Abstract
In conditions of T lymphopenia, interleukin (IL) 7 levels rise and, via T cell receptor for antigen–self–major histocompatibility complex (MHC) interaction, induce residual naive T cells to proliferate. This pattern of lymphopenia-induced “homeostatic” proliferation is typically quite slow and causes a gradual increase in total T cell numbers and differentiation into cells with features of memory cells. In contrast, we describe a novel form of homeostatic proliferation that occurs when naive T cells encounter raised levels of IL-2 and IL-15 in vivo. In this situation, CD8+ T cells undergo massive expansion and rapid differentiation into effector cells, thus closely resembling the T cell response to foreign antigens. However, the responses induced by IL-2/IL-15 are not seen in MHC-deficient hosts, implying that the responses are driven by self-ligands. Hence, homeostatic proliferation of naive T cells can be either slow or fast, with the quality of the response to self being dictated by the particular cytokine (IL-7 vs. IL-2/IL-15) concerned. The relevance of the data to the gradual transition of naive T cells into memory-phenotype (MP) cells with age is discussed.
In young life, most mature T cells are immuno logically naive and remain in interphase for prolonged periods (1–3); in mice, these cells are typified by low (lo) expression of CD44. A small proportion of T cells (10–20%) have features of antigen-specific memory cells, and these MP cells are characterized by high (hi) expression of CD44. In recent years, it has become apparent that the homeostatic control of naive and MP T cells is quite different. For naive T cells, these cells are kept alive by covert signaling induced by contact with IL-7 combined with TCR interaction with self-MHC ligands (4–8). For MP cells, a small subset of these cells is maintained in an activated state through overt recognition of self-MHC ligands (9, 10), but most MP cells are resting cells (1–3, 6, 11, 12). Both for CD4+ and CD8+ cells, typical resting MP cells divide intermittently, about once every 1–3 wk. These cells are MHC independent but are heavily dependent on contact with cytokines, notably IL-7 and IL-15 (6, 11, 12).
Although typical naive T cells rarely, if ever, divide unless confronted with antigen, naive cells enter cell cycle when total T cell numbers are reduced to low levels. Driven by the combined effects of an increase in IL-7 levels plus continuous TCR exposure to self-MHC ligands, residual naive CD44lo T cells undergo “homeostatic” expansion and differentiation into MP CD44hi cells, thereby restoring total T cell numbers toward normal (13–16). Such lymphopenia-induced proliferation typically occurs when small numbers of naive T cells are transferred to T cell–depleted hosts, e.g., irradiated normal mice or immunodeficient RAG−/− or SCID mice.
The tempo of homeostatic proliferation in T cell–depleted hosts is characteristically slow and leads to only gradual expansion of the cells without differentiation into effector cells. In contrast, we describe in this paper a florid form of homeostatic expansion driven by elevated levels of IL-2 and IL-15. Similar to contact with foreign antigen, naive T cells undergo massive expansion and differentiation into effector cells.
RESULTS
Proliferation of T cell subsets transferred to CD122−/− hosts
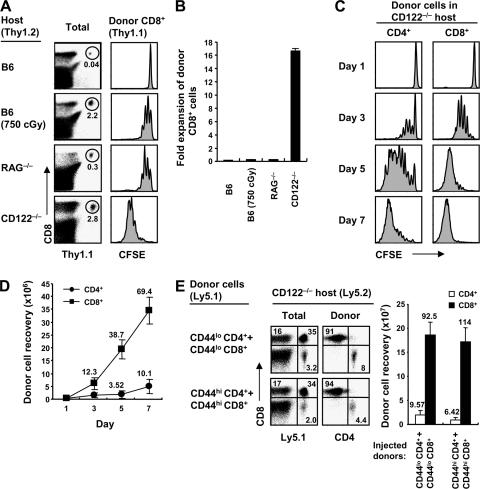
The requirements for inducing the homeostatic proliferation of naive CD8+ cells were examined by transferring small numbers of FACS-purified, CFSE-labeled CD44lo B6 CD8+ cells into various mouse strains. Confirming previous findings (13, 14), the allotype (Thy1.1)-marked donor cells remained in interphase in normal B6 hosts but underwent two to three rounds of division over 4 d in T cell–depleted hosts, i.e., irradiated (750 cGy) B6 mice or RAG−/− hosts (Fig. 1, A and B). As mentioned in the previous section above, this pattern of slow proliferation in T cell–depleted hosts is recognized as a hallmark of homeostatic proliferation and is driven largely by elevated concentrations of IL-7 combined with TCR contact with self–MHC-I ligands (7, 8, 13, 15–19).
Figure 1.
Proliferation of normal T cells in CD122−/− hosts. (A) FACS-sorted naive (CD44lo) CD8+ T cells (Thy1.1) were CFSE labeled and transferred i.v. at 106 cells per mouse into normal B6, irradiated (750 cGy) B6, RAG−/−, and CD122−/− mice. Spleen and LN cells were analyzed 4 d later by flow cytometry after staining for Thy1.1 and CD8 (left). Numbers in the dot plot indicate percentages of donor CD8+ (Thy1.1+ CD8+) cells within the total lymphocyte populations. CFSE profiles show the proliferation of gated donor CD8+ cells from the indicated hosts (right). (B) Fold expansion of donor cells recovered from pooled spleen and LN of mice in A (mean ± SD of two mice per group). (C) A mixture of FACS-sorted CD44lo CD4+ and CD8+ cells (Thy1.1) was CFSE labeled and transferred into CD122−/− mice (0.5 × 106 cells of each population per mouse). At the indicated time points, spleen and LN cells were analyzed by flow cytometry. Shown are CFSE profiles of gated donor CD4+ (Thy1.1+ CD8−) and CD8+ (Thy1.1+ CD8+) cells. (D) Total donor cell recoveries from mice in C (mean ± SD of two to three mice at each time point). Numbers indicate fold expansion of donor cells at each time point. (E) Mixtures of FACS-sorted CD44lo or CD44hi CD4+ and CD8+ cells (Ly5.1) were transferred into CD122−/− mice (2 × 106 cells of each population per mouse). Spleen and LN cells were analyzed 7 d later by flow cytometry. Dot plots show Ly5.1 versus CD8 profiles (left) of total lymphocytes and CD4 versus CD8 profiles (right) of gated donor (Ly5.1+) CD44lo (top) and CD44hi cells (bottom), and the bar graph shows total donor cell recoveries from the indicated hosts (mean ± SD of two mice per group). Numbers in the dot plots and bar graph indicate percentages of cells in the quadrants and the fold expansion of recovered donor cells, respectively.
Surprisingly, a much more prominent form of proliferation occurred after T cell transfer to CD122−/− mice, i.e., mice that lack the β chain (CD122) of the receptor (CD122/common γ chain [γc]) for IL-2 and IL-15 (20). When small numbers (106) of normal T cells were injected into unmanip ulated (nonirradiated) CD122−/− mice, total yields of donor CD8+ cells at 4 d after transfer were ∼100-fold higher than in irradiated B6 hosts (Fig. 1, A and B). Proliferation of naive T cells in CD122−/− hosts was much more pronounced for CD8+ than CD4+ cells (Fig. 1, C and D). For CD8+ cells, proliferation in CD122−/− hosts was almost as high with CD44lo cells as with CD44hi cells (Fig. 1 E and see below). All of the data in Fig. 1 refer to LN T cells. Marked (>100-fold) expansion of naive CD8+ cells also applied to mature thymocytes. Thus, transferring 107 unseparated thymocytes, which contained ∼1% CD8+ CD4− cells, yielded ∼1.5 × 107 mature donor CD8+ cells on day 7 (Fig. S1, A and B, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1). For donor CD8+ cells from the LN, it is worth noting that the donor cells (Ly5.1+) outnumbered host CD8+ cells (Ly5.1−) by 1 wk after transfer (Fig. 1 E), which is striking when it is kept in mind that CD122−/− mice have massive enlargement of the spleen and LN (20).
Surface markers
For LN CD8+ cells, proliferation of CD44lo cells in CD122−/− hosts led to rapid conversion to CD44hi cells, accompanied by up-regulation of activation markers such as CD25 and CD69 on day 3 (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1). Expression of these markers then declined. By day 7, many of the proliferating cells showed down-regulation of CD62L and up-regulation of CD122 and markers found on NK cells and memory cells, such as CD94, NKG2A, and NKG2D. In contrast, proliferation failed to cause up-regulation of activation markers or expression of NK cell markers in irradiated B6 mice (Fig. S2). In terms of effector function, the proliferating CD8+ cells showed strong staining for granzyme B and, upon restimulation in vitro, strong synthesis of IFN-γ (see below; unpublished data).
Role of CD28
In contrast to responses to foreign antigens, CD28 expression on T cells plays no obvious role in typical slow homeostatic proliferation in B6 mice (Fig. S3, A and B, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1) (21, 22). Similar findings applied to rapid T cell proliferation in CD122−/− hosts. Thus, for naive CD8+ cells, total yields of donor cells in CD122−/− hosts injected with CD28−/− cells were as high as with the injection of WT cells (Fig. S3, A and B).
