Abstract
Specific targets of cellular immunity in human premalignancy are largely unknown. Monoclonal gammopathy of undetermined significance (MGUS) represents a precursor lesion to myeloma (MM). We show that antigenic targets of spontaneous immunity in MGUS differ from MM. MGUS patients frequently mount a humoral and cellular immune response against SOX2, a gene critical for self-renewal in embryonal stem cells. Intranuclear expression of SOX2 marks the clonogenic CD138− compartment in MGUS. SOX2 expression is also detected in a proportion of CD138+ cells in MM patients. However, these patients lack anti-SOX2 immunity. Cellular immunity to SOX2 inhibits the clonogenic growth of MGUS cells in vitro. Detection of anti-SOX2 T cells predicts favorable clinical outcome in patients with asymptomatic plasmaproliferative disorders. Harnessing immunity to antigens expressed by tumor progenitor cells may be critical for prevention and therapy of human cancer.
The immune system has long been debated as a potential barrier to carcinogenesis and may provide a valuable approach to early detection and prevention of cancer (1). Studies have documented the ability of the immune system to respond to antigens expressed by tumor cells in cancer patients (2). However, the specific nature of antigenic targets of T cell immunity in human premalignancy is largely unknown (3, 4). Understanding the specific targets of immune recognition of the earliest human tumors and their precursors directly in patients is therefore a critical first step for harnessing the immune system to detect and prevent human cancer. Monoclonal gammopathy of undetermined significance (MGUS) occurs in 3% of the population >50 yr of age and represents a precursor lesion to myeloma (MM) (5). Tumor cells in MGUS carry most of the known cytogenetic and genomic abnormalities found in MM (6), but only a small proportion transform into clinical malignancy, suggesting a role for additional events, including those involving the host, in regulating malignant transformation.
Studies have recently provided experimental evidence for the concept that the growth of several human tumors may depend on a small proportion of clonogenic or “cancer stem cells” (7). Although the bulk tumor in MM consists of plasma cells that express syndecan-1 (CD138), recent studies have suggested that the clonogenic growth may be enriched in a fraction missing this marker (8, 9). However, specific markers to identify this population are lacking. Whether the immune system has the capacity to specifically target antigens expressed on cancer stem cells in humans is also not known.
In this paper, we show that the expression of an embryonal stem cell marker, SOX2, specifically marks the clonogenic CD138− compartment in MGUS patients, and these patients frequently mount humoral and cellular immunity to this antigen. These data demonstrate the capacity of the human immune system to spontaneously target antigens expressed on tumor progenitors and the association of spontaneous immunity against this target with an improved clinical outcome.
RESULTS
Detection of anti-SOX2 IgG antibodies in MGUS but not MM patients or healthy donors
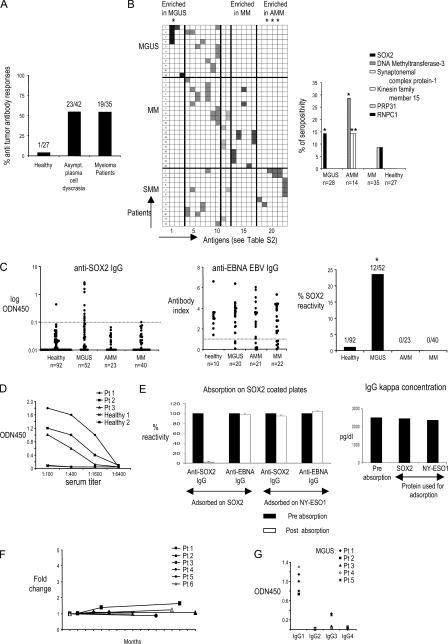
In prior studies, we have shown that the immune system is capable of recognizing the preneoplastic lesions in MGUS (10). To begin a systematic analysis of antigenic targets of antitumor immunity in MGUS/MM, we initially analyzed sera from patients with MM (n = 35), MGUS (n = 28), and asymptomatic MM (AMM; n = 14) for the presence of IgG antibodies against a panel of 83 serological expression of cDNA expression libraries (SEREX)–defined tumor antigens using a serum antibody detection array (SADA; Fig. 1 A and Table S1, available at http://www.jem.org/cgi/content/full/jem.20062387). Reactivity against 23 of the antigens in this panel was detected in the sera from MGUS, AMM, or MM patients but only in 1 out of 27 sera from normal blood donors with this assay. Interestingly, the pattern of antigenic reactivity differed between these cohorts (Fig. 1 B and Table S2). Immune responses to Sry-HMG-box 2 (SOX2) protein were seen only in MGUS, whereas antibodies against certain other antigens (DNA methyltransferase 3, synaptonemal complex protein 1, and kinesin family member 15) were only detected in AMM (Fig. 1 B). To validate and quantify the presence of anti-SOX2 antibodies in MGUS, we reanalyzed a larger cohort of patients and age-matched healthy controls with an ELISA-based assay (Fig. 1 C). Overall, anti-SOX2 IgG antibodies were detected in 12 out of 52 (23%) MGUS patients but in none of the AMM (n = 23) and MM (n = 40) patients and in only 1 out of 92 healthy donors tested (P < 0.001). As controls, immune responses to Epstein-Barr nuclear antigen 1 (EBNA-1; Fig. 1 C) and tetanus toxoid (not depicted) were comparably detectable in all cohorts. Anti-SOX2 antibodies were present at a high titer and were detectable at a dilution of ≥1:400 (Fig. 1 D). No anti-SOX2 IgM antibodies were detected in any cohort (unpublished data). Anti-SOX2 IgG antibodies were of both κ and λ light chain specificity and were detected in both IgG and non-IgG gammopathies (unpublished data). Preabsorption of sera with recombinant SOX2 protein abrogated anti-SOX2 reactivity without affecting the monoclonal paraprotein (Fig. 1 E). Therefore, the observed reactivity is not caused by the monoclonal Ig found in these patients. For six MGUS patients with high titers of anti-SOX2 antibodies, follow-up samples over 2 yr revealed that the antibody titers, as well as the clinical status, remain stable over time (Fig. 1 F). In all SOX2-reactive MGUS sera, antibodies were predominantly of the IgG1 subclass (Fig. 1 G). Collectively, these data demonstrate the presence of anti-SOX2 IgG1 antibodies in a substantial proportion of MGUS patients.
