Abstract
Mammalian Toll-like receptors (TLRs) recognize microbial pathogen-associated molecular patterns and are critical for innate immunity against microbial infection. Diacylglycerol (DAG) kinases (DGKs) regulate the intracellular levels of two important second messengers involved in signaling from many surface receptors by converting DAG to phosphatidic acid (PA). We demonstrate that the ζ isoform of the DGK family (DGKζ) is expressed in macrophages (Mφ) and dendritic cells. DGKζ deficiency results in impaired interleukin (IL) 12 and tumor necrosis factor α production following TLR stimulation in vitro and in vivo, increased resistance to endotoxin shock, and enhanced susceptibility to Toxoplasma gondii infection. We further show that DGKζ negatively controls the phosphatidylinositol 3–kinase (PI3K)–Akt pathway and that inhibition of PI3K activity or treatment with PA can restore lipopolysaccharide-induced IL-12 production by DGKζ-deficient Mφ. Collectively, our data provide the first genetic evidence that an enzyme involved in DAG/PA metabolism plays an important role in innate immunity and indicate that DGKζ promotes TLR responses via a pathway involving inhibition of PI3K.
Toll-like receptors (TLRs) recognize specific microbial pathogen–associated molecular patterns and constitute a major mechanism to respond to microbial infection. Engagement of TLRs activates the production of proinflammatory cytokines, such as IL-12 and TNFα, and up-regulates co-stimulatory molecules on APCs. TLR-induced responses are important not only for innate immunity but also for generation of proper adaptive immunity against microbial infection (1, 2). TLR signals are mainly transduced through two pathways, the myeloid differentiation primary response protein 88 (MyD88)–dependent and the Toll/Il-1 receptor domain–containing adaptor–inducing interferon-β–dependent pathways. The MyD88 pathway activates IκB kinase α/β/γ, resulting in IκB degradation and nuclear translocation of NF-κB to activate transcription of proinflammatory cytokines (3–5). In addition, MyD88 is required for activation of the c-Jun N-terminal kinase and p38 mitogen-associated protein kinases (MAPKs) that are also important for TLR-induced inflammatory responses (6–9). The Toll/Il-1 receptor domain–containing adaptor–inducing interferon-β–dependent pathway, which is activated by TLR3 and TLR4, leads to phosphorylation and activation of IFN regulatory factor 3 (IRF3) and late-phase NF-κB activation (10–14).
Interestingly, both diacylglycerol (DAG) and phosphatidic acid (PA) are induced after stimulation of macrophages (Mφ) with lipopolysaccharide (LPS) and lipopeptide, ligands for TLR4 and TLR2, respectively. Inhibition of DAG and PA production by chemicals reduces TNFα and nitric oxide production in Mφ treated with LPS or lipopeptide, suggesting that these second messengers may participate in TLR signal transduction (15–17). However, there is no reported genetic evidence that the modulation of DAG and/or PA concentrations may affect TLR signaling and innate immunity.
TLR-mediated responses are critical for host defense against microbial organisms such as Toxoplasma gondii. T. gondii is an intracellular opportunistic protozoan that causes widespread infection in humans and animals. It can establish life-long chronic infection in immune-competent hosts but causes serious health problems in immunocompromised individuals, such as HIV patients (18–20). T. gondii is recognized by several TLRs (such as TLR2 and TLR11) and the CC chemokine receptor 5 (9, 21–26). TLR signaling and the subsequent production of IL-12 by DCs, Mφ, and neutrophils induce Th1 adaptive immune responses and IFN-γ production (27), which is critical for resistance to T. gondii infection (28–30). In mouse models, deficiency of MyD88 expression results in lethality after T. gondii infection (22) and impairment of host defense against other microbes caused by the inhibition of both innate and adaptive immunity (11, 31, 32). Mechanisms that regulate TLR signaling during innate immune responses to T. gondii are not well understood.
Given their abilities to induce potent proinflammatory responses, TLR signals must be tightly controlled. The class IA family of PI3Ks, which promote cell survival and regulate transcription and other cellular processes, negatively regulate TLR2-, TLR4-, and TLR9-induced IL-12 production in DCs (33–35). Although multiple mechanisms may mediate the inhibitory effects of PI3K on TLR responses, recent evidence indicates that PI3K may do so through Akt-mediated phosphorylation and inactivation of glycogen synthase kinase–3β (35, 36). Glycogen synthase kinase–3β is critical for inflammatory responses induced by several TLRs (35). An important question raised is how PI3K activity is regulated to ensure productive TLR-induced responses.
DAG kinases (DGKs) are a family of enzymes that catalyze the conversion of DAG to PA by phosphorylation. DGKs may play important roles in signaling from many receptors and modulate diverse cellular processes, because their enzyme activity influences both DAG and PA levels (37, 38). We have previously demonstrated that DGKζ is an important negative regulator of T cell receptor signaling and T cell function in mice (39, 40). More recent experiments have revealed that DGKζ negatively regulates FcɛRI-induced cytokine production but positively controls mast cell degranulation in vitro and in vivo (41). In this paper, we demonstrate that DGKζ is expressed in DCs and Mφ and that its expression is up-regulated after LPS stimulation. DGKζ deficiency leads to impaired TNFα and IL-12 production after TLR stimulation both in vitro and in vivo. Mice deficient of DGKζ show decreased susceptibility to LPS-induced shock, fail to mount effective innate and adaptive immune responses against T. gondii, and manifest enhanced susceptibility to toxoplasmosis. Mechanistically, DGKζ deficiency results in the elevation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) levels and Akt activation in Mφ but does not inhibit IκB degradation or activation of p38 and Erk1/2 MAPKs. Inhibition of PI3K activity or treatment with PA can restore LPS-induced IL-12 production by DGKζ-deficient Mφ. These observations provide the first genetic evidence that an enzyme involved in DAG and/or PA metabolism plays an important role in TLR signaling and innate immunity against a parasitic pathogen and reveal that DGKζ is a novel negative regulator of the PI3K–Akt pathway.
RESULTS
Expression of DGKζ in DCs and Mφ
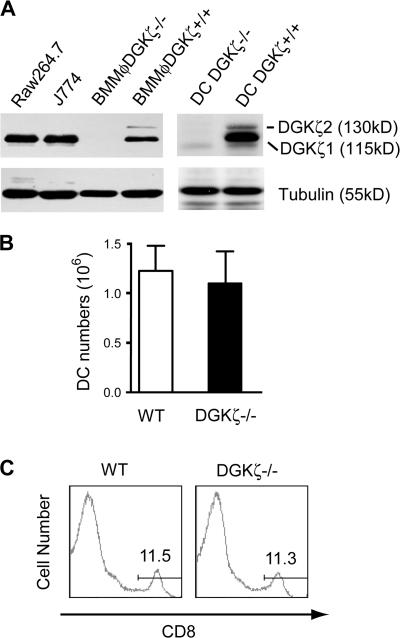
Two DGKζ isoforms differing only at their N-termini have been identified. The molecular masses of the ζ1 and ζ2 isoforms are 115 and 130 kD, respectively (42). Using Western blotting, we revealed that DGKζ1 is expressed in the Mφ cell lines Raw264.7 and J774. In bone marrow–derived Mφ (BMMφ) and DCs generated from WT mice, both the ζ1 and ζ2 isoforms were detected, with the ζ1 isoform predominantly expressed (Fig. 1 A). None of these DGKζ isoforms were detected in DGKζ-deficient Mφ and DCs, confirming the successful targeting of the DGKζ locus. Deficiency of DGKζ appeared not to globally perturb DCs and myeloid cell development, because similar numbers of CD11c+CD8+ DCs (Fig. 1, B and C), as well as CD11b+ cells (40), were present in both WT and DGKζ-deficient mice.
Figure 1.