Proliferation of TCR transgenic (Tg) cells
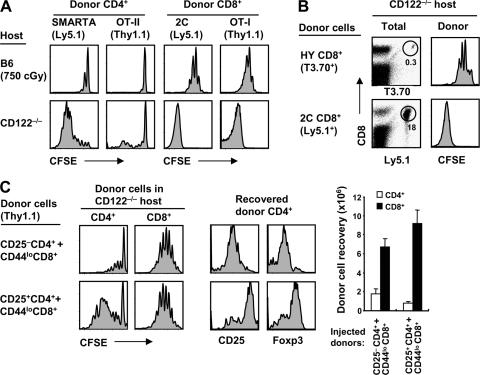
As for polyclonal B6 T cells, marked proliferation of naive T cells in CD122−/− hosts applied to TCR Tg cells (Fig. 2 A). For each TCR Tg line tested, CD44lo T cell proliferation in CD122−/− hosts was far stronger than in irradiated B6 mice. Importantly, as for homeostatic proliferation in irradiated B6 hosts, the intensity of donor proliferation in CD122−/− mice varied considerably from one TCR Tg line to another. Thus, for naive OT-I (23) and 2C (24) CD8+ cells, and also for SMARTA CD4+ cells (25), moderate proliferation of these cells in irradiated B6 mice correlated with massive proliferation in CD122−/− mice (Fig. 2 A). Conversely, for HY CD8+ cells (26) and OT-II CD4+ cells (27), little or no proliferation in irradiated B6 mice (Fig. 2 A) (19, 28–30) correlated with weak but notable proliferation in CD122−/− hosts (Fig. 2, A and B). For HY cells, yields of clonotype-positive (T3.70+) CD8+ cells in CD122−/− hosts were 50–100-fold lower on day 7 than with the transfer of OT-I or 2C cells to these hosts (Fig. 2 B and not depicted).
Figure 2.
Proliferation of TCR Tg T cells in CD122−/− mice and the influence of T reg cells. (A) Mixtures of FACS-sorted CD44lo SMARTA (Ly5.1) and OT-II (Thy1.1) CD4+ cells or CD44lo 2C (Ly5.1) and OT-I (Thy1.1) CD8+ cells were CFSE labeled and transferred into irradiated (750 cGy) B6 (top) and CD122−/− (bottom) mice (0.5–1 × 106 cells of each population per mouse). Spleen and LN cells were analyzed 7 d later by flow cytometry. Shown are CFSE profiles of gated donor SMARTA (Ly5.1+ Thy1.1−) and OT-II (Ly5.1− Thy1.1+) CD4− cells or donor 2C (Ly5.1+ Thy1.1−) and OT-I (Ly5.1− Thy1.1+) CD8+ cells. (B) FACS-sorted CD44lo HY (T3.70) or 2C (Ly5.1) CD8+ cells were CFSE labeled and transferred into CD122−/− mice (0.4–1 × 106 cells per mouse). Spleen and LN cells were analyzed 7 d later by flow cytometry. Shown are T3.70 versus CD8 profiles (top) or Ly5.1 versus CD8 profiles (bottom) of total lymphocytes (left) and CFSE profiles of gated donor HY (T3.70+ CD8+) and 2C (Ly5.1+ CD8+) cells (right). Numbers in the dot plots indicate percentages of cells in the gates. (C) Using normal B6 mice as donors, 0.5 × 106 FACS-sorted CFSE-labeled CD44lo CD8+ cells (Thy1.1) were cotransferred with either 106 CFSE-labeled CD25− CD4+ (top) or CD25+ CD4+ (T reg) cells (bottom) into CD122−/− mice. Spleen and LN cells were analyzed 3 d later by flow cytometry. Histograms show CFSE profiles (left) of gated donor CD4+ (Thy1.1+ CD8−; left) and CD8+ (Thy1.1+ CD8+; right) cells and expression of CD25 and Foxp3 (middle) on gated donor CD25− CD4+ (top) and CD25+ CD4+ cells (bottom). Bar graph shows total donor cell recoveries from the indicated hosts (mean ± SD of two mice per group).
Influence of CD25+ CD4+ T reg cells
As mentioned above, CD122−/− mice show prominent lymphadenopathy and splenomegaly. These mice lack typical CD25+ Foxp3+ CD4+ T reg cells (31), presumably because these IL-2–responsive cells cannot respond to IL-2 in the host environment. Hence, the intense proliferation of normal T cells in CD122−/− hosts might reflect the absence of T reg cells. To investigate this possibility, small numbers of CD44lo B6 CD8+ cells were transferred to CD122−/− hosts together with a twofold higher dose of CD25+ CD4+ cells versus CD25− CD4+ cells. Notably, proliferation of the donor CD8+ cells measured on day 3 was as high with cotransfer of donor CD25+ CD4+ cells as with CD25− CD4+ cells (Fig. 2 C). This lack of inhibition by the T reg cells correlated with prominent proliferation of these cells, presumably because of the raised levels of IL-2 in CD122−/− mice (see below). It should be noted that the proliferating T reg cells recovered from CD122−/− hosts remained CD25+ and Foxp3+ (Fig. 2 C).
Comparison with responses to foreign antigens
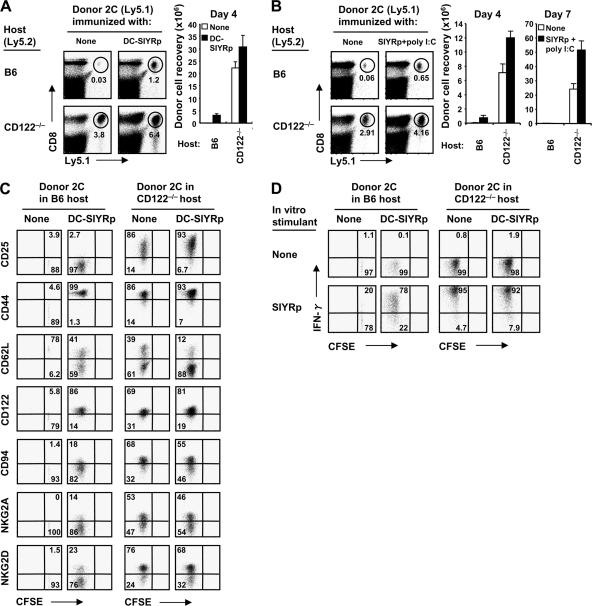
The rapid proliferation of naive CD8+ cells in CD122−/− hosts raised the question of how this type of proliferation compared with typical responses of CD8+ cells to foreign antigens. To examine this issue, we used the fact that 2C CD8+ cells give strong responses to Kb-restricted 8-mer SIYR peptide (SIYRp) in B6 hosts (32). When naive 2C cells were transferred to nonirradiated B6 mice together with SIYR-loaded syngenic BM-derived DCs, total yields of the donor T cells from spleen on day 4 were approximately fourfold higher than the number initially injected (Fig. 3 A). Similar findings occurred when 2C cells were transferred to B6 mice plus soluble SIYRp and poly I:C as adjuvant (Fig. 3 B). In contrast, when 2C cells were transferred to CD122−/− mice without SIYRp, yields of donor 2C cells on day 4 were much greater, i.e., 20–30-fold higher than the number initially injected (Fig. 3, A and B). Yields were further improved mildly (by 20–50%) when 2C cells were cotransferred with SIYRp to CD122−/− hosts (Fig. 3, A and B). In these hosts, the strong expression of activation markers and NK cell markers on the donor cells was largely unaffected by the presence of SIYRp (Fig. 3 C). Responses to peptide in B6 hosts, in contrast, led to only limited expression of activation and NK cell markers. In terms of effector function, IFN-γ synthesis after TCR ligation in vitro was stronger for 2C cells transferred to CD122−/− hosts with or without peptide than for 2C cells exposed to peptide in B6 hosts (Fig. 3 D). Collectively, these findings suggest that the “antigen-independent” proliferative response of naive CD8+ cells in CD122−/− hosts was as, or more, intense than the response to specific antigen in normal B6 hosts.
Figure 3.
Comparison with responses to foreign antigens. 106 MACS-purified CFSE-labeled (A) or 0.5 × 106 FACS-sorted unlabeled (B) CD44lo 2C cells (Ly5.1) were transferred into normal B6 (top) and CD122−/− (bottom) mice and were either unimmunized (left) or immunized (right) with either 2 × 106 BM-derived DCs pulsed with SIYRp (DC-SIYRp; A) or with soluble SIYRp plus poly I:C (B), as described in Materials and methods. Spleen and LN cells were analyzed on day 4 (A) and days 4 and 7 (B) by flow cytometry. Dot plots in A and B show Ly5.1 versus CD8 profiles of total lymphocytes on day 4 in the indicated hosts, and numbers in the plots indicate percentages of cells in the gates. Bar graphs show total recoveries of donor 2C (Ly5.1+ CD8+) cells on day 4 (A) and days 4 and 7 (B) from the indicated hosts (mean ± SD of two to three mice per group). (C and D) Expression of various surface markers (C) and intracellular staining of IFN-γ production (D) on gated donor 2C cells from B6 (left) versus CD122−/− (right) mice shown in A. IFN-γ production was measured by 5-h in vitro stimulation without (top) or with (bottom) SIYRp, as described in Materials and methods. Numbers in the dot plots indicate percentages of cells in the quadrants.