Figure 1.
Antibody responses in patients with monoclonal gammopathies. (A) Preabsorbed serum samples from patients with MGUS, AMM, and MM were evaluated by SADA for the presence of IgG antibodies against a panel of 83 SEREX-defined antigens. The frequency of antibody responses within the cohorts of patients with asymptomatic plasma cell disease and multiple MM is shown. Numbers indicate the patients with positive antibody response out of absolute numbers of patients evaluated by SADA for each group. (B, left) Patterns of antigenic reactivity in patients with MGUS, MM, and AMM. Rows depict individual patients according to their diagnosis, and columns show antibody reactivity against 23 tumor antigens with seropositivity in this cohort. Specific antigens in each column correspond numerically to antigens 1–23 in Table S2. (right) Shown are specific antigens inducing differential antibody response between subgroups of monoclonal gammopathies and the frequency of antibody reactivity as detected by SADA. *, P < 0.05. PRPF31, pre-mRNA–processing factor 31 homologue; RNPC1, RNA-binding region (RNP1, RRM)–containing 1. (C) ELISA for detection of SOX2-specific (left) and EBNA EBV–specific (middle) IgG antibodies in sera of MGUS, AMM, and MM patients. The distribution of antibody titers within specific groups is shown. (right) Overall SOX2 reactivity. Dotted lines represent the cutoff values for seropositivity. *, P < 0.05. (D) Titers of SOX2 antibodies in serial dilutions of sera from SOX2-reactive patients. (E) Sera from SOX2-positive patients were absorbed on SOX2-coated plates (or NY-ESO1–coated plates as irrelevant controls) and evaluated for anti-SOX2 IgG (or anti-EBNA IgG as a control) antibodies by ELISA (left) or detection of monoclonal Ig (by serum protein electrophoresis). Prior absorption on SOX2-abrogated anti-SOX2 reactivity without affecting anti-EBNA IgG reactivity (left) and monoclonal paraprotein concentration (right) are shown. Absorption on NY-ESO1 protein was performed as a control. Data represent the mean ± SEM. (F) Long-term persistence of anti-SOX2 IgG response in follow-up samples. Titers of anti-SOX2–specific IgG antibodies were detected in follow-up samples from patients with MGUS (patients 1–6). (G) IgG subclass– specific analysis of SOX2-specific antibody response in patients with MGUS.
Detection of anti-SOX2 T cell responses in MGUS but not MM patients or healthy donors
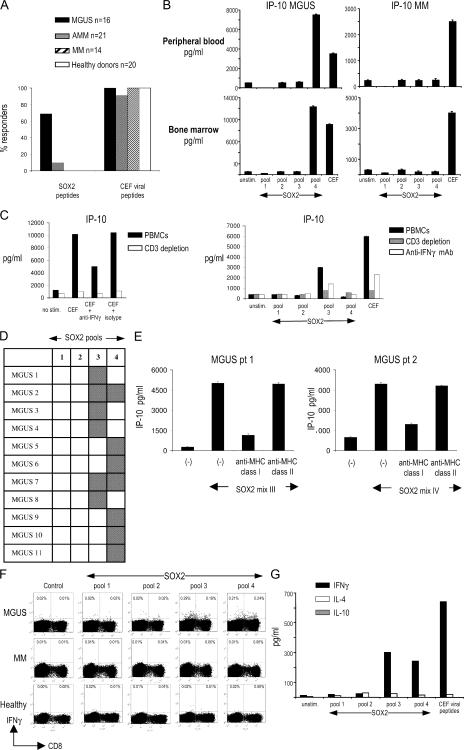
We examined if SOX2 was also a target of antitumor T cell response in these patients. Freshly isolated PBMCs were stimulated with a library of overlapping 15-mer peptides spanning the entire SOX2 protein (Table S3, available at http://www.jem.org/cgi/content/full/jem.20062387/DC1). Peptide-reactive chemokine production (IFN-γ–inducible protein 10 [IP-10]) was monitored by Luminex analysis. Using this assay, SOX2-specific T cells were detected in fresh PBMCs from 11 out of 16 MGUS patients tested but in none of the MM patients (n = 14) or healthy donors (n = 20; P < 0.05; Fig. 2, A and B). Anti-SOX2–specific T cells were also detected in 2 out of 21 patients with AMM. T cell reactivity against a pool of MHC class I–restricted viral antigen peptides (CEF, derived from CMV, EBV, and influenza virus) was comparable in all four cohorts. SOX2-specific T cells were detected in both blood and bone marrow in four patients tested (Fig. 2 B). In this assay, the production of IP-10, a potent antiangiogenic chemokine, in response to viral or SOX2 peptides is a sensitive readout for T cell–derived IFN-γ, as it is abrogated by prior depletion of CD3+ T cells and substantially reduced in the presence of neutralizing anti–IFN-γ mAb (Fig. 2 C). SOX2-specific reactivity in MGUS patients targeted peptides in pools 3 or 4 of the SOX2 peptide library. In two patients, SOX2-specific T cells were detected in response to both pools 3 and 4 (Fig. 2 D). SOX2-reactive T cells detected in this assay were predominantly MHC class I restricted, as the response was inhibited by anti–MHC class I blocking antibody (Fig. 2 E). To further analyze the nature of this T cell response, PBMCs from MGUS patients were stimulated for 2 wk with autologous DCs loaded with the SOX2 peptide library and analyzed for the presence of SOX2-specific T cells by intracellular IFN-γ flow cytometry. These experiments demonstrated that SOX2-reactive T cells included both CD4+ and CD8+ T cells (Fig. 2 F). In contrast, SOX2-specific T cells could not be detected from MM patients or healthy donors, even after four restimulations with peptide-pulsed DCs (Fig. 2 F and not depicted). Expanded SOX2-reactive CD4+ T cells were of the Th1 cell phenotype, as they mainly produced IFN-γ, but not IL-4 or IL-10, upon SOX2 stimulation (Fig. 2 G). SOX2-specific T cells in MGUS were detected in seven out of nine patients with positive anti-SOX2 antibodies tested, as well as in four of seven patients without detectable anti-SOX2 antibodies. Therefore, SOX2 is a frequent target for specific T cell immunity in MGUS patients but not in MM or healthy donors, and the detection of specific T cell responses may be more sensitive than current assays in detecting humoral responses.