DC development in DGKζ-deficient mice. (A) Expression of DGKζ in Mφ and DCs. DGKζ protein expression in Raw264.7 and J774 Mφ cell lines, BMMφ, and DCs from WT and DGKζ-deficient mice was detected by Western blot analysis using an anti-DGKζ antibody (reference 69). The blots were stripped and reprobed with an antitubulin antibody to determine equal protein loading. BMMφ and DCs were differentiated from bone marrow progenitor cells using M-CSF– and GM-CSF–containing media, respectively. (B) Quantification of CD11c+ cells in WT and DGKζ-deficient spleens. BSA gradient–enriched WT and DGKζ−/− splenic DCs were stained for CD11c and CD8. Total CD11c+ cells were calculated after FACS analysis (n = 3). Data shown are the mean ± SD. (C) CD8 staining of splenic CD11c+ cells as described in B. Numbers indicate the percentages of gated cell populations.
Impairment of IL-12 p40 and TNFα production in DGKζ-deficient DCs and Mφ after TLR stimulation
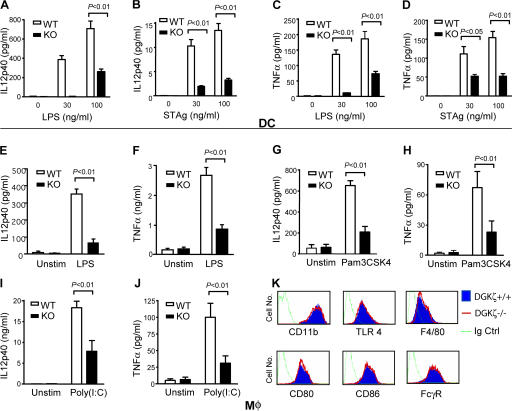
We examined the effects of DGKζ deficiency on IL-12 p40 and TNFα production in DCs and Mφ. Splenic DCs from WT and DGKζ-deficient mice were isolated and left unstimulated or stimulated with LPS to activate via TLR4 or T. gondii–soluble tachyzoite Ag (STAg), which activates via several pathways, including TLR2 and TLR11 (21). After 24 h of stimulation, IL-12 p40 (Fig. 2, A and B) and TNFα (Fig. 2, C and D) production by DGKζ-deficient DCs were considerably lower than by WT DCs. Similar to DCs, DGKζ-deficient BMMφ produced less IL-12 p40 and TNFα than WT BMMφ after stimulation with LPS (Fig. 2, E and F), Pam3CSK4 (Fig. 2, G and H), and Poly(I:C) (Fig. 2, I and J). Both WT and DGKζ-deficient BMMφ and DCs expressed similar levels of TLR4 and other molecules (Fig. 2 K), suggesting that the impaired production of these proinflammatory cytokines was not caused by decreased TLR expression or global alteration of Mφ properties. Because LPS, Pam3CSK4, and Poly(I:C) activates cells via TLR4, TLR1/2, and TLR3, respectively (43–45), these data indicate that DGKζ is involved in signal transduction downstream of multiple TLR receptors.
Figure 2.
Impairment of proinflammatory cytokine production in DGKζ−/− DCs and BMMφ. (A–D) Measurement of cytokine production in DCs. Splenic DCs from WT and DGKζ−/− mice were left unstimulated or stimulated with 30 or 100 ng/ml LPS or 30 or 100 ng/ml STAg at 37°C for 24 h. IL-12 p40 (A and B) and TNFα (C and D) levels in culture supernatants were determined by ELISA. (E–J) Measurement of cytokine production by BMMφ. BMMφ from WT and DGKζ−/− mice were left unstimulated or stimulated with 100 ng/ml LPS (E and F), 50 ng/ml Pam3CSK4 (G and H), or 50 μg/ml Poly(I:C) (I and J) for 24 h. IL-12 p40 (E, G, and I) and TNFα (F, H, and J) levels in supernatants were detected by ELISA. (K) Cell surface expression of CD11b, TLR4, and other molecules on WT and DGKζ−/− BMMφ. WT and DGKζ−/− BMMφ were stained with a control antibody (dotted green line) or a biotin-labeled anti-TLR4 antibody, followed by PE-conjugated streptavidin staining or fluorescently labeled anti-CD11b, F4/80, CD80, CD86, or FcγR antibodies (WT, blue shaded region; DGKζ−/−, red line). Data shown in A–J are the mean ± SD of each group and represent three experiments.
Decreased production of IL-12 p40 and TNFα in DGKζ-deficient mice after in vivo TLR stimulation
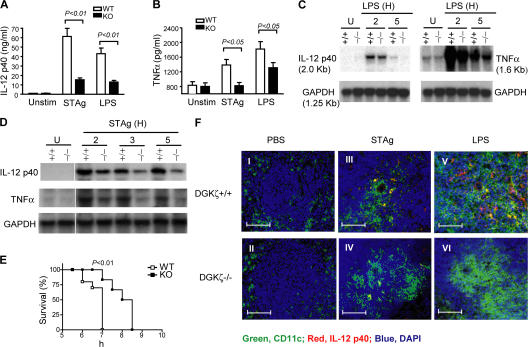
In addition to DCs and Mφ, many other cell lineages such as neutrophils produce proinflammatory cytokines in response to TLR stimulation. To determine how DGKζ deficiency may affect TLR-induced responses in vivo, WT and DGKζ-deficient mice on a C57BL/6 genetic background were injected intraperitoneally with LPS, STAg, or PBS as a control. 6 h after injection, serum IL-12 and TNFα concentrations in DGKζ-deficient mice were lower than in WT control mice (Fig. 3, A and B). Northern blot analysis revealed decreased expression of both IL-12 p40 and TNFα mRNA in DGKζ-deficient spleens as compared with WT spleens after injection of LPS (Fig. 3 C) or STAg (Fig. 3 D), suggesting that reduced IL-12 p40 and TNFα production by DGKζ-deficient mice is likely caused by decreased transcription or mRNA stability. Consistent with decreased TNFα production, DGKζ-deficient mice were less susceptible to LPS-induced shock than WT mice (Fig. 3 E).
Figure 3.
Impaired TNFα and IL-12 production in DGKζ-deficient mice after TLR-stimulation. (A and B) Measurement of serum cytokine concentrations by ELISA. WT and DGKζ−/− mice were intraperitoneally injected with 10 μg LPS or 5 μg STAg. 6 h after injection, sera were collected for measurement of IL-12 p40 and TNFα production by ELISA. Data shown are the mean ± SD for each group and are representative of three experiments. (C and D) Measurement of cytokine messengers by Northern blot. WT and DGKζ−/− mice were intraperitoneally injected with 10 μg LPS or 5 μg STAg. 2, 3, and 5 h after injection, total splenic RNA was isolated, and IL-12 p40 and TNFα transcripts were measured by Northern blot analysis. Data shown are representative of three experiments. (E) Increased resistance to LPS-induced shock in DGKζ−/− mice. Age- and sex-matched WT mice (n = 10) and DGKζ−/− mice (n = 6) were intraperitoneally injected with 20 mg d-galactosamine and 2 μg LPS per mouse, and their survival was observed at the indicated time points. Data shown are representative of two independent experiments. (F) Detection of IL-12 production by immunofluorescence. Spleens were removed from mice 6 h after injection of PBS, LPS, or STAg. Frozen sections of spleens were processed and stained with a goat anti–mouse IL-12 p40 antibody, followed by a double incubation with Alexa Fluor 488–conjugated anti–mouse CD11c and Alexa Fluor 594–conjugated anti–goat antibodies. The cells were counterstained for nuclei using DAPI. Images were captured using an Axiovert microscope with AxioVision software (see Materials and methods). Green, red, and blue represent CD11c+ DCs, IL-12, and nuclei, respectively; yellow represents CD11c+IL-12+ after overlay. Bars, 50 μm.
Immunofluorescence staining revealed similar numbers and distribution of CD11c+ DCs (green) in WT and DGKζ-deficient spleens before STAg or LPS injection (Fig. 3 F, I and II). WT and DGKζ-deficient CD11c+ DCs migrated toward T cell areas of the spleen after injection of STAg (Fig. 3 F, III and IV) or LPS (Fig. 3 F, V and VI). However, fewer CD11c+ cells expressed IL-12 (yellow) in DGKζ-deficient spleens compared with WT spleens after STAg or LPS injection. These data further confirm the requirement of DGKζ for TLR-induced IL-12 p40 production and indicate that deficiency of DGKζ selectively affects cytokine production without inhibition of DC migration.