Stimuli for proliferation
Because CD122−/− mice contain large numbers of activated host cells, the marked proliferation of donor T cells in these mice might reflect exposure to high levels of proinflammatory cytokines. This possibility is unlikely, however, because proliferation of donor T cells was also conspicuous (though slightly reduced) in 2C or OT-I mice crossed to a CD122−/− background (Fig. S4, A and B, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1); these mice display minimal lymphadenopathy, and nearly all of their T cells are CD44lo. For the experiment shown, we used a mixture of either allotype-marked CD44lo and CD44hi CD8+ cells (for 2C/CD122−/− hosts) or CD44lo CD4+ and CD8+ cells (for OT-I/CD122−/− hosts) as donor cells.
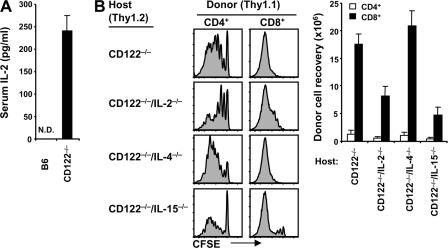
With regard to other stimuli, serum levels of IL-2 were undetectable in normal B6 mice but were very high, ∼200 pg/ml, in CD122−/− mice (Fig. 4 A). Because CD122/γc is the receptor for IL-15 as well as IL-2, one would expect both cytokines to be elevated in CD122−/− mice. However, we were unable to detect IL-15 in serum (unpublished data), which is in line with the recent view that, for the most part, IL-15 is not secreted but is held on the cell surface bound to IL-15Rα (33). Hence, the failure to detect IL-15 in serum does not exclude the possibility that CD122−/− mice have high levels of cell-associated IL-15.
Figure 4.
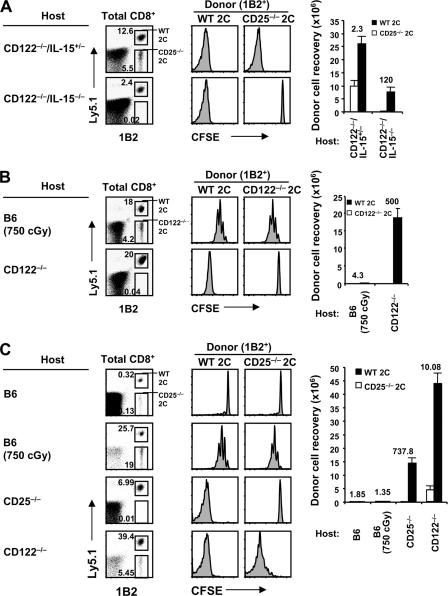
Cytokines as a stimulus in CD122−/− mice. (A) Serum IL-2 protein levels were tested from normal B6 versus CD122−/− mice by ELISA, as described in Materials and methods. Means ± SD of three to five mice per group are shown. N.D., not detected. (B) A mixture of FACS-sorted CD44lo CD4+ and CD8+ cells (Thy1.1) was CFSE labeled and transferred into CD122−/−, CD122−/−/IL-2−/−, CD122−/−/IL-4−/−, and CD122−/−/IL-15−/− mice (106 cells of each subset per mouse). Spleen and LN cells were analyzed 4 d later by flow cytometry. Shown are CFSE profiles (left) of gated donor CD4+ (Thy1.1+ CD8−; left) and CD8+ (Thy1.1+ CD8+; right) cells and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two to three mice per group).
To assess the stimulatory roles of IL-2 and IL-15 in CD122−/− mice, we examined the proliferation of naive B6 T cells in CD122−/− mice crossed to an IL-2−/−, IL-4−/−, or IL-15−/− background. The notable finding in this instance was that, both for donor CD4+ and CD8+ cells, total recoveries of the proliferating cells were two- to threefold lower in combined CD122−/− IL-2−/− and CD122−/− IL-15−/− hosts than in CD122−/− IL-4−/− or CD122−/− mice, suggesting that proliferation was driven by a combination of IL-2 and IL-15 (Fig. 4 B). To test this possibility directly, we examined the proliferation of 2C CD8+ cells crossed to a CD25−/− (IL-2Rα−/−) background, thereby limiting the response of the cells to IL-2 but preserving reactivity to IL-15. When these cells were cotransferred at a 1:1 ratio with allotype (Ly5.1)-marked WT 2C CD8+ cells, proliferation of the TCR clonotype-positive (1B2+) 2C cells in CD122−/− IL-15+/− hosts was modestly skewed to WT 2C cells, implicating IL-2 as a stimulus (Fig. 5 A). In contrast, proliferation was clearly detectable (though reduced) for WT 2C cells but was totally absent for CD25−/− 2C cells in CD122−/− IL-15−/− hosts (Fig. 5 A), thus implicating IL-15.
Figure 5.
Contribution of IL-2 and IL-15 as stimuli in CD122−/− mice. (A) A mixture of FACS-sorted CD44lo WT 2C (Ly5.1, 1B2+) and CD25−/− 2C (Ly5.2, 1B2+) CD8+ cells was CFSE labeled and transferred into CD122−/−/IL-15+/− (top) and CD122−/−/IL-15−/− (bottom) mice (0.5 × 106 cells of each population per mouse). Spleen and LN cells were analyzed 5 d later by flow cytometry. Shown are Ly5.1 and 1B2 profiles (left) of gated total CD8+ cells, CFSE profiles (middle) of gated donor WT (1B2+ Ly5.1+; left) versus CD25−/− (1B2+ Ly5.1−; right) 2C CD8+ cells, and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two mice per group). (B) A mixture of FACS-sorted CD44lo WT (Ly5.1) and CD122−/− 2C CD8+ cells (Ly5.2) was CFSE labeled and transferred into irradiated (750 cGy) B6 (top) and CD122−/− (bottom) mice (0.5 × 106 cells of each population per mouse). Spleen and LN cells were analyzed 5 d later by flow cytometry. Shown are Ly5.1 and 1B2 profiles (left) of gated total CD8+ cells, CFSE profiles (middle) of gated donor WT (left) versus CD122−/− 2C CD8+ (right) cells, and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two to three mice per group). (C) A mixture of FACS-sorted CD44lo WT (Ly5.1) and CD25−/− 2C CD8+ cells (Ly5.2) was CFSE labeled and transferred into normal B6, irradiated (750 cGy) B6, CD25−/−, and CD122−/− mice (0.5 × 106 cells of each population per mouse). Spleen and LN cells were analyzed 7 d later by flow cytometry. Shown are Ly5.1 and 1B2 profiles (left) of gated total CD8+ cells, CFSE profiles (middle) of gated donor WT (left) versus CD25−/− 2C CD8+ (right) cells, and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two to three mice per group). Numbers in the dot plots and bar graph indicate percentages of cells in the gates and the ratio of WT to either CD25−/− (A and C) or CD122−/− (B) 2C CD8+ cells recovered.
These findings provide strong support for the view that the proliferation of naive CD8+ cells in CD122−/− hosts reflected joint contact with IL-2 and IL-15. Additional support for this conclusion was obtained by backcrossing 2C mice to a CD122−/− background, thus causing unresponsiveness of 2C cells to both cytokines. Cotransfer studies with WT 2C cells showed that naive CD122−/− 2C CD8+ cells were totally unable to proliferate in CD122−/− hosts (Fig. 5 B). However, these cells retained the capacity to proliferate in irradiated B6 mice, i.e., in a situation where proliferation is driven largely by a different cytokine, IL-7 (Fig. 5 B).
Proliferation in CD25−/− hosts
Like CD122−/− mice, CD25−/− mice have lymphadenopathy and a paucity of T reg cells, reflecting unresponsiveness to IL-2 (34, 35). CD25−/− mice also resemble CD122−/− mice in having raised serum levels of IL-2 of around 100 pg/ml (references (36, 37) and data not shown). However, because CD25 expression does not influence responsiveness to IL-15, levels of IL-15 are presumably normal in CD25−/− mice. Notably, as in CD122−/− hosts, strong proliferation of normal B6 and 2C CD8+ cells occurred after transfer to CD25−/− hosts (Fig. 5 C and not depicted). In the experiment shown, a 1:1 mixture of naive WT 2C and CD25−/− 2C CD8+ cells was injected. These two populations expanded in parallel in irradiated B6 mice, presumably driven by IL-7. In contrast, there was marked expansion of WT 2C cells but no proliferation of CD25−/− 2C cells in CD25−/− hosts. Confirming earlier findings (see above), the CD25−/− 2C cells proliferated well in CD122−/− hosts (though not as vigorously as WT 2C cells), presumably in response to IL-15. These data indicate that the environment of CD25−/− mice was strongly stimulatory for naive CD8+ cells. The finding that proliferation in these hosts was high with normal T cells but undetectable with CD25−/− T cells implies that proliferation was selectively driven by raised levels of IL-2.
T cell proliferation in response to IL-2–IL-2 mAb complexes
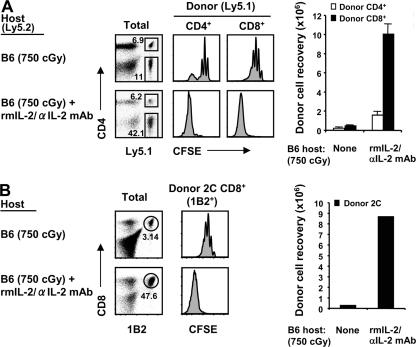
The data with CD122−/− and CD25−/− mice as hosts suggest that exposing normal T cells to a high concentration of IL-2, IL-15, or both induced massive expansion of naive CD8+ cells, as well as modest expansion of naive CD4+ cells. To seek further support for the role of IL-2, we studied T cell proliferation in normal B6 mice injected with IL-2–IL-2 mAb complexes. As previously shown, the biological activity of IL-2 in vivo is greatly increased by association with IL-2 mAbs (38). When normal T cells were transferred to normal B6 hosts, injection of IL-2–IL-2 mAb complexes led to marked proliferation of donor CD44hi CD8+ cells (which have high levels of CD122) but only very limited proliferation of CD44lo CD8+ cells, presumably because of strong competition and IL-2 consumption by the expanded host CD44hi CD8+ cells (38). To avoid the problem of IL-2 consumption by host cells, the following experiments used irradiated B6 mice as hosts.