Figure 2.
Analysis of SOX2-specific T cell responses. (A) Overall analysis of frequency of anti-SOX2 T cell responses in patients with MGUS, AMM, and MM and healthy donors. (B) Analysis of anti-SOX2 T cell reactivity in freshly isolated PBMCs and BMMNCs. Freshly isolated MNCs were stimulated with peptide pools derived from the SOX2 peptide library for 48 h, and supernatants were analyzed for IP-10 production. Data represent the mean ± SEM. (C) Depletion of CD3+ T cells or neutralization of IFN-γ decreases antigen-specific production of IP-10 in response to viral peptides (left) or SOX2-derived peptides (right). (left) PBMCs were stimulated with a cocktail of peptides derived from viral antigens (CEF) with or without prior depletion of CD3+ T cells or prior treatment with 10 μg/ml anti–IFN-γ blocking antibody or isotype control mAb. (right) PBMCs from a MGUS patient were similarly stimulated with four pools of SOX2-derived peptides. Supernatants were analyzed for IP-10 production by Luminex. (D) Patterns of anti-SOX2 T cell reactivity in MGUS patients. Gray squares represent a positive T cell response against a corresponding pool of the SOX2 peptide library. (F) Analysis of anti-SOX2 T cell reactivity in PBMCs from two patients with SOX2-specific T cells against SOX2. Stimulation with SOX2 peptides was performed in the presence of anti–MHC class I blocking antibody and supernatants analyzed for IP-10 production. Data represent the mean ± SEM. (F) Expansion of SOX2-specific CD4 and CD8 T cells in MGUS after two stimulations with SOX2 peptide library–loaded DCs evaluated by intracellular staining for IFN-γ. Representative results of one experiment out of three with similar results are shown. (G) ELISA for IFN-γ, IL-4, and IL-10 in the supernatants from cultures of SOX2-stimulated T lymphocytes.
Intranuclear SOX2 marks the clonogenic compartment in MGUS
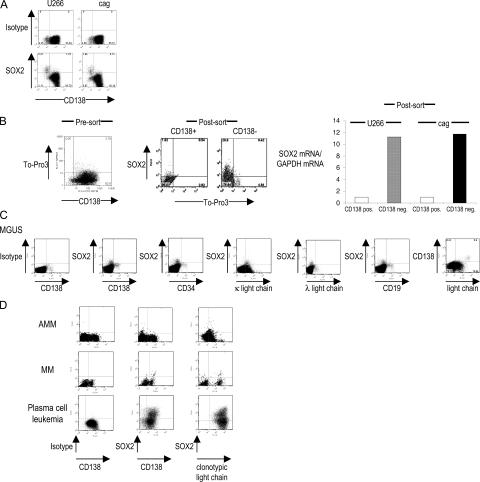
Expression of SOX2 is restricted to embryonal and neural stem cells, wherein it plays a critical role in regulating the self-renewal and pluripotency of stem cells (11–15). Recent studies in MM and MGUS have suggested that a minor CD138− subpopulation of both MM cell lines and primary cells is enriched in clonogenic progenitors, capable of growth in methylcellulose as well as in immune-deficient mice (8, 9). Analysis of intranuclear SOX2 expression in two MM cell lines by flow cytometry, as well as SOX2 mRNA by TaqMan, revealed that SOX2 indeed specifically marks this CD138− subpopulation (Fig. 3, A and B). Analysis of SOX2 expression in marrow from MGUS patients revealed that SOX2+ cells were restricted for tumor-associated Ig light chain but lacked the expression of the terminal plasma cell differentiation marker CD138 and the hematopoietic stem cell marker CD34 (Fig. 3 C). These cells expressed lower levels of Ig light chain compared with CD138+ plasma cells and lacked expression of CD19, a B cell marker. Overall, SOX2+ cells in the MGUS marrow accounted for only 0.5–1.5% of mononuclear cells (MNCs) and had a phenotype consistent with preplasma cells (CD138−CD19−IgLlo) (16). Interestingly, in patients with active MM, SOX2+ cells were also observed in the more differentiated CD138+IgLhigh compartment in five out of five patients tested (Fig. 3 D). The SOX2 expression pattern in patients with AMM was intermediate, with three out of five patients resembling the staining in MGUS (Fig. 3 D), whereas the other two were more like MM. Circulating tumor cells in a patient with advanced MM in leukemic phase showed higher reactivity, suggesting the acquisition of this marker by more differentiated cells in some patients with more aggressive disease (Fig. 3 D).
Figure 3.
Phenotype of SOX2-expressing cells in MM cell lines and in patients with MGUS, AMM, and MM. (A) Intranuclear staining for SOX2 expression in multiple MM cell lines U266 and cag. Data shown are gated on live cells based on scatter properties, and SOX2 expression was analyzed by intranuclear staining. The percentage of cells in each quadrant is indicated. (B) Analysis of SOX2 mRNA and protein in sorted CD138+ and CD138− MM cells. FACS data shown are gated for live cells based on scatter properties. Cells were stained with anti-CD138 and To-Pro-3 (to further discriminate dying cells) before the cell sort. Live (To-Pro-3–negative) cells were then sorted into CD138− and CD138+ populations by flow sorting. Each population was stained for the expression of intranuclear SOX2 expression and analyzed for the expression of SOX2 mRNA by TaqMan. The relative quantity of SOX2 transcripts (normalized to GAPDH) is shown. The percentage of cells in each quadrant is indicated. (C) SOX2 expression in BMMNCs in a patient with IgG κ MGUS. BMMNCs were stained with mAbs against SOX2 in combination with other markers. Representative results of one out of eight MGUS patients are shown. (D) SOX2 expression patterns in tumor cells from patients with AMM, MM, and plasma cell leukemia. Data are representative of five patients tested with MM and of seven patients with AMM. Three out of five AMM patients had SOX2 expression patterns similar to MGUS patients (AMM). In two out of five patients with AMM, the pattern was similar to that in MM. Clonotypic light chain refers to the light chain of the tumor-derived monoclonal Ig.