DGKζ deficiency does not cause impairment of IκB degradation or activation of p38 and Erk1/2 MAPKs
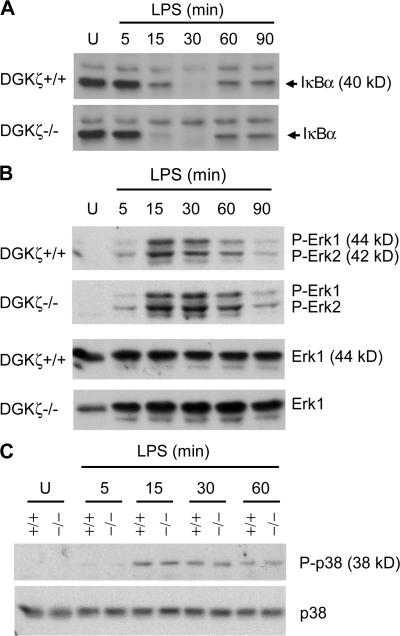
To understand how DGKζ is integrated into TLR signaling, we first examined whether DGKζ deficiency may affect IκBα degradation, an event downstream of the MyD88-dependent pathway that is critical for NF-κB activation and TLR-induced cytokine production. No decrease of TLR4-induced IκBα degradation was found in DGKζ-deficient Mφ after LPS stimulation (Fig. 4 A), suggesting that proximal signaling events leading to IκBα degradation are not inhibited and, therefore, are not likely to account for the inhibition of TLR-induced IL-12 and TNFα production in the absence of DGKζ expression. The MAPK Erk1/2 have been reported to negatively regulate IL-12 production induced by several TLR ligands (9, 46, 47). No obvious enhancement of Erk1/2 activation, as assessed by their phosphorylation, was observed in DGKζ-deficient Mφ after LPS stimulation (Fig. 4 B). In addition, LPS-induced activation of the p38 MAPK, a positive regulator of TLR-induced cytokine production, was not impaired in DGKζ-deficient Mφ (Fig. 4 C), suggesting that the impaired cytokine production caused by DGKζ deficiency is not caused by inhibition of this MAPK.
Figure 4.
Assessment of IκBα degradation and Erk1/2 and p38 activation in DGKζ-deficient Mφ after TLR4 stimulation. DGKζ+/+ and DGKζ−/− BMMφ were rested in RPMI 1640 medium supplemented with 0.5% FBS for 3 h. Cells were left unstimulated (U) or stimulated with 100 ng/ml LPS for the indicated times. Proteins in total cell lysates were separated by SDS-PAGE and levels of IκBα (A), phospho-Erk1/2 (B), and phospho-p38 (C) were determined by Western blot with anti-IκBα, anti–phospho-Erk1/2, and anti–phospho-p38 antibodies. The blots were stripped and reprobed with anti-Erk1/2 and anti-p38 antibodies for loading control.
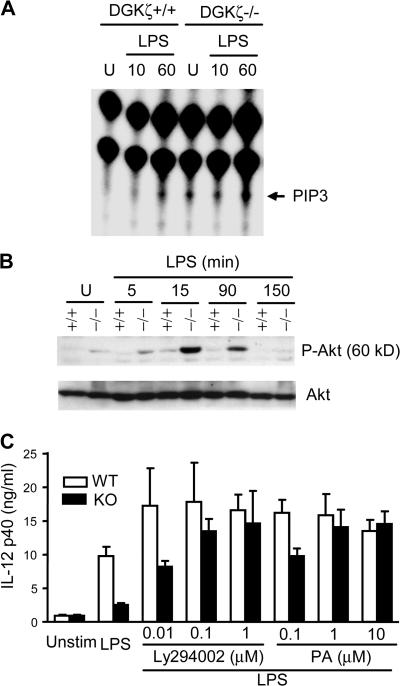
DGKζ deficiency causes increased activation of the PI3K–Akt pathway
The PI3K–Akt pathway can negatively regulate TLR-induced responses (33–35). How the PI3K–Akt pathway is controlled during TLR-induced responses is not clear. We examined the intracellular concentration of PIP3, the product of PI3K (48, 49), before and after LPS stimulation. In WT BMMφ, PIP3 was virtually undetectable before stimulation or 10 min after LPS stimulation but was detectable 60 min after LPS stimulation. In contrast, in DGKζ-deficient BMMφ, PIP3 was readily detectable both before stimulation and 10 min after LPS stimulation. Furthermore, a higher level of PIP3 was observed in DGKζ-deficient BMMφ than in WT BMMφ 60 min after LPS stimulation (Fig. 5 A). Consistent with their elevated PIP3 levels in response to LPS stimulation, DGKζ-deficient BMMφ exhibited higher levels of Akt phosphorylation, an event downstream of PI3K activation (Fig. 5 B). A similar elevation of Akt phosphorylation was observed in DGKζ-deficient splenic DCs before and after LPS stimulation (unpublished data). These observations reveal that DGKζ is a novel negative regulator of the PI3K–Akt pathway in both Mφ and DCs.
Figure 5.
Enhanced activation of the PI3K–Akt pathway in DGKζ-deficient Mφ after TLR4 stimulation. (A) Elevated LPS-induced PIP3 production in DGKζ-deficient BMMφ. WT and DGKζ-deficient BMMφ were rested in phosphate-free RPMI 1640 with 0.5% FBS at 37°C for 3 h, with the last 90 min supplemented with [32P]orthophosphate. Cells were left unstimulated or stimulated with 100 ng/ml LPS for 10 and 60 min. Total lipids were extracted and separated by TLC. PIP3 (molecular mass ≈ 1.2 kD) was visualized using a Phosphorimager (see Materials and methods). Data shown are representative of three experiments. (B) Enhanced Akt activation in DGKζ−/− Mφ. WT (+/+) and DGKζ-deficient (−/−) BMMφ were stimulated as in Fig. 4, but Western blots were probed using an anti–phospho-Akt (Ser473) antibody. The blot was stripped and reprobed with an anti-Akt antibody to determine equal protein loading. Data shown are representative of three experiments. (C) Restoration of LPS-induced IL-12 production in DGKζ-deficient Mφ by inhibition of PI3K or by exogenous PA. WT and DGKζ-deficient Mφ were left unstimulated or stimulated with 100ng/ml LPS in the absence or presence of indicated concentrations of Ly294002 or PA at 37°C for 24 h. IL-12 p40 levels in supernatants were measured with ELISA. Data shown are representative of three experiments. Data shown are the mean ± SD.
Restoration of LPS-induced IL-12 production by inhibition of PI3K and by PA
To test whether enhanced activation of the PI3K–Akt pathway contributes to impairment of TLR-induced cytokine responses in DGKζ-deficient Mφ, WT and DGKζ-deficient Mφ were treated with Ly294002, a PI3K inhibitor, during LPS stimulation. As shown in Fig. 5 C, 10 nM Ly294002 promoted IL-12 production by both WT and DGKζ-deficient Mφ. Increasing Ly294002 concentrations further enhanced IL-12 production by DGKζ-deficient Mφ. These observations, together with the biochemical studies shown in Fig. 5 (A and B), indicate that DGKζ positively contributes to TLR-induced proinflammatory cytokine production by inhibiting the function of PI3K, a natural inhibitor of TLR-induced responses.
DGKζ deficiency is expected to increase DAG levels and decrease PA levels. To test whether DGK-derived PA may contribute to TLR-induced responses, WT and DGKζ-deficient Mφ were stimulated with LPS in the absence or presence of differing concentrations of PA (Fig. 5 C). PA promoted IL-12 production by WT and DGKζ-deficient Mφ. When treated with 1 or 10μM PA, DGKζ-deficient Mφ produced similar levels of IL-12 as WT Mφ. This observation suggests that DGKζ-derived PA plays a positive role in supporting LPS-induced IL-12 production.
