Abstract
To maintain immune homeostasis, the intestinal immune system has evolved redundant regulatory strategies. In this regard, the gut is home to a large number of regulatory T (T reg) cells, including the Foxp3+ T reg cell. Therefore, we hypothesized that the gut environment preferentially supports extrathymic T reg cell development. We show that peripheral conversion of CD4+ T cells to T reg cells occurs primarily in gut-associated lymphoid tissue (GALT) after oral exposure to antigen and in a lymphopenic environment. Dendritic cells (DCs) purified from the lamina propria (Lp; LpDCs) of the small intestine were found to promote a high level of T reg cell conversion relative to lymphoid organ–derived DCs. This enhanced conversion by LpDCs was dependent on TGF-β and retinoic acid (RA), which is a vitamin A metabolite highly expressed in GALT. Together, these data demonstrate that the intestinal immune system has evolved a self-contained strategy to promote T reg cell neoconversion.
Although a role for Foxp3-expressing regulatory T (T reg) cells in the maintenance of immune tolerance has been demonstrated in both humans and mice, the origin of these cells is still not completely understood. Early neonatal thymectomy experiments in mice strongly suggested that T reg cells are generated in the thymus. Recent studies using Foxp3 reporter mice (1) and transgenic mice that express foreign antigens in thymic tissue (2, 3) have also traced the development of Foxp3+ cells to the thymus.
Aside from evidence that natural Foxp3+ T reg cells arise and mature in the thymus, there is mounting evidence that Foxp3+ T reg cells can develop extrathymically under certain conditions. Both mouse (4, 5) and human (6) CD4+CD25− T cells have been shown to express Foxp3+ and acquire suppressive activity in vitro after TCR stimulation in the presence of TGF-β. In vivo, delivery of subimmunogenic doses of antigen (7), as well as endogenous expression of foreign antigen in a lymphopenic environment (8), can also induce peripheral Foxp3+ T reg cell development.
Although results from these studies have provided promising therapeutic approaches, the occurrence of such conversion in an unmanipulated setting remains controversial. Previous works suggest that peripheral conversion does not occur under steady-state conditions or during infections (9, 10). However, these studies failed to evaluate peripheral conversion at sites that require high levels of control, such as mucosal environments.
The gastrointestinal tract is in constant contact with food proteins, commensals, and potentially pathogenic microorganisms. To maintain immune homeostasis in this environment, the intestinal immune system has evolved redundant regulatory strategies. In this regard, the gut is home to a large number of regulatory T cells, including Foxp3+ T reg cells. These cells play a central role in the control of intestinal homeostasis (11). Additionally, several subsets of DCs with regulatory properties have been described with the capacity to induce IL-10 secretion from T cells or induce oral tolerance at steady-state conditions (12–14). Some effects of gut-associated lymphoid tissue (GALT) DCs on mucosal immunity are associated with their capacity to synthesize a vitamin A metabolite, retinoic acid (RA). For instance, RA production by GALT DCs can selectively induce molecules, such as CCR9 and α4β7, on conventional T cells and T reg cells involved in directing gut tropism (15–17). RA has also been implicated in the modulation of B cell tropism and effector functions by GALT DCs (18).
We hypothesized that the gut environment, particularly small intestine lamina propria (Lp) DCs, could potentially mediate extrathymic T reg cell development. In this study, we demonstrate that peripheral conversion occurs primarily in the GALT after oral exposure to antigen in a replete host or in a lymphopenic environment. In vitro, DCs purified from the Lp of the small intestine promoted a high level of T reg cell conversion compared with DCs from the spleen. Enhanced conversion by LpDC was associated with the capacity of these cells to release RA. To our knowledge, these data provide the first evidence that DCs from a defined environment can contribute to the generation of a pool of peripherally arising T reg cells under steady-state conditions.
RESULTS
Conversion or accumulation of converted Foxp3+ T reg cells occur primarily in the GALT
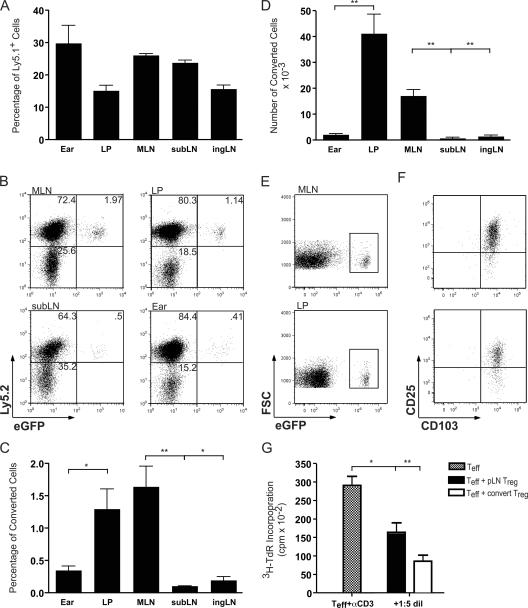
The small intestinal tissue has been shown to constitutively express large amounts of TGF-β (19) and is continuously exposed to gut flora or dietary antigens. To specifically determine if the GALT could favor peripheral conversion, we used a lymphopenic mouse transfer model. To avoid Foxp3 contamination in the initial Ly5.2+ transfer population and to formally identify converted T reg cells, we used Foxp3eGFP reporter mice. Specifically, CD4+ enhanced (e) GFP−(Ly5.2+) T cells (3 × 105) were adoptively transferred into recombination-activating gene 1–deficient (RAG-1−/−) hosts in conjunction with wild-type (wt) congenic (Ly5.1+) CD4+CD25hi cells (105) to prevent concomitant autoimmunity. At each time point examined, from as early as 4 wk to as late as 17 wk after transfer, both populations of transferred T cells could be detected in all tissues examined and were maintained at similar levels across all tissues (Fig. 1 A and not depicted).