Targeting SOX2 immunity inhibits the clonogenic growth of tumors
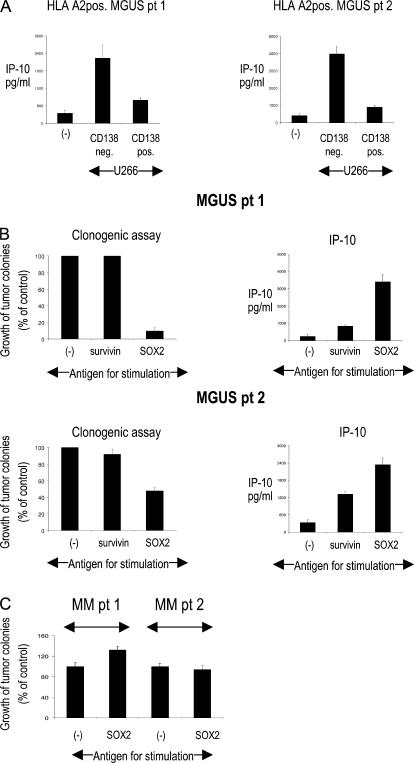
T cells from HLA-A2+ MGUS patients were capable of IP-10 secretion in response to a CD138− compartment of A2+ U266 cells (wherein the SOX2 expression is limited; Fig. 3 A), consistent with the recognition of endogenously presented antigen (Fig. 4 A). Although SOX2 is expressed only by a proportion of the bulk tumor, immunity against this antigen could still be important if this subpopulation or antigen was important for the clonogenic growth of tumors. To directly test this, marrow MNCs from MGUS patients were stimulated with the SOX2 peptide library, and SOX2 responsiveness was documented by the production of IP-10 (Fig. 4 B). Marrow MNCs (CD34−CD138−) stimulated under these conditions were plated in clonogenic assays (9). Cultures stimulated with the SOX2 peptide library demonstrated substantially inhibited clonogenic growth in three out of three MGUS patients tested (Fig. 4 B and not depicted). As noted earlier, stimulation of marrow MNCs from MM with SOX2 peptides did not lead to any detectable reactivity (Fig. 2, A and F), and consistent with this, prestimulation with the SOX2 peptide library did not lead to inhibition of clonogenic growth in MM (Fig. 4 C).
Figure 4.
Relevance of SOX2-specific T cells for the recognition of tumor cells and inhibition of clonogenic tumor growth. (A) Production of IP-10 in HLA-A2+ MGUS patients in response to MACS-sorted CD138− and CD138+ subsets of U266 cell line (HLA-A2+). Representative results for two patients are shown. (B) Inhibition of clonogenic growth of primary tumor cells after stimulation with the SOX2 peptide library in MGUS (left). BMMNCs were depleted of CD138+ and CD34+ cells, incubated with SOX2 or control peptides, and plated with 5 × 105 monocyte-derived DCs/ml at a ratio of 1:2 in Methocult. Colonies were counted by microscopy 2–3 wk after plating. Effective prestimulation with SOX2 peptides was documented by IP-10 production 48 h after stimulation (right). The relative growth of tumor colonies for two patients with SOX2-reactive T cells is shown. (C) Clonogenic growth of primary tumor cells after stimulation with the SOX2 peptide library in MM. BMMNCs were depleted of CD138+ and CD34+ cells, incubated with SOX2 or left unstimulated, and plated with 5 × 105 monocyte-derived DCs/ml at a ratio of 1:2 in Methocult. Colonies were counted by microscopy 2–3 wk after plating. Relative growth of tumor colonies for two patients with SOX2-reactive T cells is shown. Data represent the mean ± SEM.
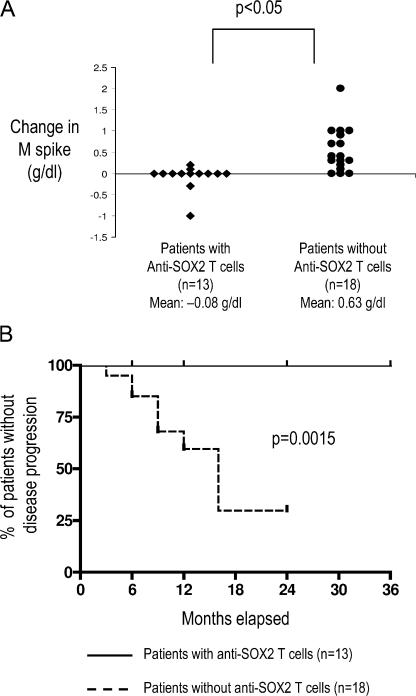
Detection of anti-SOX2 T cells predicts a favorable outcome in patients with asymptomatic plasmaproliferative disorders
Most studies evaluating the clinical significance of antitumor immunity in humans are based on retrospective data. Several of the patients analyzed in this study were enrolled in an observational trial of patients with asymptomatic plasmaproliferative disorders, performed under the auspices of the Southwest Oncology Group. This provided a unique opportunity to prospectively evaluate whether T cell immunity to a single antigen could predict tumor progression. With a median follow up of 24 mo, patients with anti-SOX2 T cells had a significantly lower net change in the level of tumor-derived monoclonal Ig (M spike) over time compared with those lacking anti-SOX2 T cells (mean increase in M spike = 0.08 g/dl vs. 0.63 g/dl, respectively; P < 0.05; Fig. 5 A). The patients with anti-SOX2 T cells also had a significantly lower likelihood of disease progression, with a 2-yr progression-free survival rate of 100 versus 30% compared with patients lacking anti-SOX2 T cells. (P < 0.01; Fig. 5 B). Therefore, immunity to SOX2 predicts the clinical outcome in patients with asymptomatic plasmaproliferative disorders.
Figure 5.
Correlation of detectable SOX2-reactive T cell immunity with clinical outcome in patients with asymptomatic plasmaproliferative disorders. (A) Net change in levels of tumor-derived monoclonal Ig (M spike) in patients with asymptomatic plasmaproliferative diseases with or without detectable SOX2-specific T cells. (B) Comparison of time-to-progression Kaplan-Meier's curves constructed for the cohorts with and without SOX2-specific T cells (P < 0.01 using the log-rank test).