When naive T cells were transferred to irradiated (750 cGy) B6 hosts followed by daily injections of IL-2–IL-2 mAb complexes, the pattern of donor T cell proliferation and differentiation into CD44hi cells was quite similar to that seen in CD122−/− hosts (Fig. 6 A and not depicted). Thus, there was conspicuous proliferation of naive CD8+ cells, with total recoveries of these cells on day 5 being ∼5-fold higher than the number initially injected and ∼20-fold higher than in hosts not injected with IL-2–IL-2 mAb. There was also considerable proliferation of naive CD4+ cells, though total yields of these cells were much less than for CD8+ cells (Fig. 6 A). Likewise, for CD8+ cells, proliferation elicited by IL-2–IL-2 mAb complexes applied to TCR Tg cells, i.e., to 2C and OT-I (Fig. 6 B and not depicted), as well as to polyclonal B6 cells. As for polyclonal B6 T cells, proliferation of naive CD44lo 2C TCR Tg CD8+ cells elicited by IL-2–IL-2 mAb complexes was intense, and total yields of donor cells were 20–30-fold higher than in irradiated mice not given these complexes (Fig. 6 B). As in CD122−/− hosts, the proliferating cells became CD44hi, up-regulated NK markers (NKG2A), and synthesized granzyme B (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1).
Figure 6.
Proliferation of naive T cells in response to IL-2–IL-2 mAb complexes. (A) A mixture of FACS-sorted CD44lo CD4+ and CD8+ cells (Ly5.1) was CFSE labeled and transferred into irradiated (750 cGy) B6 mice (1.5 × 106 cells of each subset per mouse). Host mice were either uninjected (top) or injected i.p. (bottom) daily for four consecutive days with IL-2–IL-2 mAb complexes, as described in Materials and methods. Spleen and LN cells were analyzed 1 d later (on day 5) by flow cytometry. Shown are Ly5.1 versus CD4 profiles (left) of total lymphocytes, CFSE profiles (middle) of gated donor CD4+ (Ly5.1+ CD4+; left) and CD8+ (Ly5.1+ CD4−; right) cells, and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of three mice per group). (B) 1–1.5 × 106 MACS-purified CFSE-labeled CD44lo 2C CD8+ cells (1B2+) were transferred into irradiated (750 cGy) B6 mice. Host mice were either untreated (top) or treated (bottom) with IL-2–IL-2 mAb complexes, as in A. Spleen and LN cells were analyzed on day 5 by flow cyto metry. Shown are 1B2 versus CD8 profiles (left) of total lymphocytes, CFSE profiles (middle) of gated donor 2C (1B2+ CD8+) cells, and total donor cell recoveries from the indicated hosts (bar graph; one representative of at least three independent experiments). Numbers in the dot plots indicate percentages of cells in the gates.
Role of MHC-I ligands in proliferation
Except for the marked difference in the rate of proliferation, the IL-2/IL-15–induced proliferation of naive T cells described in this paper closely resembled typical homeostatic proliferation in T cell–depleted hosts. In particular, proliferation was more prominent for CD8+ cells than CD4+ cells, and there was a similar hierarchy in the response of T cells from various TCR Tg lines. Because typical slow IL-7–dependent homeostatic proliferation requires TCR–MHC interaction, one might expect this requirement to also apply to IL-2/IL-15–driven proliferation.
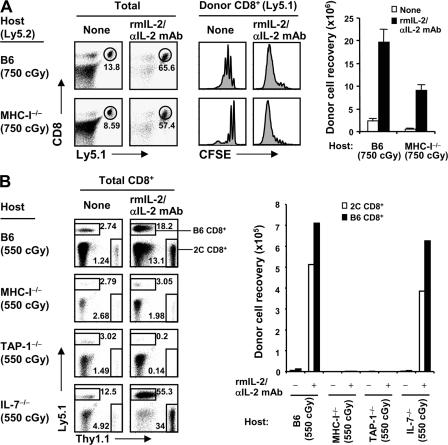
To test this possibility, we used combined H-2Kb−/− Db−/− β2M−/− (MHC-I−/−) mice on a B6 background as hosts. In initial experiments, we injected an intermediate dose of 2–3 × 106 B6 CD8+ cells into B6 mice given 750 cGy (the dose of irradiation used above), followed by IL-2–IL-2 mAb injection. Contrary to our expectations, donor cell proliferation was almost as intense in MHC-I−/− hosts as in normal B6 hosts (Fig. 7 A); similar findings applied to the injection of naive 2C CD8+ cells (not depicted). One explanation for this surprising finding is that, in MHC-I−/− hosts, the combined effects of heavy irradiation and the injection of relatively large numbers of T cells caused crowding of the donor T cells, thus leading to T–T interaction and TCR recognition of MHC-I ligands on neighboring donor T cells.
Figure 7.
MHC-I requirement for IL-2–IL-2 mAb–driven proliferation. (A) FACS-sorted CD44lo CD8+ cells (Ly5.1) were CFSE labeled and transferred into irradiated (750 cGy) B6 (top) and MHC-I−/− (bottom) mice (2.5–3 × 106 cells per mouse). Host mice were either uninjected or injected with IL-2–IL-2 mAb complexes, as in Fig. 6. Spleen and LN cells were analyzed on day 5 by flow cytometry. Shown are Ly5.1 versus CD8 profiles (left) of total lymphocytes, CFSE profiles (middle) of gated donor CD8+ (Ly5.1+ CD8+) cells, and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two mice per group). (B) A mixture of FACS-sorted CD44lo B6 (Ly5.1) and 2C CD8+ cells (Thy1.1) was transferred into various irradiated (550 cGy) B6, MHC-I−/−, TAP-1−/−, and IL-7−/− mice (105 cells of each population per mouse), followed either by no treatment or injection of IL-2–IL-2 mAb complexes, as in Fig. 6. Spleen and LN cells were analyzed on day 7 by flow cytometry. Shown are Thy1.1 versus Ly5.1 profiles (left) of total CD8+ cells and total recoveries of donor B6 (Ly5.1+ Thy1.1−) and 2C (Ly5.1− Thy1.1+) CD8+ cells from the indicated hosts (bar graph; one representative of two independent experiments). Numbers in the dot plots indicate percentages of cells in the gates.
To assess this possibility, we first injected a similar number of 2 × 106 CD8+ cells into MHC-I−/− hosts given a lower dose (550 cGy) of irradiation, thus increasing competition from radioresistant host MHC-I−/− cells in the T cell areas. In this situation, there was still proliferation of the donor CD8+ cells (a mixture of 2C and B6 cells in the experiment shown), but total yields of donor cells were considerably reduced (Fig. S6, available at http://www.jem.org/cgi/content/full/jem.20070740/DC1). We then examined the combined effects of using the low dose of 550 cGy and reducing the numbers of donor T cells by 10-fold to 2 × 105 (Fig. 7 B). Notably, under these conditions, donor naive CD8+ cell proliferation in MHC-I−/− mice, as well as in TAP-1−/− mice, was minimal, and total cell recoveries were very low (Fig. 7 B). This finding applied to both normal B6 and 2C CD8+ cells, which were coinjected at a 1:1 ratio in the experiment shown; CFSE profiles were poorly resolved in Fig. 7 B, but other experiments showed that donor CD8+ cell proliferation was barely detectable in both MHC-I−/− and TAP-1−/− hosts (Fig. S7 A). Control studies showed that strong proliferation in response to IL-2–IL-2 mAb occurred in irradiated IL-7−/− mice, implying little, if any, influence of host IL-7 (Fig. 7 B and Fig. S7 B); note that the stronger proliferation in irradiated IL-7−/− than B6 hosts probably reflected the paucity of T cells in IL-7−/− mice and, therefore, less competition from host cells. Confirming previous findings (6–8, 17), slow homeostatic proliferation of CD8+ cells in irradiated hosts not given IL-2–IL-2 mAb was abolished in MHC-I−/− hosts, as well as in IL-7−/− hosts (Fig. 7 B and Fig. S7 B).