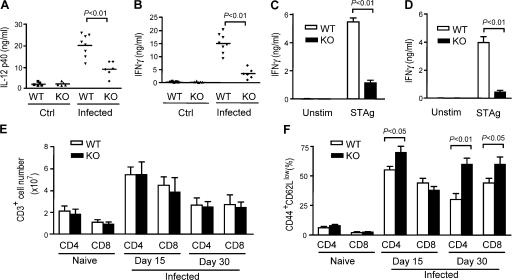
Impairment of Th1 immune responses against T. gondii in DGKζ-deficient mice
Host resistance to T. gondii requires TLR-induced IL-12 production by APCs and subsequent induction of IFN-γ production from NK cells and Th1-type CD4 T cells (22, 50, 51). As DGKζ deficiency causes decreased IL-12 p40 production after in vivo and in vitro STAg stimulation, we next examined whether DGKζ deficiency may affect host defense against T. gondii. Both C57BL/6 WT and DGKζ-deficient mice were intraperitoneally challenged with live T. gondii bradyzoite cysts (strain ME49). Serum IL-12 p40 and IFN-γ levels in T. gondii–infected DGKζ-deficient mice were much lower than in WT mice on days 5 and 7 after infection, respectively (Fig. 6, A and B), suggesting impairment of innate immune responses against T. gondii. On days 15 and 30 after infection, DGKζ-deficient and WT splenocytes were stimulated ex vivo with STAg for 24 h. DGKζ-deficient splenocytes produced less IFN-γ than WT cells after stimulation, indicating that the Th1 immune response against T. gondii was inhibited in DGKζ-deficient mice (Fig. 6, C and D). Despite this finding, there were similar total T cell numbers, as well as CD4 and CD8 T cell subsets, in DGKζ-deficient and WT spleens (Fig. 6 E). Interestingly, there were higher percentages of CD44+CD62Llow CD4 T cells in DGKζ-deficient spleens compared with WT spleens on days 15 and 30 after infection (Fig. 6 F). Although there were similar or slightly lower percentages of CD44+CD62Llow CD8 T cells in DGKζ-deficient spleens on day 15 after infection, on day 30 we observed a similar increase in the percentage of CD44+CD62Llow CD8 T cells in DGKζ-deficient spleens. These observations suggest that the decreased IFN-γ production was not caused by a decrease in T cell numbers or an inability to activate DGKζ-deficient T cells.
Figure 6.
Impaired innate and T cell responses to T. gondii infection in DGKζ-deficient mice. C57BL/6 WT and DGKζ−/− mice were intraperitoneally injected with 20 cysts of ME49 strain T. gondii. (A and B) Decreased serum IL-12 and IFN-γ concentrations in DGKζ-deficient mice. Serum IL-12 (A) was measured 5 d after infection, and IFN-γ (B) was measured 7 d after infection by ELISA. Data shown are representative of three experiments. Horizontal lines in A and B indicate the means. (C and D) Recall responses. At day 15 (C) and day 30 (D) after T. gondii infection, splenocytes from WT and DGKζ-deficient mice were left unstimulated or stimulated with STAg at 37°C for 24 h. IFN-γ in the culture supernatant was measured by ELISA. (E) Splenic CD4 and CD8 T cell numbers in WT and DGKζ-deficient mice after T. gondii infection. Splenic T cell subsets from uninfected and T. gondii–infected mice were determined after enumeration and FACS analysis. (F) CD44 and CD62L staining on splenic CD4 and CD8 T cell subsets of CD4 and CD8 T cells as in E. Data shown are representative of three experiments. Data shown in C–F are the mean ± SD.
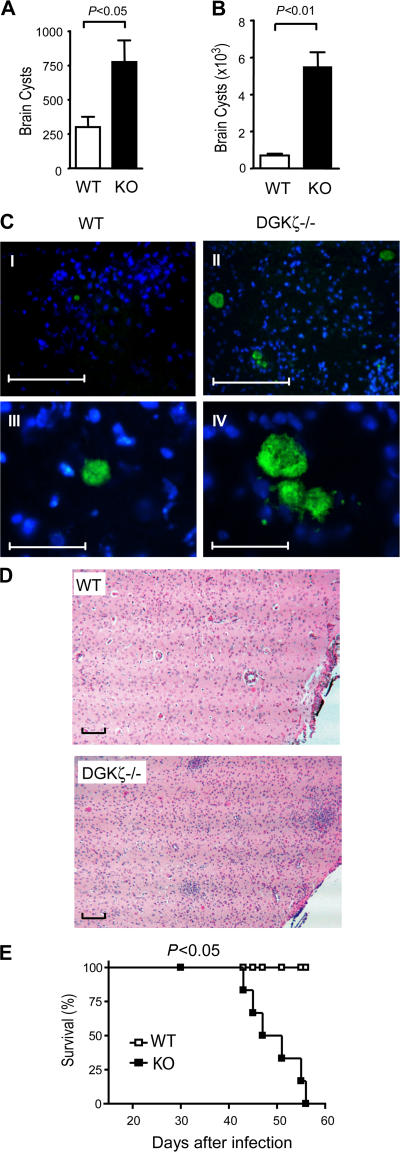
DGKζ-deficient mice succumbed to toxoplasmosis
To further determine how DGKζ deficiency may affect host resistance to T. gondii, we examined brain cyst numbers in WT and DGKζ-deficient mice after infection with this parasite. Consistent with the impairment of innate and adaptive immune responses against T. gondii, the brain parasite cyst number in infected DGKζ-deficient mice was more than twofold higher than in WT mice on day 15 after infection (Fig. 7 A). The fold increase in brain cysts in DGKζ-deficient mice was even more dramatic at a late stage of infection (Fig. 7 B). Immunofluorescence staining for T. gondii with an antibody specific for T. gondii cyclophilin-18 showed increased cyst numbers and cyst sizes in DGKζ-deficient brain tissues compared with WT brain (Fig. 7 C, I–IV), further confirming the inability of DGKζ-deficient mice to effectively control this infection. Hematoxylin and eosin (H&E) staining of paraffin-embedded sections showed a striking increase in mononuclear cell infiltration in brains from DGKζ-deficient mice compared with WT mice on day 30 after infection (Fig. 7 D), indicating the persistence of active inflammation in DGKζ-deficient brains. DGKζ-deficient mice showed signs of disease, such as increased lethargy and rough fur, starting ∼20–30 d after infection. All DGKζ-deficient mice succumbed to toxoplasmosis between 45 to 55 d after infection (Fig. 7 E). In contrast, all WT mice remained healthy and survived the infection. Collectively, these observations indicate that DGKζ deficiency results in impaired innate immunity against T. gondii, which may cause defective induction of Th1 immune responses and ineffective control of this parasitic infection.
Figure 7.
Increased susceptibility to T. gondii infection in DGKζ-deficient mice. (A and B) Brain T. gondii cyst counts in WT and DGKζ−/− mice on day 15 (A) and day 45 (B) after infection. Brain homogenates (n = 3) were examined for the presence of T. gondii cysts at the indicated times after peritoneal injection of ME49 strain parasites. Data shown are representative of three experiments. Data shown in A and B are the mean ± SD. (C) Detection of brain T. gondii cysts by immunofluorescence. Frozen brain sections from T. gondii–infected WT and DGKζ-deficient mice were stained with an anti–cyclophilin-18 antibody (1499), followed by detection with Alexa Fluor 488–conjugated secondary chicken anti–rabbit IgG. Nuclei were detected by DAPI staining. Green and blue, T. gondii cysts and nuclei, respectively. Bars: (I and II) 250 μm; (III and IV) 50 μm. (D) H&E staining of brain paraffin sections from WT and DGKζ-deficient mice 30 d after T. gondii infection. Bars, 100 μm. (E) Enhanced susceptibility of DGKζ-deficient mice to toxoplasmosis. Six WT and DGKζ-deficient mice were intraperitoneally infected with T. gondii, and survival was monitored. Data shown are representative of three experiments.