DISCUSSION
A growing body of evidence points to the capacity of the human immune system to recognize preneoplastic lesions (3, 4, 10, 17, 18). However, the nature of specific antigens recognized by T cells in the preneoplastic stage of human cancer is largely unknown. The data in this paper suggest that the pattern of antigens spontaneously recognized by the immune system in preneoplastic lesions may differ from that in clinical cancer. Therefore, these data have implications for harnessing the immune system for the early detection and prevention of cancer in humans (19, 20).
The finding (originating from an unbiased search) that immunity to SOX2 (a gene critical for the self-renewal and pluripotency of embryonic stem cells) (14, 15) predicts clinical outcome supports the importance of stem cell genes and self-renewal pathways in cancer biology. These data also show that intranuclear SOX2 specifically marks the putative MM progenitors (8). SOX2 has also been recently found to be expressed in other cancer stem cells and implicated in intestinal metaplasia in gastric cancer (12, 13). Identification of a specific marker for the putative progenitor population in MM should facilitate understanding of myelomagenesis, as well as the development of specific therapies targeting this population. A subpopulation of CD138+ cells also acquires the expression of SOX2 in patients with progressive MM. Thus, the progression from MGUS to MM may involve the acquisition of self-renewal properties by the differentiated compartment of the clone. In other words, there may be a fundamental difference in the nature of the self-renewing compartment in MGUS versus MM. Further studies are needed to evaluate this possibility. Very similar correlations have recently been described in patients with chronic myelogenous leukemia, wherein blast transformation is associated with the acquisition of self-renewal genes by the more differentiated compartment of the clone (21, 22).
In earlier experiments, we had shown that the tumor bed in MGUS is enriched for T cells reactive against autologous CD138+ preneoplastic cells (10). The data in the current paper extend these findings to show that MGUS patients also mount an immune response against antigens expressed in the CD138− compartment of the clone, thought to be enriched in clonogenic tumor progenitors. The frequency of these T cells is low (relative to an antiviral responses during acute infection), and they target an antigen expressed only in a proportion of the clone. However, stimulation of the marrow MNCs with this antigen, without prior in vitro expansion, was sufficient to inhibit the clonogenic growth of MGUS cells in vitro. One possibility is that the immune response focused against progenitors or self-renewal genes may be more efficient in suppressing clonogenic growth than in immunity against bulk tumor. Interestingly, spontaneous immunity to SOX2 was not detectable in MM patients, who also carry SOX2-expressing cells. Further studies are needed to understand the absence of spontaneous SOX2 immunity in MM patients. Importantly, MGUS patients with anti-SOX2 immunity did not develop any clinical autoimmunity. These considerations make SOX2 an attractive target for specific immunotherapy of MM.
The potential role of the immune system in regulating cancer development has been extensively debated (1, 23). It is likely that distinct components of the immune system have the capacity to both promote as well as suppress cancer. It is therefore of interest that T cell immunity to SOX2 correlates with a favorable outcome. Antibodies against SOX2 have also been detected in some patients with small cell lung cancer and impart a favorable prognosis, although SOX2-specific T cells have not yet been studied in these patients (24, 25). However, the correlation of SOX2 immunity with a favorable outcome does not establish a causal relationship. For example, the detection of SOX2-specific T cells may be reflective of altered biology of tumor progenitors in MGUS versus MM. Therefore, clinical studies to enhance SOX2 immunity are needed to directly assess whether immunity to SOX2 or other antigens on tumor progenitors can induce tumor regressions in patients with MM and other cancers. These data should also encourage a systematic search for specific targets of spontaneous T cell immunity in other human preneoplastic states.
These data also have several clinical implications. Current management of patients with asymptomatic plasmaproliferative tumors is a challenge, as it is often difficult to predict disease progression and the need for therapy in these patients (26). These data suggest that in addition to changes in tumor cells, the nature of tumor-specific host immune response may also provide a novel approach for predicting an outcome. Most studies of immunotherapy of human cancer to date have focused on trying to target antigens expressed by bulk tumors. This approach has led to generally low rates of clinical regressions, often in spite of high frequencies of immunity to vaccine antigens. These data suggest the possibility that targeting drugs or the immune system against targets critical to the biology of tumor progenitors may be needed for the effective control of cancer.
MATERIALS AND METHODS
Patient samples.
Bone marrow and peripheral blood samples used in this study were obtained from patients with a diagnosis of MGUS, AMM, and MM based on standard clinical criteria (27). All patients signed an informed consent approved by the institutional review board. Several samples were obtained under the auspices of a prospective multicenter Southwest Oncology Group observational clinical trial (S0120) of patients with asymptomatic plasmaproliferative disorders.
Cell lines and media.
Multiple MM cells lines U266 and cag have been previously described (28). For DC and T cell cultures, 5% pooled human serum (Labquip) was used.
SADA for analyzing serum reactivity.
Preabsorbed serum samples from 28 patients with MGUS, 14 patients with AMM, 35 patients with MM, and 27 healthy blood donors were evaluated by SADA for the presence of IgG antibody to a panel of 83 SEREX-defined antigens, as previously described (29). In brief, precut nitrocellulose membranes (80 × 120 mm) were precoated with a layer (≈0.2 mm) of growth media and placed on a reservoir layer of media in Omni Tray (86 × 128 mm; Nalge Nunc International Corp.). A total of 105 PFU of bacteriophage-encoding individual SEREX-defined tumor antigens (Table S1) in a volume of 20 μl was mixed with 20 μl of exponentially growing Escherichia coli XL-1 Blue MRF′ and spotted on the precoated nitrocellulose membranes. 30 SEREX-defined antigens were spotted in duplicate on each nitrocellulose membrane. Membranes were incubated for 15 h at 37°C and processed as per the standard SEREX protocol (29). In brief, membranes were blocked in 0.5% nonfat dried milk, incubated in 10 ml of a 1:200 dilution of sera at room temperature for 15 h, and incubated in a 1:3,000 dilution of alkaline phosphatase–conjugated, Fc fragment–specific, goat anti–human IgG (Jackson ImmunoResearch Laboratories). Serum IgG reactivity was detected with the alkaline phosphatase substrate 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate (Biosource International). Positive assays were repeated in a blinded manner to confirm reactivity.
ELISA for the detection of anti-SOX2, EBV (EBNA-1) antibodies.
