cunningham-Rundles, L.B. Ivashkiv, L.M. Staudt, J.W. Young, and M.C. Nussenzweig. 2000. Circulating human B cells that express surrogate light chains and edited receptors. Nat. Immunol. 1:207–213. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki, N., T. Harada, S. Mihara, and T. Sakane. 1996. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J. Clin. Invest. 98:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monestier, M., and M. Zouali. 2002. Receptor revision and systemic lupus. Scand. J. Immunol. 55:425–431. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y., H. Li, D. Ni, and M. Weigert. 2002. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J. Exp. Med. 196:1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, Y., H. Li, and M. Weigert. 2002. Autoreactive B cells in the marginal zone that express dual receptors. J. Exp. Med. 195:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter, R.H., and D.T. Fearon. 1992. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 256:105–107. [DOI] [PubMed] [Google Scholar]
- 29.Rickert, R.C., K. Rajewsky, and J. Roes. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 376:352–355. [DOI] [PubMed] [Google Scholar]
- 30.Engel, P., L.J. Zhou, D.C. Ord, S. Sato, B. Koller, and T.F. Tedder. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 3:39–50. [DOI] [PubMed] [Google Scholar]
- 31.Shivtiel, S., N. Leider, O. Sadeh, Z. Kraiem, and D. Melamed. 2002. Impaired light chain allelic exclusion and lack of positive selection in immature B cells expressing incompetent receptor deficient of CD19. J. Immunol. 168:5596–5604. [DOI] [PubMed] [Google Scholar]
- 32.Verkoczy, L., B. Duong, P. Skog, D. Ait-Azzouzene, K. Puri, J.L. Vela, and D. Nemazee. 2007. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J. Immunol. 178:6332–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Culton, D.A., M.W. Nicholas, D.O. Bunch, Q.L. Zhen, T.B. Kepler, M.A. Dooley, C. Mohan, P.H. Nachman, and S.H. Clarke. 2007. Similar CD19 dysregulation in two autoantibody-associated autoimmune diseases suggests a shared mechanism of B-cell tolerance loss. J. Clin. Immunol. 27:53–68. [DOI] [PubMed] [Google Scholar]
- 34.Enyedy, E.J., J.P. Mitchell, M.P. Nambiar, and G.C. Tsokos. 2001. Defective FcgammaRIIb1 signaling contributes to enhanced calcium response in B cells from patients with systemic lupus erythematosus. Clin. Immunol. 101:130–135. [DOI] [PubMed] [Google Scholar]
- 35.Liossis, S.N., B. Kovacs, G. Dennis, G.M. Kammer, and G.C. Tsokos. 1996. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J. Clin. Invest. 98:2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipsky, P.E. 2001. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat. Immunol. 2:764–766. [DOI] [PubMed] [Google Scholar]
- 37.Vratsanos, G.S., S. Jung, Y.M. Park, and J. Craft. 2001. CD4+ T cells from lupus-prone mice are hyperresponsive to T cell receptor engagement with low and high affinity peptide antigens: a model to explain spontaneous T cell activation in lupus. J. Exp. Med. 193:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benschop, R.J., E. Brandl, A.C. Chan, and J.C. Cambier. 2001. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J. Immunol. 167:4172–4179. [DOI] [PubMed] [Google Scholar]
- 39.Chung, J.B., M.A. Baumeister, and J.G. Monroe. 2001. Cutting edge: differential sequestration of plasma membrane-associated B cell antigen receptor in mature and immature B cells into glycosphingolipid-enriched domains. J. Immunol. 166:736–740. [DOI] [PubMed] [Google Scholar]
- 40.Kono, D.H., and A.N. Theofilopoulos. 2006. Genetics of SLE in mice. Springer Semin. Immunopathol. 28:83–96. [DOI] [PubMed] [Google Scholar]
- 41.Fairhurst, A.M., A.E. Wandstrat, and E.K. Wakeland. 2006. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv. Immunol. 92:1–69. [DOI] [PubMed] [Google Scholar]
- 42.Kumar, K.R., L. Li, M. Yan, M. Bhaskarabhatla, A.B. Mobley, C. Nguyen, J.M. Mooney, J.D. Schatzle, E.K. Wakeland, and C. Mohan. 2006. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 312:1665–1669. [DOI] [PubMed] [Google Scholar]
- 43.Wandstrat, A.E., C. Nguyen, N. Limaye, A.Y. Chan, S. Subramanian, X.H. Tian, Y.S. Yim, A. Pertsemlidis, H.R. Garner Jr., L. Morel, and E.K. Wakeland. 2004. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 21:769–780. [DOI] [PubMed] [Google Scholar]