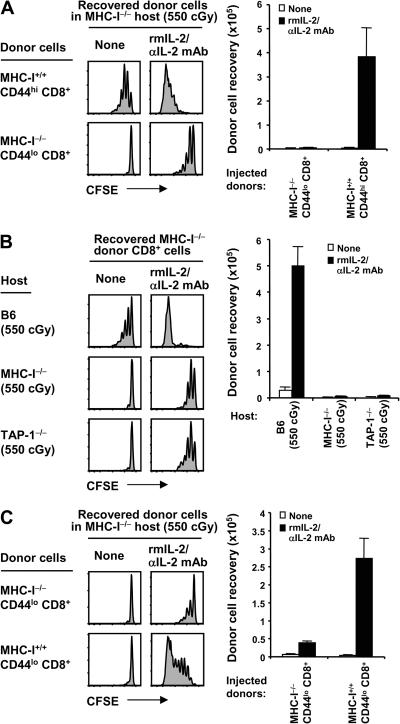
Despite prior irradiation of the hosts, it was conceivable that transferring normal T cells to MHC-I−/− hosts led to a host-versus-graft reaction directed to donor MHC-I. This possibility seemed unlikely, because unlike naive cells, normal MP CD44hi CD8+ cells proliferated well to IL-2–IL-2 mAb in MHC-I−/− hosts (Fig. 8 A). Nevertheless, to eliminate the possibility of rejection, we generated BM chimeras by reconstituted heavily irradiated B6 mice with MHC-I−/− BM. Doses of 2 × 105 naive CD44lo CD8+ T cells from these chimeras were then exposed to IL-2–IL-2 mAb on adoptive transfer. Unexpectedly, there was a small degree of proliferation of donor MHC-I−/− CD8+ cells in both MHC-I−/− and TAP-1−/− hosts (Fig. 8, A and B). However, proliferation was far less than in B6 hosts, and cell recoveries were very low. With a higher dose of 2 × 106 donor CD8+ cells, IL-2–induced proliferation in MHC-I−/− hosts was ∼10-fold higher with MHC-I+/+ donor cells than with MHC-I−/− cells (Fig. 8 C), further supporting the view that T cell crowding leads to TCR–MHC-I interaction via T–T interaction (see above). Collectively, the data indicate that proliferation of naive CD8+ cells induced by IL-2–IL-2 mAb injection is strongly MHC-I dependent.
Figure 8.
Response of MHC-I−/− CD8+ cells to IL-2–IL-2 mAb in MHC-I−/− hosts. (A) MHC-I+/+ CD44hi (Ly5.1; top) or MHC-I−/− CD44lo (Thy1.2; bottom) CD8+ cells were purified by FACS sorting from normal B6.SJL and MHC-I−/− BM→B6 chimeras, as described in Materials and methods, respectively, and transferred into irradiated (550 cGy) MHC-I−/− mice (2 × 105 cells per mouse). Host mice were either uninjected or injected with IL-2–IL-2 mAb complexes, as in Fig. 6. Spleen and LN cells were analyzed on day 5 by flow cytometry. Shown are CFSE profiles (left) of gated donor MHC-I+/+ CD44hi CD8+ (Ly5.1+ Kb+ Db+ CD8+; top) and MHC-I−/− CD44lo CD8+ (Thy1.2+ CFSE+ Kb− Db− CD8+; bottom) cells and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two mice per group). (B) FACS-sorted MHC-I−/− CD44lo CD8+ cells were obtained as in A, CFSE labeled, transferred into irradiated (550 cGy) B6.PL (Thy1.1; top), MHC-I−/− (middle), and TAP-1−/− mice (bottom; 2 × 105 cells per mouse), and followed either by no treatment or injection of IL-2–IL-2 mAb complexes, as in Fig. 6. Host mice were also treated with anti-NK1.1 mAb (days −1, 0, 2, and 4), as described in Materials and methods. Spleen and LN cells were analyzed on day 5 by flow cytometry. Shown are CFSE profiles (left) of gated donor MHC-I−/− CD44lo CD8+ cells (Thy1.2+ Kb− Db− CD8+ for B6.PL hosts; Thy1.2+ CFSE+ Kb− Db− CD8+ for both MHC-I−/− and TAP-1−/− hosts) and total donor cell recoveries (bar graph; mean ± SD of two mice per group). (C) MHC-I−/− CD44lo (Thy1.2; top) or MHC-I+/+ CD44lo (Ly5.1; bottom) CD8+ cells were purified by FACS sorting from MHC-I−/− BM→B6 chimeras and normal B6.SJL mice, respectively, and transferred into irradiated (550 cGy) MHC-I−/− mice (2 × 106 cells per mouse). Host mice were either uninjected or injected with IL-2–L-2 mAb complexes, as in Fig. 6. Spleen and LN cells were analyzed on day 5 by flow cytometry. Shown are CFSE profiles (left) of gated donor MHC-I+/+ CD44lo CD8+ (Ly5.1+ Kb+ Db+ CD8+; bottom) and MHC-I−/− CD44lo CD8+ (Thy1.2+ CFSE+ Kb− Db− CD8+; top) cells and total donor cell recoveries from the indicated hosts (bar graph; mean ± SD of two mice per group).
DISCUSSION
The strong stimulatory effects of IL-2 on naive T cells described in this paper stemmed from the initial observation that normal T cells underwent massive expansion after transfer to CD122−/− hosts. This finding might not seem surprising, because CD122−/− mice have prominent lymphadenopathy and most of their T cells are in an activated state (20). So, stimulation of both donor and host T cells could be attributed to some form of “cytokine storm,” perhaps reflecting the lack of T reg cells in CD122−/− mice (31). In this case, one can consider two scenarios. The simplest idea is that T reg cells act by inhibiting responses to self-components, the florid proliferation of host (and donor) T cells in CD122−/− mice reflecting unrestrained antiself-responses. Therefore, our expectation was that the proliferation of donor T cells in CD122−/− hosts would be substantially reduced by cotransfer of normal T reg cells. Surprisingly, this was not the case. Thus, proliferation of donor CD8+ cells in CD122−/− mice was as rapid with cotransfer of donor T reg cells as with transfer of control normal CD25− CD4+ cells, even at a high (2:1) ratio of CD4+ to CD8+ cells.
The second scenario is that the lack of T reg cells and other CD25+ (IL-2Rα+) cells in CD122−/− mice leads to a failure to use IL-2, causing host IL-2 levels to rise and thereby provide a powerful stimulus for IL-2–responsive donor T cells. In fact, serum IL-2 levels in CD122−/− mice were found to be markedly elevated, presumably reflecting the combined effects of the lack of IL-2Rβ (CD122) expression by host cells plus strong IL-2 synthesis as a by-product of the antiself-response; high IL-2 levels may also reflect lack of feedback inhibition of IL-2 synthesis by IL-2 (39). Conceivably, high IL-2 expression could explain the apparent dysfunction of normal T reg cells transferred to CD122−/− hosts. Further studies will be needed to answer this question.
The presence of high levels of IL-2 in CD122−/− mice did not necessarily indicate that donor cell proliferation was elicited by IL-2 by itself. IL-2 was clearly important, because proliferation was reduced with the transfer of CD25−/− T cells to CD122−/− mice and also with the transfer of normal T cells to combined CD122−/− IL-2−/− hosts. Though reduced, proliferation in these situations was still substantial, however, implicating other cytokines. IL-15 was a logical possibility because CD122 is a known receptor for IL-15 and IL-2 (40–45). Strong support for this idea came from the finding that donor T cell proliferation was even lower in CD122−/− IL-15−/− mice than in CD122−/− IL-2−/− hosts. The key finding, however, was that proliferation was undetectable with the transfer of CD122−/− T cells, i.e., T cells that were unresponsive to both IL-2 and IL-15; in this case, to avoid T cell hyperplasia, we used naive 2C CD8+ cells crossed to a CD122−/− background. Collectively, these findings indicate that donor T cell proliferation in CD122−/− hosts is limited to just two cytokines, IL-2 and IL-15. The prominent role of IL-15 is surprising, because like others (33, 46, 47), we failed to detect IL-15 in the serum of CD122−/− hosts. Hence, IL-15 presumably accumulated in these mice in cell-associated rather than soluble form. It should be noted that strong proliferation of naive CD8+ cells also occurred in CD25−/− hosts. These mice resemble CD122−/− mice in having raised levels of IL-2 (36, 37), but IL-15 levels are probably normal. This follows from the finding that CD25−/− 2C CD8+ cells proliferated only in CD122−/− and not CD25−/− hosts.
The conclusion that naive donor cell proliferation in CD122−/− hosts is driven by a mixture of IL-2 and IL-15 predicts that these cytokines would have comparable effects on T cells in normal mice. For IL-15, this is indeed the case. Thus, when complexed with soluble IL-15Rα, IL-15 was recently found to be stimulatory for naive T cells (CD8+ cells) in vivo, as well as for MP cells (48, 49). Stimulation of naive CD8+ cells was also seen with the injection of IL-2, in the form of IL-2–IL-2 mAb complexes (38). However, such proliferation was very limited and dwarfed by the massive proliferation of MP cells. These findings applied in normal mice as hosts, and as shown here, quite different results occurred with irradiated hosts. In these hosts, IL-2–IL-2 mAb injection elicited strong proliferation of naive T cells, presumably reflecting reduced consumption of IL-2 by host cells.
Both with IL-2 injection and cell transfer to CD122−/− hosts, proliferation of naive T cells applied to both CD4+ and CD8+ cells but was heavily skewed to CD8+ cells. In part, this finding can be attributed to appreciably higher expression of CD122 (at the intermediate rather than high level) on CD8+ cells than CD4+ cells (50). However, it also appears that CD8+ cells are intrinsically more sensitive to cytokines than CD4+ cells. This is apparent from several findings, including the faster tempo of proliferation of CD8+ than CD4+ cells for lymphopenia-induced homeostatic proliferation (14–17, 29) and the marked overrepresentation of CD8+ cells in IL-7 Tg mice (12, 51) and suppressor of cytokine signaling–1−/− mice (52, 53).