Sera were screened for the presence of anti-SOX2 antibodies by standard ELISA. A 96-well microtiter polystyrene half-area immunoassay plate (Corning) was coated in PBS with 1 μg/ml of recombinant human SOX2 protein overnight at 4°C. Plates were washed with PBS and blocked with 5% nonfat dry milk in PBS for 2 h. After washing, serum samples at 1:100 and 1:400 dilutions in the blocking solution were added and incubated for 1 h at room temperature. Plates were extensively washed with 0.05% Tween 20 in PBS, and the secondary antibody, horseradish peroxidase–labeled goat anti–human IgG, IgG1, IgG2, IgG3, or IgG4 (Southern Biotechnology Associates, Inc.), in blocking solution was added. Plates were incubated for 1 h at room temperature and washed, and SOX2 reactivity was revealed by the addition of substrate solution (Biosource International). After 30 min of incubation in the dark, the reaction was ended with Stop solution (Biosource International), and the absorbance at 450 nm was measured using an ELISA plate reader (MultiSkan Plus; Thermo Fisher Scientific). The threshold for seropositivity for SOX2 antibodies was determined as ODN450 at 0.11, based on the mean background for no antigen controls plus 4 SD. Seropositivity for anti–EBNA-1 IgG was determined using a commercial kit (SCIMEDX) according to the manufacturer's instructions. Samples with an antibody index ≥1 were considered to be seropositive for EBNA IgG.
Detection of cytokines by ELISA.
Commercial ELISA kits (Biosource International) were used to measure IFN-γ, IL-4, and IL-10 concentrations in the cell-culture supernatants, according to the manufacturer's recommendations.
Preabsorption of anti-SOX2–positive samples.
Before ELISA analysis for SOX2 reactivity, samples with anti-SOX2 IgGs were preabsorbed overnight at 4°C in 96-well plates previously coated with 30 μg/ml of recombinant SOX2 protein and blocked with 5% nonfat dry milk in PBS.
Synthesis of the SOX-2 peptide library.
Peptides were synthesized in collaboration with the Proteomics Resource Center at the Rockefeller University. Overlapping sequences from the Sox-2 protein were determined and optimized for synthesis by using the epitope library fragment generation program PeptGen, developed by Los Alamos National Laboratories, as part of the HIV Immunology Database (available at http://www.hiv.lanl.gov). All peptides were created in a microtiter plate (96 well) format using a parallel peptide synthesizer/spotter (MultiPep; Intavis) on resin (TentaGel R RAM; Rapp Polymere) loaded at 5 μm per well, using Fmoc-protected amino acids (Anaspec). Deprotection of the amine was accomplished with 20% piperidine (Sigma-Aldrich) in N-methylpyrrolidinone (NMP; EMD Biosciences, Inc.). Repetitive coupling reactions were conducted using 0.3 M HATU/ HOBt and 0.4 M NMM using NMP as the primary solvent. Simultaneous resin cleavage and side-chain deprotection were achieved by treatment with 0.8 ml/well of concentrated, sequencing grade trifluoroacetic acid (Fisher Scientific) with triisopropylsilane, water, and DODT in a ratio of 95:2:2:1 for 2 h. After vacuum filtration to a collection plate, centrifugal evaporation (Genevac) was used to remove TFA from the plate containing the soluble peptides. Peptides were treated with 8 M acetic acid, and the acidic mixture was evaporated and redissolved in 20% acetonitrile and HPLC (Waters Chromatography)-grade water and dried twice more. All crude products were subsequently analyzed by reversed-phase HPLC using a C18 column (Chromolith Performance; Merck). Individual peptide integrity was verified by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry using a delayed extraction spectrometer system (Voyager; PerSeptive/Applied Biosystems).
Screening for SOX2-reactive T cells in fresh PBMCs.
MNCs from blood or bone marrow were separated by density gradient centrifugation using Ficoll-Hypaque (GE Healthcare). 2 × 105 PBMCs or BMMCs in 200 μl of media were cultured in the presence of 2.5 μg/ml of peptide pools derived from the SOX2 peptide library or with a mixture of MHC class I–restricted peptides derived from CMV, EBV, and influenza virus (CEF mix) (30). The composition of the SOX2 peptide library pools is noted in Table S3. After 48 h, supernatants were collected and assayed for the production of IP-10 by Luminex, using the manufacturer's directions (Upstate Biotechnology), and analyzed by Beadview software (Upstate Biotechnology). In some experiments, CD3 T cells were depleted by negative selection, or PBMC stimulation by specific antigens was performed in the presence of IFN-γ or anti–MHC class I blocking mAbs (Biolegend) to confirm the specificity of IP-10 production. For some experiments, CD138+ and CD138− fractions of U266 (HLA-A2+ cell line) were also used for the stimulation of fresh PBMCs from HLA-A2+ MGUS patients with documented anti-SOX2 T cell reactivity, and IP-10 production was analyzed by Luminex 48 h later. A twofold or greater increase in IP-10 production relative to control was considered as positive for the presence of antigen-specific T cells.
DC generation and expansion of SOX2-specific T cells.
MNCs from blood or bone marrow were separated by density gradient centrifugation using Ficoll-Hypaque. DCs were generated from monocytes isolated by CD14 magnetic beads (Miltenyi Biotec) and cultured for 5 d in the presence of GM-CSF (Immunex) and IL-4 (R&D Systems), as previously described (30). Day-5 DCs were matured overnight with 100 ng/ml LPS (Sigma-Aldrich) and pulsed for 2 h with peptide pools derived from the SOX2 peptide library (15-mer peptides overlapping by 11 aa) at 2 μg/ml. A CD14− T cell–enriched fraction was added to a U-bottom 96-well plate at 2 × 105 cells/well in 200 μl of medium and stimulated with mature peptide-pulsed DCs at a ratio of 1 DC per 20 CD14− cells. Recombinant IL-2 was added at 15 U/ml every third day. Antigen-specific T cells were restimulated with the same antigen-pulsed DCs every week and tested for IFN-γ production at the time of restimulation by intracellular cytokine flow cytometry.
Flow cytometry for the detection of intracellular cytokines.