DISCUSSION
In this report, we show that DGKζ deficiency causes impaired innate immune responses to T. gondii and decreased susceptibility to endotoxin-induced shock, as well as decreased IL-12 and TNFα production in response to LPS and several other TLR ligands. These alterations in immune cell function correlate with increased activation of the PI3K–Akt pathway in DGKζ-deficient Mφ. Inhibition of PI3K in DGKζ-deficient Mφ can restore IL-12 production. Thus, our data indicate that DGKζ positively contributes to TLR-induced responses by negatively regulating the PI3K–Akt pathway. The conclusion is consistent with the reported observations that class IA PI3Ks negatively control TLR-mediated responses (33–35). Of note, inhibition of the PI3K–Akt pathway by DGKζ is not limited to Mφ and DCs. In mast cells, DGKζ deficiency enhances Akt phosphorylation and cell survival in vitro in the presence or absence of surviving cytokines (41). Thus, DGKζ may have a broader role as a negative regulator of PI3K in different cell lineages.
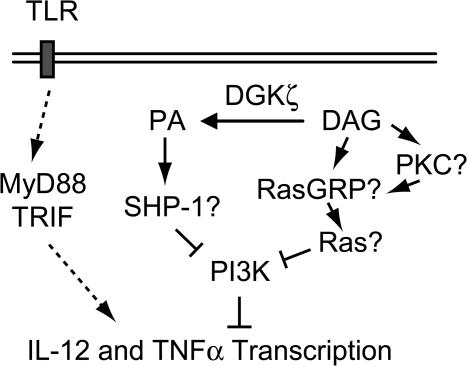
At present, it is not known how DGKζ controls PI3K activation, but it is possible that both DAG- and PA-mediated mechanisms could be involved (Fig. 8). On one hand, DGKζ could negatively control PI3K through its product, PA. The Src homology 2 domain–containing phosphatase 1 (SHP-1) is a PA effector molecule that can bind to PA. Association with PA may increase the enzyme activity of SHP-1 (52). In T cells and osteoclasts, SHP-1 can dephosphorylate the p85α subunit to inhibit activation of the class IA PI3K (53, 54). DGKζ deficiency may decrease PA production and SHP-1 activity, leading to alleviation of the negative control of PI3K by SHP-1. In support of this hypothesis, we have found that treatment of DGKζ-deficient Mφ with exogenous PA restores TLR-induced IL-12 production, suggesting that DGKζ-derived PA promotes TLR-induced proinflammatory responses. On the other hand, DGKζ deficiency could lead to accumulation of DAG and subsequent activation of Ras through Ras guanyl nucleotide-releasing protein (RasGRP) or protein kinase C (PKC), two important families of DAG effector molecules (55–57). PKCα and β have been shown to be required for STAg-induced IL-12 production (58). Based on this finding, it is unlikely that these PKC isoforms mediate signaling events that inhibit IL-12 production in DGKζ-deficient Mφ and DCs. It has been previously shown that enhanced Ras activity can promote PI3K activation (59). In this regard, it is interesting to note that Ras can be activated by TLR2 (60). Therefore, it is possible that an increase of RasGRP and Ras activity in DGKζ-deficient Mφ and DCs could increase PI3K activity during TLR stimulation. Studies are ongoing to distinguish between these possibilities.
Figure 8.
Potential mechanisms of DGKζ function in TLR-induced proinflammatory responses. DGKζ activity could negatively control PI3K through its product, PA. PA may associate with and increase SHP-1 activity. SHP-1 may dephosphorylate PI3K and inhibit its activation to promote TLR-induced proinflammatory cytokine production. Alternatively, DGKζ could inactivate DAG and subsequently inhibit Ras activation through RasGRPs, leading to a decrease of PI3K activity.
It is intriguing that DGKζ deficiency causes enhanced immune responses against lymphocytic choriomeningitis virus (LCMV) (40) but decreases host resistance to T. gondii. These seemingly opposite effects could be caused by the differential requirements of innate immunity and microbial recognition mechanisms for effective clearance of these pathogens. For example, TLR-independent viral recognition mechanisms, such as the RNA helicase retinoid acid inducible gene I–mediated pathway (61, 62), have been identified and contribute to host defense against viral infection. At present, whether DGKζ is involved in TLR-independent viral recognition is not known. Furthermore, IL-12 appears to have different roles in host resistance to LCMV and T. gondii. Loss of IL-12 in mice dramatically reduces host resistance to T. gondii (51, 63). However, immune responses to LCMV infection and clearance of the virus are not impaired in IL-12–deficient mice (64). Our data showing that DGKζ is required for mice to survive T. gondii infection are consistent with the role of DGKζ for optimal IL-12 production in response to T. gondii and the requirement of IL-12 for host resistance against this parasite. The impairment of IL-12 production in DGKζ-deficient mice may not substantially affect host immune responses against LCMV infection. LCMV-specific CD8 T cells are massively expanded and activated in response to LCMV infection and are critical in host defense against LCMV (65). The enhanced immune responses against LCMV infection in DGKζ-deficient mice are likely caused by elevated expansion and activation of IFN-γ–producing CD8 T cells (40).
It is important to note that both WT and DGKζ-deficient CD4 and CD8 T cells manifest a similar kinetics of expansion and contraction during the course of T. gondii infection. Although DGKζ-deficient mice are susceptible to toxoplasmosis, T cell activation, as assessed by increased expression of CD44 and reduced expression of CD62L, appears enhanced in DGKζ-deficient mice compared with WT mice. Despite signs of activation, Th1 immune responses are dramatically inhibited in DGKζ-deficient T cells, as seen by their reduced production of IFN-γ in response to ex vivo stimulation with STAg. These observations suggest that the impairment of TLR-induced IL-12 production may selectively inhibit Th1 immune responses but not globally inhibit T cell activation. The increase of T cell activation during T. gondii infection could be caused by the intrinsic hyperresponsiveness of DGKζ-deficient T cells or the persistence of T. gondii infection in DGKζ-deficient mice.
DGKζ-deficient mice succumbed to toxoplasmosis with a delayed kinetics when compared with MyD88- or IL-12–deficient mice (22, 63). This observation is consistent with our data showing that DGKζ deficiency causes a substantial decrease, but not complete abolishment of, TLR-induced IL-12 production. Partial inhibition of TLR-induced cytokine production could be caused by the expression of other DGK isoforms in DCs and Mφ (unpublished data), which may compensate for the loss of DGKζ expression. Additionally, PA and DAG can be produced by other enzymes such as phosphatidylcholine-specific phospholipase C and D, which may also contribute to TLR-induced cytokine production (15–17). However, even if these enzymes participate in TLR-induced innate immune responses, they are not able to completely compensate for DGKζ deficiency.
Although DGKζ deficiency causes enhanced activation of the PI3K–Akt pathway, it does not globally inhibit TLR signaling. For example, TLR-induced IκBα degradation and activation of Erk1/2 and p38 MAPKs are not altered in DGKζ-deficient Mφ. The selective role of DGKζ on specific signaling events may differentially affect TLR-induced responses. As we have shown in Fig. 3, loss of DGKζ expression inhibits TLR-induced IL-12 p40 and TNFα production but not DC migration. These differential effects of DGKζ on TLR responses are consistent with the proposed roles of PI3K during TLR-induced cytokine production and DC migration. Specifically, PI3K is believed to positively regulate DC migration but inhibit IL-12 production after TLR engagement (44, 46, 47). Therefore, in the absence of DGKζ, enhanced PI3K signals would be expected to support DC migration but negatively influence proinflammatory cytokine production.