IL-2/IL-15–mediated stimulation of naive CD8+ cells in vivo was strikingly rapid and was particularly prominent in CD122−/− hosts. In this case, even small numbers of donor cells proliferated extensively for many days and outnumbered host cells within 1–2 wk. Based on studies with TCR Tg cells, the tempo of proliferation in CD122−/− hosts was as high or higher than the response of naive T cells to foreign antigen (through definitive data would require studies on responses to infectious agents). Also, as with responses to foreign antigens, proliferation driven by IL-2/IL-15 led to overt activation and differentiation of naive CD8+ cells into effector cells. A key issue is whether the cells were stimulated solely by cytokines or, as for lymphopenia-induced proliferation, required a combination of cytokines and a TCR signal. TCR recognition of foreign antigens was unlikely, because proliferation applied to naive T cells from several TCR Tg lines. It is notable that proliferation was far weaker, though detectable, with T3.70+ CD8+ T cells from the HY TCR Tg line. This line is unique in being unable to undergo lymphopenia-induced proliferation (19, 28, 29), which is presumed to reflect that, unlike OT-I and 2C CD8+ cells, HY CD8+ cells have below-average TCR affinity for self–MHC-I ligands (19) and/or low “TCR promiscuity” (54).
The different rates of proliferation of various TCR Tg cells in CD122−/− hosts provided strong, if indirect, support for the notion that proliferation of naive donor CD8+ cells in CD122−/− hosts is MHC-I dependent. In favor of this idea, preliminary experiments still in progress showed that proliferation of donor CD8+ cells was virtually abolished in combined CD122−/− TAP-1−/− mice (unpublished data). Similar findings applied to proliferation induced by IL-2–IL-2 mAb complexes, though these results critically depended on the dose of T cells injected. With transfer of small numbers of CD8+ cells, IL-2–IL-2 mAb–induced proliferation was barely detectable in both MHC-I−/− and TAP-1−/− hosts, thus clearly implicating a requirement for TCR–MHC-I interaction. Surprisingly, however, proliferation in these hosts was quite high when large numbers of T cells were transferred. A likely explanation for this finding is that crowding of the donor T cells in the host lymphoid tissues facilitated T–T interaction, thus leading to significant TCR contact with MHC-I on neighboring T cells. This problem was avoided by generating MHC-I−/− CD8+ cells in MHC-I−/− BM→B6 chimeras. With these cells, IL-2–IL-2 mAb–induced proliferation of naive CD8+ cells was far lower in MHC-I−/− and TAP-1−/− hosts than normal B6 hosts.
Collectively, the data make a strong case that stimulation of naive CD8+ cells by IL-2 (and probably IL-15 as well) in vivo is MHC-I dependent. Nevertheless, it should be noted that, even with MHC-I−/− T cells, naive CD8+ cells did divide several times when exposed to IL-2–IL-2 mAb in an MHC-I−/− environment. Whether this finding indicates that a portion of the response of naive CD8+ cells to IL-2 is totally MHC-I independent or has other explanations remains to be elucidated.
The biological significance of IL-2/IL-15–induced proliferation of naive CD8+ cells in vivo is a matter for speculation. In certain respects, this form of proliferation is similar to lymphopenia-induced proliferation, notably with regard to (a) the requirement for TCR interaction with self-ligands plus contact with a γc cytokine (IL-7 vs. IL-2/IL-15), (b) a hierarchy in the response of TCR Tg T cells, and (c) preferential stimulation of CD8+ cells over CD4+ cells. In fact, the only major difference is in the intensity of the response: lymphopenia-induced proliferation is typically slow, whereas the IL-2–induced proliferation described is very rapid and leads to overt T cell activation. This difference may reflect that the cytokines concerned, IL-7 and IL-2, have essentially different functions. Thus, at least in mice, IL-7 acts largely by maintaining cell survival and is much weaker than IL-2 in inducing T cell proliferation (38, 43–45, 55, 56).
With regard to physiological importance, lymphopenia-induced proliferation is clearly practical whenever T cell numbers are low, e.g., in the neonatal period or after treatment of adults with cytotoxic drugs. Under these conditions, raised levels of IL-7 cause T cell numbers to expand gradually. For the rapid type of proliferation described in this study, however, whether the marked elevation of IL-2 (and IL-15) found in CD122−/− mice ever occurs naturally is doubtful. This issue raises the question of how and why naive T cells gradually differentiate into MP cells, with the latter becoming a dominant population in old age. The prevailing view is that this transition reflects a lifetime of exposure to various environmental antigens. However, it is striking that, at least in young animals, the proportion of MP cells in germ-free and “antigen-free” mice is not obviously lower than in normal mice (57–59). Hence, most of the MP cells in normal mice are presumably stimulated by self-ligands rather than foreign antigens. In neonatal life, this transition could be mediated largely by IL-7 during the period of lymphopenia when T cells are just beginning to be exported from the thymus. But how can one account for the transition of naive T cells into MP cells in adult life? At this stage, lymphopenia-induced proliferation via IL-7 seems unlikely. The possibility we are exploring is that the naive→MP cell transition in adults is elicited by transient elevation of IL-2 and/or IL-15, and perhaps other γc cytokines synthesized during responses to various infectious agents. In these situations, one can envisage that bursts of γc cytokines released by antigen-specific T cells at the sites of infection act on bystander naive T cells: combined with continued TCR contact with self-MHC ligands, the naive T cells then divide and differentiate into MP cells. This scenario is still hypothetical, but it is of interest that, in one study, exposing TCR Tg cells to a bystander infectious agent, Mycobacterium tuberculosis, caused the T cells to divide briefly (60). Whether the cells differentiated into MP cells was not discussed.
In conclusion, we have described a novel form of MHC-I–dependent homeostatic proliferation in which exposure to high levels of IL-2 or IL-15 in vivo causes massive proliferation of naive CD8+ cells and differentiation into effector cells and memory cells. Except for the marked difference in the rate of proliferation, IL-2/IL-15–induced proliferation closely resembles typical IL-7–mediated lymphopenic proliferation. We suggest that both forms of proliferation fall into the category of “homeostatic” proliferation: each type of proliferation represents a TCR-driven response to self-components, with the quality of the response being dictated by the differing stimulatory properties of the γc cytokines concerned.
MATERIALS AND METHODS
Mice.
C57BL/6 (B6), B6.PL (Thy1.1), B6.SJL (Ly5.1), IL-4−/−, IL-2+/−, CD25+/−, CD122+/−, TAP-1−/−, and RAG−/− mice, all on a B6 background, were purchased from the Jackson Laboratory. Sources of 2C, OT-I, HY.RAG−/−, OT-II, and SMARTA TCR Tg mice were previously described (17, 19, 25, 29). Origins of IL-15−/− and MHC-I−/− mice, all on a B6 background, were previously described (11, 17). 2C.Ly5.1 (or Thy1.1), OT-I.Thy1.1, SMARTA.Ly5.1, and OT-II.Thy1.1 mice were generated by being crossed to B6.SJL or B6.PL mice. IL-2−/−, CD25−/−, and CD122−/− mice were obtained from heterozygote breeders and screened by PCR methods using a primer provided by the Jackson Laboratory. 2C/CD25−/−, 2C/CD122−/−, and OT-I/CD122−/− mice were made from heterozygote breeders (2C/CD25+/−, 2C/CD122+/−, and OT-I/CD122+/− mice, respectively). CD122−/−/IL-2−/−, CD122−/−/IL-4−/−, and CD122−/−/IL-15−/− mice were generated from heterozygote breeders (CD122+/−/IL-2+/−, CD122+/−/IL-4+/−, and CD122+/−/IL-15+/− mice, respectively). All mice were maintained under specific pathogen-free conditions and, unless otherwise described, used at 4–6 wk of age. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at the Scripps Research Institute and the Animal Ethics and Experimentation Committee at Garvan Institute of Medical Research.
T cell purification and adoptive transfer.
Polyclonal naive CD4+ and CD8+ cells used for adoptive transfer experiments were purified from pooled (inguinal, axillary, lateral axillary, cervical, mesenteric, and periarterial) LN by cell sorting. In brief, LN cells were first enriched for CD3+ T cells by using a mouse T cell enrichment column (R&D Systems). Enriched T cells were labeled with antibodies and further purified by cell sorting for obtaining CD4+ CD44lo and CD8+ CD44lo T cells. In some experiments, a similar protocol was used to isolate CD4+ CD44hi, CD8+ CD44hi, CD25− CD4+, and CD25+ CD4+ T cells. All cells described were isolated from normal young (4–6 wk of age, for naive T cells) or old (6–8 mo of age, for MP T cells) B6, B6.PL, or B6.SJL mice, as indicated in the figures. CD44lo TCR Tg cells (OT-II, SMARTA, HY. RAG−/−, 2C, and OT-I) were isolated by direct cell sorting without prior enrichment steps. For the experiments using HY, CD8+ cells from female HY.RAG−/− mice were used as donors and transferred into female recipients, as indicated in the figures. Cell sorting was performed using a FACSAria (BD Biosciences). The purity of sorted cells was routinely tested after sorting and was >98%. In some experiments, naive 2C T cells were purified by MACS using a CD8+ T cell isolation kit (Miltenyi Biotec), according to the manufacturer's instructions, and, for deleting CD44hi cells, were further selected by MACS using pretitrated amount of biotin-conjugated anti-CD44 mAb plus antibiotin microbeads (Miltenyi Biotec). For adoptive transfer experiments, unless otherwise stated, purified T cells were labeled with 2.5–5 μM CFSE (Invitrogen) as previously described (11). Various numbers of unlabeled or CFSE-labeled purified T cells were transferred i.v. into different mice, as indicated in the figures. In some experiments, normal B6 mice given 750 cGy of whole-body irradiation 1 d before adoptive transfer were used as hosts. At the time points indicated in the figures, spleen and LN cells were analyzed with various fluorochrome-conjugated antibodies by flow cytometry, as described in the following section.