Antigen-specific cells were analyzed by a flow cytometry–based assay for the detection of intracellular cytokines, as described previously (31). In brief, blood or bone marrow T cells were cultured for 12 h with autologous unpulsed mature DCs, or DCs loaded with specific peptide mixtures, in the presence of Golgistop (Cytofix/CytoPerm Plus Kit; BD Biosciences). Cells were fixed and permeabilized in 100 μl Cytofix/Cytoperm solution using the manufacturer's instructions and stained for intracellular cytokines (IFN-γ) and surface markers (CD3 and CD8).
Intranuclear SOX2 staining.
5 × 105 cells were fixed in Cytofix/Cytoperm overnight at 4°C. Cells were permeabilized in Cytoperm solution, refixed in Cytofix/Cytoperm for 5 min on ice, and treated with 300 μg/ml DNase I for 45 min at 37°C. Cells were stained with PE-SOX2 mAb (R&D Systems) for 20 min at room temperature, washed, and stained for surface or intracellular markers. Samples were acquired on an instrument (FACSCalibur; BD Biosciences) using CellQuest software (BD Biosciences) and analyzed with FlowJo software (TreeStar Inc.). Typically, 1–5 × 105 events were collected per sample.
Cell sorting and analysis of SOX2 mRNA by TaqMan.
CD138− and CD138+ fractions of the U266 and cag cell lines were sorted on a FACSVantage (BD Biosciences). The purity of sorted populations used in real-time PCR experiments exceeded 95%. RNA was isolated with the RNeasy Mini Kit (QIAGEN), and RT-PCR was conducted with the Assays-on-Demand (Applied Biosystems) primer probe for SOX2 using a sequence detection system (ABI PRISM 7700; Applied Biosystems). Expression of GAPDH was monitored as a housekeeping gene. Reactions were set up in triplicates using EZ PCR Core Reagents (Applied Biosystems), according to the manufacturer's instructions, with 20 ng of total RNA. The relative expression of target genes was calculated using the comparative threshold cycle method.
Clonogenic assays on primary tumor cells.
Bone marrow MNCs (BMMNCs) were isolated from marrow samples using density gradient centrifugation. CD138+ and CD138− fractions were isolated using CD138 microbeads (Miltenyi Biotec), and the CD138− fraction was further depleted of normal hematopoietic progenitors using CD34 microbeads (Miltenyi Biotec). CD138−CD34− cells were incubated overnight with SOX2 or control peptides and plated (5 × 105 cells/ml) with monocyte-derived DCs at a ratio of 1:2 in methylcellulose containing 5% leukocyte-conditioned media (Methocult; Stem Cell Technologies, Inc.), as described previously (9). Cells were plated in 35-mm2 tissue culture dishes in triplicates and incubated at 37°C and 5% CO2. Colonies consisting of >40 cells were counted by microscopy 2–3 wk after plating.
Statistical analysis.
Differences in frequencies were assessed with Fisher's exact test for two groups and the χ2 test for three or more groups. Differences in change in M protein over time between those with and without SOX2 immunity were tested using the Wilcoxon test. The criteria for disease progression required an increase in M protein ≥0.5 g/dl, an increase in marrow plasmacytosis ≥10%, or a development of symptomatic disease requiring initiation of therapy (32). Progression-free survival curves were constructed using the Kaplan-Meier method (33) and tested using the log-rank test (34).
Online supplemental material.
Table S1 provides a list of tumor antigens tested in the SADA. Table S2 shows a list of tumor antigens inducing IgG antibody responses in patients with plasma cell diseases. Table S3 provides the sequences of peptides used in the SOX2 peptide library. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20062387/DC1.
Supplemental Material
Acknowledgments
This paper is dedicated to our patients for their encouragement of this work and to the loving memory of Matt Scanlan.
The authors thank Dr. R.M. Steinman for critical reading of the manuscript; Drs. L.J. Old and K.L. Calame for helpful discussions; J. Krasovsky, A. Hutchinson, and A. Murray for excellent technical assistance; A. Hurley for help with clinical aspects; and J. Adams for help with illustrations.
This work was supported in part by funds from the National Institutes of Health (grants CA106802, CA109465, P50-AT02779, MO1-RR00102, and PO1-CA55819), an Eli Lilly-Damon Runyon Clinical Investigator Award, the Dana Foundation, the Irma T. Hirschl Foundation, the Fund to Cure Myeloma, and the Southwest Oncology Group.
The authors have no conflicting financial interests.
Abbreviations used: AMM, asymptomatic MM; BMMNC, bone marrow MNC; EBNA, Epstein-Barr nuclear antigen; IP-10, IFN-γ–inducible protein 10; MGUS, monoclonal gammopathy of undetermined significance; MM, myeloma; MNC, mononuclear cell; SADA, serum antibody detection array; SEREX, serological expression of cDNA expression libraries.
Dr. Scanlan died on 12 March 2004.