It is interesting to note that DGKζ performs a variety of roles in response to different receptors within the immune system. In T cells, DGKζ functions as a negative regulator of T cell receptor signaling and T cell activation by inhibiting DAG-mediated activation of the Ras–Erk1/2 pathway (39, 40, 66). In mast cells, DGKζ performs both positive and negative roles in FcɛRI-induced responses. It positively contributes to FcɛRI-induced mast cell degranulation by promoting Ca2+ influx but negatively controls FcɛRI-induced IL-6 production and survival via inhibition of Ras, Erk, and Akt (41). In DCs and Mφ, DGKζ positively contributes to TLR-induced cytokine responses, which correlates with inhibition of PI3K activity. The differential effects of DGKζ deficiency on FcɛRI-induced IL-6 production in mast cells and on LPS-induced IL-12 production in DCs and Mφ may reflect differences in transcriptional activation between these two genes. Such differential effects could also be caused by the distinct roles of signaling pathways downstream of different receptors in activating transcription of these two cytokines. Collectively, these observations reveal the importance of DGKζ in these lineages and suggest that by differential modulation of DAG and/or PA-mediated signaling pathways, DGKζ regulates diverse cellular processes.
In summary, the current study provides the first genetic evidence that an enzyme involved in DAG and PA metabolism is important for TLR-induced innate immune responses in vitro and in vivo and for host defense against T. gondii. DGKζ negatively regulates the PI3K–Akt pathway in response to TLR4 stimulation. We propose that DGKζ may function as a positive regulator of TLR-induced proinflammatory responses by facilitating PA-dependent signaling events.
MATERIALS AND METHODS
Mice.
Previously described DGKζ-deficient mice (40) were backcrossed to the C57BL/6 background for more than seven generations. Mice used for experiments were between 6–12 wk old. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Duke University.
Isolation of splenic DCs.
Spleens from WT and DGKζ−/− mice were digested with 1 mg/ml collagenase D (0.5ml/spleen; Roche Diagnostics) in a digestion buffer (10 mM Hepes-NaOH [pH 7.4], 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2) for 30 min at 37°C. The spleens were then smashed into a single-cell suspension, and the low-density leukocytes were obtained by centrifuging the cell suspension on a dense BSA gradient, as previously described (67). DCs were further purified from splenic low-density leukocytes by MACS using anti-CD11c microbeads according to the manufacturer's protocol (Miltenyi Biotec). The purified DCs were routinely 70–85% CD11c+, as determined by FACS analysis.
Generation of BMMφ.
Bone marrow cells from femurs and tibias were plated into Petri dishes containing RPMI 10 (RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 1,000 U/ml streptomycin, and 20 mM l-glutamine) and 20% L929 cell- conditioned medium. After 2–3 d of culture at 37°C in CO2 incubator, nonadherent cells were colleted and cultured in fresh medium for another 3–5 d before being used for experiments. More than 95% of cells were CD11b+ using flow cytometry analysis.
In vitro stimulation for cytokine production.
Splenic DCs and BMMφ were cultured in 96-well plates at a concentration of 2 × 105 cells/well and 105 cells/well, respectively, in 200 μl RPMI 10. Cells were left unstimulated or stimulated with 30 or 100 ng/ml STAg, 100 ng/ml LPS, 50 ng/ml Pam3CSK4, or 50 μg/ml Poly(I:C) for 24 h. STAg was prepared from sonicated T. gondii (RH strain) tachyzoites, as previously described (68). Synthetic bacterial lipopeptide Pam3CSK4 and Poly(I:C) were purchased from Apotech. LPS from E. coli serotype 0111:B4 was obtained from both Apotech and Sigma-Aldrich. IL-12 p40 and TNFα levels in the culture supernatants were measured by ELISA (R&D Systems).
In vivo LPS and STAg stimulation.
DGKζ-deficient and C57BL/6 mice were intraperitoneally injected with 10 μg LPS or 5 μg STAg. To measure TNFα and IL-12 p40, sera were collected 6 h after injection, and cytokine concentrations were determined by ELISA. For analysis of TNFα and IL-12 p40 mRNA levels, total splenic RNA was isolated before and after LPS or STAg injection using the TRIzol reagent, according to the manufacturer's protocol (Invitrogen). 10 μg of total RNA from each sample was resolved in a formaldehyde-containing agarose gel, transferred to a nylon membrane, and hybridized with 32P-labeled probes for IL-12 p40 and TNFα. The blots were stripped and probed with a 32P-labeled GAPDH probe for loading control.
Endotoxic shock.
6-wk-old sex-matched C57BL/6 and DGKζ-deficient mice were intraperitoneally injected with 20 mg d-galactosamine (Sigma-Aldrich) and 2 μg LPS (Sigma-Aldrich) in 0.2 ml PBS per mouse. Survival of mice was monitored over time at 30-min intervals.
Measurement of intracellular PIP3 levels.
PIP3 level was measured as previously described, with modifications (48). In brief, BMMφ were plated in 60-mm dishes at the density of two million cells per dish. After overnight incubation at 37°C, culture medium was replaced with 2 ml of phosphate-free medium with 0.5% FBS and cultured for another 3 h, with the last 90 min supplemented with 100 μCi [32P]orthophosphate. Cells were left unstimulated or stimulated with 100 ng/ml of LPS for 10 and 60 min. Total lipids were extracted with methanol/chloroform (2:1, vol/vol), dried, redissolved in chloroform/methanol (1:1), and separated by TLC using a solvent (chloroform/acetone/methanol/acetic acid/water [80:30:26:24:14]). PIP3 was identified by the use of a co-migrating standard with iodine vapor staining and revealed by a Phosphorimager (Typhoon 9410; Amersham Biosciences).
Measurement of p38 and Erk1/2 MAPK activation, Akt phosphorylation, and IκB degradation.
WT and DGKζ-deficient BMMφ were plated on 6-cm dishes at a concentration of 2 × 106 cells/dish and incubated at 37°C overnight. Cells were rested in 0.5% FBS-RPMI at 37°C for 3 h and were left unstimulated or stimulated with 100 ng/ml LPS for the times indicated in the figures. Akt, Erk1/2, and p38 phosphorylation and IκBα protein levels were determined by Western blot using anti–phospho-Akt (Ser473), anti–phospho-Erk1/2, anti–phospho-p38, and anti-IκBα antibodies, respectively (Cell Signaling Technology). The blots were probed with anti-Akt, anti-Erk1/2, and anti-p38 antibodies for loading control (Cell Signaling Technology).
T. gondii infection.
T. gondii infection was performed as previously described (51). In brief, WT and DGKζ-deficient mice were intraperitoneally injected with 20 or 40 cysts of ME49 strain T. gondii. On days 5 and 7 after infection, serum samples were collected to measure IL-12 p40 and IFN-γ production, respectively, by ELISA. During the course of infection, T. gondii cysts in brain homogenates were counted by light microscopy. Survival of mice was monitored over time.
To measure T cell recall responses, spleens were harvested from mice that had been infected with T. gondii 15 or 30 d earlier. Total splenocytes were enumerated and analyzed by FACScan after staining with antibodies for CD4, CD8, CD3, CD44, and CD62L. Splenocytes were also seeded in U-bottom 96-well plates at a concentration of 5 × 105 cells/well in 200 μl RPMI 10 with or without 100 ng/ml STAg. After 24 h incubation at 37°C, IFN-γ concentrations in the culture supernatants were determined by ELISA.
Microscopy.
Spleens were removed from mice before or 6 h after STAg or LPS stimulation, and frozen sections were processed and stained with a goat anti–mouse IL-12 p40 (BD Biosciences), followed by a double incubation with Alexa Fluor 488–conjugated anti–mouse CD11c and Alexa Fluor 594–conjugated anti–goat antibodies (Invitrogen). The cells were counterstained for nuclei with DAPI (Invitrogen). Images were captured using a microscope (Axiovert 200M; Carl Zeiss MicroImaging, Inc.) with the AxioVision software (Carl Zeiss MicroImaging, Inc.).
To visualize T. gondii cysts by immunofluorescence microscopy, frozen brain sections from T. gondii–infected WT and DGKζ-deficient mice were stained with an anti–cyclophilin-18 antibody (1499) (24), followed by detection with Alexa Fluor 488–conjugated secondary chicken anti–rabbit IgG (Invitrogen). Nuclei were stained with DAPI, and images were captured as described in the previous paragraph.
H&E staining.