Antibodies and flow cytometry.
Cell suspensions of spleen and LN were prepared according to standard protocols and stained for FACS analysis using PBS containing 1% FCS, 2 mM EDTA, and 0.05% sodium azide with the following mAbs (obtained from eBioscience unless otherwise stated): FITC- or Alexa Fluor 405–conjugated anti-CD4 (RM4-5; BD Biosciences and Caltag Laboratories, respectively); PE-, PerCP-Cy5.5–, or allophycocyanin (APC)-conjugated anti-CD8α (53–6.7; BD Biosciences); PE-conjugated anti-CD25 (PC61.5), anti-CD69 (H1.2F3), anti-CD62L (MEL-14), anti-CD122 (TM-β1), anti-CD94 (18d3), anti-NKG2A (16a11), anti-NKG2D (A10 and CX5), anti-Foxp3 (FJK-16s), anti–granzyme B (GB11; Caltag Laboratories), and anti-HY TCR (T3.70); PE-, APC-, or Alexa Fluor 405–conjugated anti-CD44 (IM7; Caltag Laboratories); FITC-, PE-, or APC-conjugated anti-CD45.1 (A20); FITC-, PE-, or APC-conjugated anti-CD90.1 (HIS51); FITC- or biotin-conjugated anti-NKG2ACE (20d5); and biotin-conjugated 2C TCR clonotype 1B2 antibody (11). Biotin-conjugated antibodies were developed with PE- or APC-conjugated streptavidin. Intracellular granzyme B and Foxp3 staining were performed according to the manufacturer's recommendations. In brief, cells were stained first for cell surface markers, then fixed using 2% paraformaldehyde and permeabilized using saponin before intracellular staining. Flow cytometry samples were analyzed using a digital flow cytometer (LSR II; BD Biosciences). Data were analyzed using FlowJo software (TreeStar, Inc.).
In vivo antigenic stimulation and intracellular IFN-γ staining.
0.5–106 unlabeled or CFSE-labeled purified 2C CD8+ cells were injected i.v. into normal B6 and CD122−/− mice. On the same day of adoptive transfer, the mice were immunized either i.p. with 50 μM H-2Kb–restricted SIYRp plus 50 μg poly I:C or i.v. with 2 × 106 BM-derived DCs pulsed with 10 μM SIYRp; these cells were prepared by a 7-d in vitro culture of B6 BM cells with 20 ng/ml GM-CSF, as previously described (61). Spleen and LN cells were analyzed by flow cytometry at the time points indicated in the figures. For intracellular IFN-γ staining, spleen cells were plated at 106 cells per well in 96-well plates with or without 0.1 μM SIYRp and were treated with GolgiStop (BD Biosciences) during the last 3 h of culture. 5 h later, cells were stained with cell-surface markers and fixed/permeabilized using buffer (Cytofix/Cytoperm; BD Biosciences), stained with PE-conjugated anti–IFN-γ mAb (XMG1.2; eBioscience) using buffer (Perm/Wash; BD Biosciences), and analyzed by flow cytometry, as described in the previous section.
Generation of BM chimeras.
BM cells were obtained from MHC-I−/− mice (Ly5.2), and mature T cells were depleted using anti-Thy1.2 mAb (J1j) plus Low-Tox Guinea Pig Complement (Cedarlane Laboratories), as previously described (9, 11). 5 × 106 T cell–depleted MHC-I−/− BM cells were injected i.v. into irradiated (1,200 cGy) B6.SJL mice that received 100 μg anti-NK1.1 mAb (PK136) 1 d before transfer. 2 mo later, MHC-I−/− CD44lo CD8+ cells (Ly5.2) were obtained from these MHC-I−/− BM→B6 chimeras by FACS sorting with a purity of >98% (Ly5.2+ Kb− Db− CD44lo CD8+). It is noted that the levels of all tested surface markers (CD25, CD43, CD44, CD62L, CD69, CD122, CD127, and CD45RA) on MHC-I−/− CD8+ cells from these chimeras were similar to control naive B6 CD8+ cells. 2 × 105 purified MHC-I−/− CD44lo CD8+ cells per mouse were labeled with CFSE and injected i.v. into the mice indicated in the figures that were irradiated (550 cGy) 1 d before transfer. The host mice were also treated i.p. with anti-NK1.1 mAb (PK136) 1 d before transfer (day −1; 100 μg) and days 0, 2, and 4 (50 μg) thereafter.
Injection of IL-2–IL-2 mAb complexes.
IL-2–anti–IL-2 mAb complexes were prepared as previously described (38), with slight modifications. In brief, 1 μg recombinant mouse IL-2 (PeproTech) was mixed with 25 μg anti–IL-2 mAb (S4B6) purified from hybridomas, incubated for 10 min on ice and for 30 min at 37°C, and injected i.p. daily for four consecutive days into the irradiated (550–750 cGy) mice indicated in the figures that had previously received various populations of purified donor T cells.
ELISA.
Blood from 4–6-wk-old WT B6, CD122−/−, and CD25−/− mice was collected to obtain serum, which was then tested in a standard IL-2 sandwich ELISA, as previously described (9)
Online supplemental material.
Fig. S1 shows the proliferation and expansion of donor thymocytes in normal B6 versus CD122−/− hosts. Fig. S2 shows expression of various surface markers on donor CD8+ cells in normal B6 and irradiated B6 hosts versus CD122−/− hosts. Fig. S3 depicts proliferation and expansion of donor WT versus CD28−/− CD8+ cells in irradiated B6 and CD122−/− hosts. Fig. S4 shows massive donor T cell proliferation even in CD122−/− hosts on a 2C or OT-I TCR Tg background. Fig. S5 depicts activation of naive CD8+ cells by IL-2–IL-2 mAb injection in irradiated B6 hosts. Fig. S6 and Fig. S7 show the effects of large and small doses of donor T cell transfer, respectively, on MHC-I dependency of IL-2–IL-2 mAb–induced proliferation in irradiated MHC-I−/− hosts. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20070740/DC1.
Supplemental Material
Acknowledgments
We acknowledge support from the Flow Cytometry Core Facility. This work was supported by National Institutes of Health and National Health and Medical Research Council grants. O. Boyman was supported by the Swiss National Science Foundation and the Novartis Foundation.
J. Sprent, C.D. Surh, M.P. Rubinstein, and O. Boyman have submitted a patent application on the capacity of anticytokine mAbs to increase the biological activity of cytokines. This patent was based entirely on a paper published previously by these coauthors. The authors have no other conflicting financial interests.
Abbreviations used: APC, allophycocyanin; γc, common γ chain; MP, memory phenotype; MHC-I−/−, Kb−/− Db−/− β2M−/−; SIYRp, SIYR peptide; Tg, transgenic.