References
- 1.Dunn, G.P., A.T. Bruce, H. Ikeda, L.J. Old, and R.D. Schreiber. 2002. Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol. 3:991–998. [DOI] [PubMed] [Google Scholar]
- 2.Blattman, J.N., and P.D. Greenberg. 2004. Cancer immunotherapy: a treatment for the masses. Science. 305:200–205. [DOI] [PubMed] [Google Scholar]
- 3.Finn, O.J. 2003. Premalignant lesions as targets for cancer vaccines. J. Exp. Med. 198:1623–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhodapkar, M.V. 2005. Harnessing host immune responses to preneoplasia: promise and challenges. Cancer Immunol. Immunother. 54:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle, R.A., T.M. Therneau, S.V. Rajkumar, D.R. Larson, M.F. Plevak, J.R. Offord, A. Dispenzieri, J.A. Katzmann, and L.J. Melton III. 2006. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 354:1362–1369. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca, R., B. Barlogie, R. Bataille, C. Bastard, P.L. Bergsagel, M. Chesi, F.E. Davies, J. Drach, P.R. Greipp, I.R. Kirsch, et al. 2004. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 64:1546–1558. [DOI] [PubMed] [Google Scholar]
- 7.Reya, T., S.J. Morrison, M.F. Clarke, and I.L. Weissman. 2001. Stem cells, cancer, and cancer stem cells. Nature. 414:105–111. [DOI] [PubMed] [Google Scholar]
- 8.Matsui, W., C.A. Huff, Q. Wang, M.T. Malehorn, J. Barber, Y. Tanhehco, B.D. Smith, C.I. Civin, and R.J. Jones. 2004. Characterization of clonogenic multiple myeloma cells. Blood. 103:2332–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kukreja, A., A. Hutchinson, K. Dhodapkar, A. Mazumder, D. Vesole, R. Angitapalli, S. Jagannath, and M.V. Dhodapkar. 2006. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J. Exp. Med. 203:1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhodapkar, M.V., J. Krasovsky, K. Osman, and M.D. Geller. 2003. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J. Exp. Med. 198:1753–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegner, M., and C.C. Stolt. 2005. From stem cells to neurons and glia: a Soxist's view of neural development. Trends Neurosci. 28:583–588. [DOI] [PubMed] [Google Scholar]
- 12.Tatematsu, M., T. Tsukamoto, and K. Inada. 2003. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 94:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmati, H.D., I. Nakano, J.A. Lazareff, M. Masterman-Smith, D.H. Geschwind, M. Bronner-Fraser, and H.I. Kornblum. 2003. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA. 100:15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyer, L.A., T.I. Lee, M.F. Cole, S.E. Johnstone, S.S. Levine, J.P. Zucker, M.G. Guenther, R.M. Kumar, H.L. Murray, R.G. Jenner, et al. 2005. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 122:947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. [DOI] [PubMed] [Google Scholar]
- 16.McHeyzer-Williams, L.J., and M.G. McHeyzer-Williams. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487–513. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki, H., D.F. Graziano, J. McKolanis, and O.J. Finn. 2005. T cell-dependent antibody responses against aberrantly expressed cyclin B1 protein in patients with cancer and premalignant disease. Clin. Cancer Res. 11:1521–1526. [DOI] [PubMed] [Google Scholar]
- 18.Comtesse, N., A. Zippel, S. Walle, D. Monz, C. Backes, U. Fischer, J. Mayer, N. Ludwig, A. Hildebrandt, A. Keller, et al. 2005. Complex humoral immune response against a benign tumor: frequent antibody response against specific antigens as diagnostic targets. Proc. Natl. Acad. Sci. USA. 102:9601–9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spisek, R., and M.V. Dhodapkar. 2006. Immunoprevention of cancer. Hematol. Oncol. Clin. North Am. 20:735–750. [DOI] [PubMed] [Google Scholar]
- 20.Forni, G., P.L. Lollini, P. Musiani, and M.P. Colombo. 2000. Immunoprevention of cancer: is the time ripe? Cancer Res. 60:2571–2575. [PubMed] [Google Scholar]
- 21.Jamieson, C.H., L.E. Ailles, S.J. Dylla, M. Muijtjens, C. Jones, J.L. Zehnder, J. Gotlib, K. Li, M.G. Manz, A. Keating, et al. 2004. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351:657–667. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson, C.H., I.L. Weissman, and E. Passegue. 2004. Chronic versus acute myelogenous leukemia: a question of self-renewal. Cancer Cell. 6:531–533. [DOI] [PubMed] [Google Scholar]
- 23.de Visser, K.E., A. Eichten, and L.M. Coussens. 2006. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 6:24–37. [DOI] [PubMed] [Google Scholar]
- 24.Gure, A.O., E. Stockert, M.J. Scanlan, R.S. Keresztes, D. Jager, N.K. Altorki, L.J. Old, and Y.T. Chen. 2000. Serological identification of embryonic neural proteins as highly immunogenic tumor antigens in small cell lung cancer. Proc. Natl. Acad. Sci. USA. 97:4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vural, B., L.C. Chen, P. Saip, Y.T. Chen, Z. Ustuner, M. Gonen, A.J. Simpson, L.J. Old, U. Ozbek, and A.O. Gure. 2005. Frequency of SOX Group B (SOX1, 2, 3) and ZIC2 antibodies in Turkish patients with small cell lung carcinoma and their correlation with clinical parameters. Cancer. 103:2575–2583. [DOI] [PubMed] [Google Scholar]
- 26.Kyle, R.A. 2004. New Strategies for MGUS and Smoldering Multiple Myeloma. Clin. Adv. Hematol. Oncol. 2:507–509. [PubMed] [Google Scholar]
- 27.Durie, B.G., R.A. Kyle, A. Belch, W. Bensinger, J. Blade, M. Boccadoro, J.A. Child, R. Comenzo, B. Djulbegovic, D. Fantl, et al. 2003. Myeloma management guidelines: a consensus report from the Scientific Advisors of the International Myeloma Foundation. Hematol. J. 4:379–398. [PubMed] [Google Scholar]
- 28.Dhodapkar, K., J. Krasovsky, B. Williamson, and M. Dhodapkar. 2002. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of tumor-specific killer T cells by dendritic cells. J. Exp. Med. 195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen, Y.T., A.O. Gure, and M.J. Scanlan. 2005. Serological analysis of expression cDNA libraries (SEREX): an immunoscreening technique for identifying immunogenic tumor antigens. Methods Mol. Med. 103:207–216. [PubMed] [Google Scholar]
- 30.Dhodapkar, K.M., J.L. Kaufman, M. Ehlers, D.K. Banerjee, E. Bonvini, S. Koenig, R.M. Steinman, J.V. Ravetch, and M.V. Dhodapkar. 2005. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl. Acad. Sci. USA. 102:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang, D.H., K. Osman, J. Connolly, A. Kukreja, J. Krasovsky, M. Pack, A. Hutchinson, M. Geller, N. Liu, R. Annable, et al. 2005. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl–ceramide–loaded mature dendritic cells in cancer patients. J. Exp. Med. 201:1503–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durie, B.G., J.L. Harousseau, J.S. Miguel, J. Blade, B. Barlogie, K. Anderson, M. Gertz, M. Dimopoulos, J. Westin, P. Sonneveld, et al. 2006. International uniform response criteria for multiple myeloma. Leukemia. 20:1467–1473. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan, E.L., and P. Meier. 1958. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53:457–481. [Google Scholar]
- 34.Mantel, N. 1966. Evaluation of survival data and two new rank order statistics arising in its evaluation. Cancer Chemother. Rep. 50:163–170. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.