30 d after T. gondii infection, WT and DGKζ−/− mice were sequentially perfused with 10 ml PBS and 20 ml 4% paraformaldehyde. The brains were then harvested and fixed with 10% formalin overnight. After dehydration with increased concentrations of ethanol and embedment in paraffin, thin sections were cut and stained with H&E according to standard procedures (70).
Statistical analysis.
Experimental data are expressed as the mean ± SD. The statistical significance of differences in mean values of cytokine concentrations and cyst numbers was determined using the Student's t test. Survival data are presented as a Kaplan Meier survival curve and analyzed using the log-rank test. Differences having a p-value <0.05 are considered significant.
Acknowledgments
We thank J. Harris Carpenter for technical assistance.
This work is partly supported by an award from the American Heart Association (to X.P. Zhong). X.P. Zhong thanks Dr. Gary Koretzky for his continuous support.
All authors declare no conflict of interest related to the studies.
Abbreviations used: BMMφ, bone marrow–derived Mφ DAG, diacylglycerol; DGK, DAG kinase; H&E, hematoxylin and eosin; LCMV, lymphocytic choriomeningitis virus; MAPK, mitogen-associated protein kinase; Mφ, macrophage(s); MyD88, myeloid differentiation primary response protein 88; PA, phosphatidic acid; PI3K, phosphatidylinositol 3–kinase; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; PKC, protein kinase C; RasGRP, Ras guanyl nucleotide-releasing protein; SHP-1, Src homology 2 domain–containing phosphatase 1; STAg, T. gondii–soluble tachyzoite Ag; TLR, Toll-like receptor.
C.-H. Liu, F.S. Machado, and R. Guo contributed equally to this work.
References
- 1.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- 3.Wesche, H., W.J. Henzel, W. Shillinglaw, S. Li, and Z. Cao. 1997. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 7:837–847. [DOI] [PubMed] [Google Scholar]
- 4.Muzio, M., J. Ni, P. Feng, and V.M. Dixit. 1997. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 278:1612–1615. [DOI] [PubMed] [Google Scholar]
- 5.Burns, K., F. Martinon, C. Esslinger, H. Pahl, P. Schneider, J.L. Bodmer, F. Di Marco, L. French, and J. Tschopp. 1998. MyD88, an adapter protein involved in interleukin-1 signaling. J. Biol. Chem. 273:12203–12209. [DOI] [PubMed] [Google Scholar]
- 6.Huang, Q., J. Yang, Y. Lin, C. Walker, J. Cheng, Z.G. Liu, and B. Su. 2004. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol. 5:98–103. [DOI] [PubMed] [Google Scholar]
- 7.Lu, H.T., D.D. Yang, M. Wysk, E. Gatti, I. Mellman, R.J. Davis, and R.A. Flavell. 1999. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18:1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuguchi, T., A. Masuda, K. Sugimoto, Y. Nagai, and Y. Yoshikai. 2003. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 22:4455–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason, N.J., J. Fiore, T. Kobayashi, K.S. Masek, Y. Choi, and C.A. Hunter. 2004. TRAF6-dependent mitogen-activated protein kinase activation differentially regulates the production of interleukin-12 by macrophages in response to Toxoplasma gondii. Infect. Immun. 72:5662–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto, M., S. Sato, H. Hemmi, S. Uematsu, K. Hoshino, T. Kaisho, O. Takeuchi, K. Takeda, and S. Akira. 2003. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4:1144–1150. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643. [DOI] [PubMed] [Google Scholar]
- 12.Han, K.J., X. Su, L.G. Xu, L.H. Bin, J. Zhang, and H.B. Shu. 2004. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-κB activation and apoptosis pathways. J. Biol. Chem. 279:15652–15661. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald, K.A., S.M. McWhirter, K.L. Faia, D.C. Rowe, E. Latz, D.T. Golenbock, A.J. Coyle, S.M. Liao, and T. Maniatis. 2003. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491–496. [DOI] [PubMed] [Google Scholar]
- 14.Kawai, T., O. Takeuchi, T. Fujita, J. Inoue, P.F. Muhlradt, S. Sato, K. Hoshino, and S. Akira. 2001. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 167:5887–5894. [DOI] [PubMed] [Google Scholar]
- 15.Zhang, F., G. Zhao, and Z. Dong. 2001. Phosphatidylcholine-specific phospholipase C and D in stimulation of RAW264.7 mouse macrophage-like cells by lipopolysaccharide. Int. Immunopharmacol. 1:1375–1384. [DOI] [PubMed] [Google Scholar]
- 16.Monick, M.M., A.B. Carter, G. Gudmundsson, R. Mallampalli, L.S. Powers, and G.W. Hunninghake. 1999. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide-stimulated human alveolar macrophages. J. Immunol. 162:3005–3012. [PubMed] [Google Scholar]
- 17.Zhang, F., G. Zhao, and Z. Dong. 2001. Phosphatidylcholine-specific phospholipase C regulates activation of RAW264.7 macrophage-like cells by lipopeptide JBT3002. J. Leukoc. Biol. 69:1060–1066. [PubMed] [Google Scholar]
- 18.Hill, D.E., S. Chirukandoth, and J.P. Dubey. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim. Health Res. Rev. 6:41–61. [DOI] [PubMed] [Google Scholar]
- 19.Aliberti, J. 2005. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat. Rev. Immunol. 5:162–170. [DOI] [PubMed] [Google Scholar]
- 20.Kasper, L., N. Courret, S. Darche, S. Luangsay, F. Mennechet, L. Minns, N. Rachinel, C. Ronet, and D. Buzoni-Gatel. 2004. Toxoplasma gondii and mucosal immunity. Int. J. Parasitol. 34:401–409. [DOI] [PubMed] [Google Scholar]
- 21.Yarovinsky, F., D. Zhang, J.F. Andersen, G.L. Bannenberg, C.N. Serhan, M.S. Hayden, S. Hieny, F.S. Sutterwala, R.A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 308:1626–1629. [DOI] [PubMed] [Google Scholar]
- 22.Scanga, C.A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E.Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997–6001. [DOI] [PubMed] [Google Scholar]
- 23.Mun, H.S., F. Aosai, K. Norose, M. Chen, L.X. Piao, O. Takeuchi, S. Akira, H. Ishikura, and A. Yano. 2003. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 15:1081–1087. [DOI] [PubMed] [Google Scholar]
- 24.Aliberti, J., J.G. Valenzuela, V.B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J.M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485–490. [DOI] [PubMed] [Google Scholar]
- 25.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G.B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1:83–87. [DOI] [PubMed] [Google Scholar]
- 26.Khan, I.A., S.Y. Thomas, M.M. Moretto, F.S. Lee, S.A. Islam, C. Combe, J.D. Schwartzman, and A.D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133–146. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, Y., F.K. Conley, and J.S. Remington. 1989. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045–2050. [PubMed] [Google Scholar]
- 29.Lieberman, L.A., and C.A. Hunter. 2002. The role of cytokines and their signaling pathways in the regulation of immunity to Toxoplasma gondii. Int. Rev. Immunol. 21:373–403. [DOI] [PubMed] [Google Scholar]
- 30.Denkers, E.Y., B.A. Butcher, L. Del Rio, and S. Bennouna. 2004. Neutrophils, dendritic cells and Toxoplasma. Int. J. Parasitol. 34:411–421. [DOI] [PubMed] [Google Scholar]
- 31.Koedel, U., T. Rupprecht, B. Angele, J. Heesemann, H. Wagner, H.W. Pfister, and C.J. Kirschning. 2004. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 127:1437–1445. [DOI] [PubMed] [Google Scholar]