References
- 1.Sprent, J. 1993. Lifespans of naive, memory and effector lymphocytes. Curr. Opin. Immunol. 5:433–438. [DOI] [PubMed] [Google Scholar]
- 2.Freitas, A.A., and B.B. Rocha. 1993. Lymphocyte lifespans: homeostasis, selection and competition. Immunol. Today. 14:25–29. [DOI] [PubMed] [Google Scholar]
- 3.Tough, D.F., and J. Sprent. 1994. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanchot, C., F.A. Lemonnier, B. Perarnau, A.A. Freitas, and B. Rocha. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 276:2057–2062. [DOI] [PubMed] [Google Scholar]
- 5.Kirberg, J., A. Berns, and H. von Boehmer. 1997. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex–encoded molecules. J. Exp. Med. 186:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murali-Krishna, K., L.L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 286:1377–1381. [DOI] [PubMed] [Google Scholar]
- 7.Schluns, K.S., W.C. Kieper, S.C. Jameson, and L. Lefrancois. 2000. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1:426–432. [DOI] [PubMed] [Google Scholar]
- 8.Tan, J.T., E. Dudl, E. LeRoy, R. Murray, J. Sprent, K.I. Weinberg, and C.D. Surh. 2001. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc. Natl. Acad. Sci. USA. 98:8732–8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyman, O., J.H. Cho, J.T. Tan, C.D. Surh, and J. Sprent. 2006. A major histocompatibility complex class I–dependent subset of memory phenotype CD8+ cells. J. Exp. Med. 203:1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purton, J.F., J.T. Tan, M.P. Rubinstein, D.M. Kim, J. Sprent, and C.D. Surh. 2007. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 204:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judge, A.D., X. Zhang, H. Fujii, C.D. Surh, and J. Sprent. 2002. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196:935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kieper, W.C., J.T. Tan, B. Bondi-Boyd, L. Gapin, J. Sprent, R. Ceredig, and C.D. Surh. 2002. Overexpression of interleukin (IL)-7 leads to IL-15–independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 195:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldrath, A.W., and M.J. Bevan. 1999. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 11:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surh, C.D., and J. Sprent. 2000. Homeostatic T cell proliferation: how far can T cells be activated to self-ligands? J. Exp. Med. 192:F9–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho, B.K., V.P. Rao, Q. Ge, H.N. Eisen, and J. Chen. 2000. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 192:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stockinger, B., G. Kassiotis, and C. Bourgeois. 2004. Homeostasis and T cell regulation. Curr. Opin. Immunol. 16:775–779. [DOI] [PubMed] [Google Scholar]
- 17.Tan, J.T., B. Ernst, W.C. Kieper, E. LeRoy, J. Sprent, and C.D. Surh. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 195:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seddon, B., and R. Zamoyska. 2002. TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J. Immunol. 169:3752–3759. [DOI] [PubMed] [Google Scholar]
- 19.Kieper, W.C., J.T. Burghardt, and C.D. Surh. 2004. A role for TCR affinity in regulating naive T cell homeostasis. J. Immunol. 172:40–44. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, H. Griesser, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. [DOI] [PubMed] [Google Scholar]
- 21.Prlic, M., B.R. Blazar, A. Khoruts, T. Zell, and S.C. Jameson. 2001. Homeostatic expansion occurs independently of costimulatory signals. J. Immunol. 167:5664–5668. [DOI] [PubMed] [Google Scholar]
- 22.Hagen, K.A., C.T. Moses, E.F. Drasler, K.M. Podetz-Pedersen, S.C. Jameson, and A. Khoruts. 2004. A role for CD28 in lymphopenia-induced proliferation of CD4 T cells. J. Immunol. 173:3909–3915. [DOI] [PubMed] [Google Scholar]
- 23.Hogquist, K.A., S.C. Jameson, W.R. Heath, J.L. Howard, M.J. Bevan, and F.R. Carbone. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27. [DOI] [PubMed] [Google Scholar]
- 24.Sha, W.C., C.A. Nelson, R.D. Newberry, D.M. Kranz, J.H. Russell, and D.Y. Loh. 1988. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 335:271–274. [DOI] [PubMed] [Google Scholar]
- 25.Oxenius, A., M.F. Bachmann, R.M. Zinkernagel, and H. Hengartner. 1998. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28:390–400. [DOI] [PubMed] [Google Scholar]
- 26.Kisielow, P., H. Bluthmann, U.D. Staerz, M. Steinmetz, and H. von Boehmer. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 333:742–746. [DOI] [PubMed] [Google Scholar]
- 27.Barnden, M.J., J. Allison, W.R. Heath, and F.R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34–40. [DOI] [PubMed] [Google Scholar]
- 28.Rocha, B., and H. von Boehmer. 1991. Peripheral selection of the T cell repertoire. Science. 251:1225–1228. [DOI] [PubMed] [Google Scholar]
- 29.Ernst, B., D.S. Lee, J.M. Chang, J. Sprent, and C.D. Surh. 1999. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 11:173–181. [DOI] [PubMed] [Google Scholar]
- 30.Kondrack, R.M., J. Harbertson, J.T. Tan, M.E. McBreen, C.D. Surh, and L.M. Bradley. 2003. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek, T.R., A. Yu, V. Vincek, P. Scibelli, and L. Kong. 2002. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 17:167–178. [DOI] [PubMed] [Google Scholar]
- 32.Udaka, K., K.H. Wiesmuller, S. Kienle, G. Jung, and P. Walden. 1996. Self-MHC-restricted peptides recognized by an alloreactive T lymphocyte clone. J. Immunol. 157:670–678. [PubMed] [Google Scholar]
- 33.Dubois, S., J. Mariner, T.A. Waldmann, and Y. Tagaya. 2002. IL-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity. 17:537–547. [DOI] [PubMed] [Google Scholar]
- 34.Willerford, D.M., J. Chen, J.A. Ferry, L. Davidson, A. Ma, and F.W. Alt. 1995. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 3:521–530. [DOI] [PubMed] [Google Scholar]
- 35.Papiernik, M., M.L. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378. [DOI] [PubMed] [Google Scholar]
- 36.Tsunobuchi, H., H. Nishimura, F. Goshima, T. Daikoku, Y. Nishiyama, and Y. Yoshikai. 2000. Memory-type CD8+ T cells protect IL-2 receptor alpha-deficient mice from systemic infection with herpes simplex virus type 2. J. Immunol. 165:4552–4560. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi, K., Z.X. Lian, Y. Moritoki, R.Y. Lan, K. Tsuneyama, Y.H. Chuang, G.X. Yang, W. Ridgway, Y. Ueno, A.A. Ansari, et al. 2006. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 44:1240–1249. [DOI] [PubMed] [Google Scholar]
- 38.Boyman, O., M. Kovar, M.P. Rubinstein, C.D. Surh, and J. Sprent. 2006. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 311:1924–1927. [DOI] [PubMed] [Google Scholar]
- 39.Villarino, A.V., C.M. Tato, J.S. Stumhofer, Z. Yao, Y.K. Cui, L. Hennighausen, J.J. O'Shea, and C.A. Hunter. 2007. Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J. Exp. Med. 204:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carson, W.E., J.G. Giri, M.J. Lindemann, M.L. Linett, M. Ahdieh, R. Paxton, D. Anderson, J. Eisenmann, K. Grabstein, and M.A. Caligiuri. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giri, J.G., M. Ahdieh, J. Eisenman, K. Shanebeck, K. Grabstein, S. Kumaki, A. Namen, L.S. Park, D. Cosman, and D. Anderson. 1994. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 13:2822–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reya, T., J.A. Yang-Snyder, E.V. Rothenberg, and S.R. Carding. 1996. Regulated expression and function of CD122 (interleukin-2/interleukin-15R-beta) during lymphoid development. Blood. 87:190–201. [PubMed] [Google Scholar]
- 43.Li, X.C., G. Demirci, S. Ferrari-Lacraz, C. Groves, A. Coyle, T.R. Malek, and T.B. Strom. 2001. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat. Med. 7:114–118. [DOI] [PubMed] [Google Scholar]
- 44.Cornish, G.H., L.V. Sinclair, and D.A. Cantrell. 2006. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 108:600–608. [DOI] [PubMed] [Google Scholar]
- 45.Waldmann, T.A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6:595–601. [DOI] [PubMed] [Google Scholar]
- 46.Tsunobuchi, H., H. Nishimura, F. Goshima, T. Daikoku, H. Suzuki, I. Nakashima, Y. Nishiyama, and Y. Yoshikai. 2000. A protective role of interleukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology. 275:57–66. [DOI] [PubMed] [Google Scholar]
- 47.Bulanova, E., V. Budagian, E. Duitman, Z. Orinska, H. Krause, R. Ruckert, N. Reiling, and S. Bulfone-Paus. 2007. Soluble IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J. Biol. Chem. 282:13167–13179. [DOI] [PubMed] [Google Scholar]
- 48.Rubinstein, M.P., M. Kovar, J.F. Purton, J.H. Cho, O. Boyman, C.D. Surh, and J. Sprent. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha }. Proc. Natl. Acad. Sci. USA. 103:9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stoklasek, T.A., K.S. Schluns, and L. Lefrancois. 2006. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, X., S. Sun, I. Hwang, D.F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 8:591–599. [DOI] [PubMed] [Google Scholar]
- 51.Mertsching, E., C. Burdet, and R. Ceredig. 1995. IL-7 transgenic mice: analysis of the role of IL-7 in the differentiation of thymocytes in vivo and in vitro. Int. Immunol. 7:401–414. [DOI] [PubMed] [Google Scholar]
- 52.Starr, R., D. Metcalf, A.G. Elefanty, M. Brysha, T.A. Willson, N.A. Nicola, D.J. Hilton, and W.S. Alexander. 1998. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA. 95:14395–14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davey, G.M., R. Starr, A.L. Cornish, J.T. Burghardt, W.S. Alexander, F.R. Carbone, C.D. Surh, and W.R. Heath. 2005. SOCS-1 regulates IL-15–driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J. Exp. Med. 202:1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao, Y., N. Legrand, and A.A. Freitas. 2006. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J. Exp. Med. 203:1643–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khaled, A.R., and S.K. Durum. 2002. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2:817–830. [DOI] [PubMed] [Google Scholar]
- 56.Blattman, J.N., J.M. Grayson, E.J. Wherry, S.M. Kaech, K.A. Smith, and R. Ahmed. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9:540–547. [DOI] [PubMed] [Google Scholar]
- 57.Pereira, P., L. Forni, E.L. Larsson, M. Cooper, C. Heusser, and A. Coutinho. 1986. Autonomous activation of B and T cells in antigen-free mice. Eur. J. Immunol. 16:685–688. [DOI] [PubMed] [Google Scholar]
- 58.Min, B., H. Yamane, J. Hu-Li, and W.E. Paul. 2005. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J. Immunol. 174:6039–6044. [DOI] [PubMed] [Google Scholar]
- 59.Kieper, W.C., A. Troy, J.T. Burghardt, C. Ramsey, J.Y. Lee, H.Q. Jiang, W. Dummer, H. Shen, J.J. Cebra, and C.D. Surh. 2005. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J. Immunol. 174:3158–3163. [DOI] [PubMed] [Google Scholar]
- 60.Gilbertson, B., S. Germano, P. Steele, S. Turner, B. Fazekas de St Groth, and C. Cheers. 2004. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect. Immun. 72:6884–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hahm, B., M.J. Trifilo, E.I. Zuniga, and M.B. Oldstone. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 22:247–257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.