- 32.Scanga, C.A., A. Bafica, C.G. Feng, A.W. Cheever, S. Hieny, and A. Sher. 2004. MyD88-deficient mice display a profound loss in resistance to Mycobacterium tuberculosis associated with partially impaired Th1 cytokine and nitric oxide synthase 2 expression. Infect. Immun. 72:2400–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukao, T., M. Tanabe, Y. Terauchi, T. Ota, S. Matsuda, T. Asano, T. Kadowaki, T. Takeuchi, and S. Koyasu. 2002. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3:875–881. [DOI] [PubMed] [Google Scholar]
- 34.Guha, M., and N. Mackman. 2002. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277:32124–32132. [DOI] [PubMed] [Google Scholar]
- 35.Martin, M., K. Rehani, R.S. Jope, and S.M. Michalek. 2005. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 6:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monick, M.M., A.B. Carter, P.K. Robeff, D.M. Flaherty, M.W. Peterson, and G.W. Hunninghake. 2001. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J. Immunol. 166:4713–4720. [DOI] [PubMed] [Google Scholar]
- 37.van Blitterswijk, W.J., and B. Houssa. 2000. Properties and functions of diacylglycerol kinases. Cell. Signal. 12:595–605. [DOI] [PubMed] [Google Scholar]
- 38.Luo, B., D.S. Regier, S.M. Prescott, and M.K. Topham. 2004. Diacylglycerol kinases. Cell. Signal. 16:983–989. [DOI] [PubMed] [Google Scholar]
- 39.Zhong, X.P., E.A. Hainey, B.A. Olenchock, H. Zhao, M.K. Topham, and G.A. Koretzky. 2002. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase ζ. J. Biol. Chem. 277:31089–31098. [DOI] [PubMed] [Google Scholar]
- 40.Zhong, X.P., E.A. Hainey, B.A. Olenchock, M.S. Jordan, J.S. Maltzman, K.E. Nichols, H. Shen, and G.A. Koretzky. 2003. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat. Immunol. 4:882–890. [DOI] [PubMed] [Google Scholar]
- 41.Olenchock, B.A., R. Guo, M.A. Silverman, J.N. Wu, J.H. Carpenter, G.A. Koretzky, and X.-P. Zhong. 2006. Impaired degranulation but enhanced cytokine production after FcɛRI stimulation of diacylglycerol kinase ζ–deficient mast cells. J. Exp. Med. 203:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding, L., M. Bunting, M.K. Topham, T.M. McIntyre, G.A. Zimmerman, and S.M. Prescott. 1997. Alternative splicing of the human diacylglycerol kinase ζ gene in muscle. Proc. Natl. Acad. Sci. USA. 94:5519–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poltorak, A., X. He, I. Smirnova, M.-Y. Liu, C.V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, et al. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 44.Alexopoulou, L., A.C. Holt, R. Medzhitov, and R.A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 413:732–738. [DOI] [PubMed] [Google Scholar]
- 45.Aliprantis, A.O., R.B. Yang, M.R. Mark, S. Suggett, B. Devaux, J.D. Radolf, G.R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 285:736–739. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984–4989. [DOI] [PubMed] [Google Scholar]
- 47.Dillon, S., A. Agrawal, T. Van Dyke, G. Landreth, L. McCauley, A. Koh, C. Maliszewski, S. Akira, and B. Pulendran. 2004. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 172:4733–4743. [DOI] [PubMed] [Google Scholar]
- 48.Klinghoffer, R.A., B. Duckworth, M. Valius, L. Cantley, and A. Kazlauskas. 1996. Platelet-derived growth factor-dependent activation of phosphatidylinositol 3-kinase is regulated by receptor binding of SH2-domain-containing proteins which influence Ras activity. Mol. Cell. Biol. 16:5905–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deane, J.A., and D.A. Fruman. 2004. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu. Rev. Immunol. 22:563–598. [DOI] [PubMed] [Google Scholar]
- 50.Gazzinelli, R.T., S. Hieny, T.A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA. 90:6115–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gazzinelli, R.T., M. Wysocka, S. Hayashi, E.Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533–2543. [PubMed] [Google Scholar]
- 52.Frank, C., H. Keilhack, F. Opitz, O. Zschornig, and F.D. Bohmer. 1999. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 38:11993–12002. [DOI] [PubMed] [Google Scholar]
- 53.Cuevas, B., Y. Lu, S. Watt, R. Kumar, J. Zhang, K.A. Siminovitch, and G.B. Mills. 1999. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J. Biol. Chem. 274:27583–27589. [DOI] [PubMed] [Google Scholar]
- 54.Zhang, Z., E. Jimi, and A.L. Bothwell. 2003. Receptor activator of NF-κB ligand stimulates recruitment of SHP-1 to the complex containing TNFR-associated factor 6 that regulates osteoclastogenesis. J. Immunol. 171:3620–3626. [DOI] [PubMed] [Google Scholar]
- 55.Lorenzo, P.S., M. Beheshti, G.R. Pettit, J.C. Stone, and P.M. Blumberg. 2000. The guanine nucleotide exchange factor RasGRP is a high-affinity target for diacylglycerol and phorbol esters. Mol. Pharmacol. 57:840–846. [PubMed] [Google Scholar]
- 56.Ebinu, J.O., D.A. Bottorff, E.Y. Chan, S.L. Stang, R.J. Dunn, and J.C. Stone. 1998. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science. 280:1082–1086. [DOI] [PubMed] [Google Scholar]
- 57.Roose, J.P., M. Mollenauer, V.A. Gupta, J. Stone, and A. Weiss. 2005. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol. Cell. Biol. 25:4426–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Masek, K.S., J. Fiore, M. Leitges, S.F. Yan, B.D. Freedman, and C.A. Hunter. 2006. Host cell Ca2+ and protein kinase C regulate innate recognition of Toxoplasma gondii. J. Cell Sci. 119:4565–4573. [DOI] [PubMed] [Google Scholar]
- 59.Jimenez, C., C. Hernandez, B. Pimentel, and A.C. Carrera. 2002. The p85 regulatory subunit controls sequential activation of phosphoinositide 3-kinase by Tyr kinases and Ras. J. Biol. Chem. 277:41556–41562. [DOI] [PubMed] [Google Scholar]
- 60.Pathak, S.K., A. Bhattacharyya, S. Pathak, C. Basak, D. Mandal, M. Kundu, and J. Basu. 2004. Toll-like receptor 2 and mitogen- and stress-activated kinase 1 are effectors of Mycobacterium avium-induced cyclooxygenase-2 expression in macrophages. J. Biol. Chem. 279:55127–55136. [DOI] [PubMed] [Google Scholar]
- 61.Seth, R.B., L. Sun, and Z.J. Chen. 2006. Antiviral innate immunity pathways. Cell Res. 16:141–147. [DOI] [PubMed] [Google Scholar]
- 62.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K.J. Ishii, et al. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 441:101–105. [DOI] [PubMed] [Google Scholar]
- 63.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628–631. [DOI] [PubMed] [Google Scholar]
- 64.Oxenius, A., U. Karrer, R.M. Zinkernagel, and H. Hengartner. 1999. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 162:965–973. [PubMed] [Google Scholar]
- 65.Murali-Krishna, K., J.D. Altman, M. Suresh, D.J. Sourdive, A.J. Zajac, J.D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 8:177–187. [DOI] [PubMed] [Google Scholar]
- 66.Olenchock, B.A., R. Guo, J.H. Carpenter, M. Jordan, M.K. Topham, G.A. Koretzky, and X.P. Zhong. 2006. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat. Immunol. 7:1174–1181. [DOI] [PubMed] [Google Scholar]
- 67.Reis e Sousa, C., S. Hieny, T. Scharton-Kersten, D. Jankovic, H. Charest, R.N. Germain, and A. Sher. 1997. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grunvald, E., M. Chiaramonte, S. Hieny, M. Wysocka, G. Trinchieri, S.N. Vogel, R.T. Gazzinelli, and A. Sher. 1996. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect. Immun. 64:2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bunting, M., W. Tang, G.A. Zimmerman, T.M. McIntyre, and S.M. Prescott. 1996. Molecular cloning and characterization of a novel human diacylglycerol kinase ζ. J. Biol. Chem. 271:10230–10236. [PubMed] [Google Scholar]
- 70.Kiernan, J.A. 1999. Histological and Histochemical Methods: Theory and Practice. Third edition. Butterworth Heinemann Medical Books, Great Britian. 111–113 pp.