Figure 1.
Conversion or accumulation of converted Foxp3+ CD4+ T cells occurs primarily in the GALT. 4–17 wk after transfer of Ly5.1+CD4+CD25hi T reg cells and Ly5.2+CD4+eGFP− T cells into RAG-1−/− hosts, T reg cell conversion was assessed in various tissues by surface staining and flow cytometric analysis. (A) Homeostatic proliferation of Ly5.1+ T reg cell cells and Ly5.2+ cells was similar in all tissues examined. (B–D) Conversion of Ly5.2+eGFP− cells into eGFP+ T reg cells occurred mainly in the MLN and LP, in both percentage (C) and total number (D) of cells. Data in A–D are from the 8-wk time point. The experiment was repeated at least four times with similar results. (E and F) CD25 and CD103 expression on converted eGFP+ T cells in the MLN and LP. Cells gated on Ly5.2 and CD4 that express eGFP (E) also express CD25 and CD103 (F). Data in E and F are from the 17-wk time point and the experiment was repeated at least three times. (G) Converted eGFP+ T reg cells are able to suppress Teff proliferation. Converted Ly5.2+eGFP+ T reg cells were isolated from host peripheral LN and cultured in vitro with freshly isolated splenic DCs and peripheral LN CD4+eGFP− Teff cells stimulated with α-CD3. Freshly isolated peripheral LN CD4+eGFP+ T reg cells were used as controls. Suppressive function was assessed at a ratio of 1 T reg cell: 5 Teff. The representative experiment shown was performed twice, with identical results. Results of student's t test: *, P < 0.05; **, P < 0.001. Error bars represent the SD.
At 4 wk after transfer, Foxp3-expressing Ly5.2+CD4+ cells were detected as determined by eGFP fluorescence (Fig. 1 B and not depicted). Notably, the percentage of converted T reg cells was increased up to four to five times in the mesenteric LN (MLN) over other peripheral lymph nodes (pLNs) and was increased up to three times in the small intestinal Lp over other tissue sites, such as the ear dermis (Fig. 1, B and C). Conversely, the naturally occurring (Ly5.1) T reg cells disseminated in a nonpreferential manner to all tissues examined, indicating that the presence of converted cells was not an artifact of a potential contaminant in the initial transfer population (Fig. 1 A). The percentage of increased conversion in the MLN was statistically significant compared with the submandibular LN (subLN) and to the inguinal LN (ingLN; Fig. 1 C). Additionally, increased conversion in the Lp was statistically significant compared with the ear dermis (Fig. 1 C). The GALT contains the largest number of lymphocytes in the body; thus, the absolute number of converted cells in the MLN or Lp was dramatically increased compared with other tissues (Fig. 1 D). The number of converted cells found in the Lp was 18 times that found in the ear dermis and the number of converted cells in the MLN was 11–33 times that found in other LNs examined (Fig. 1 D). Between 4 and 8 wk, the number of converted cells increased in the MLN and Lp, suggesting that there was continual conversion or accumulation of converted cells (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20070663/DC1). Additionally, pheno typic surface staining revealed that, in addition to CD25, in every tissue that we examined, converted T reg cells expressed high levels of the integrin chain CD103 (αE), a marker typically found in cells that have contacted epithelial surfaces (Fig. 1, E and F, and Fig. S1 B) (20).
To confirm that cells converted in vivo were functionally regulatory T cells, we isolated CD4+eGFP+ cells from RAG-1−/− mice that had been transferred with CD4+eGFP− T cells 9 wk before (Fig. 1 G). In vivo–converted CD4+eGFP+ T reg cells were able to suppress Teff proliferation as effectively as freshly isolated CD4+eGFP+ T reg cells (Fig. 1 G). Together, these data indicate that under lymphopenic conditions, the GALT promotes peripheral conversion or accumulation of a population of functional T reg cells.
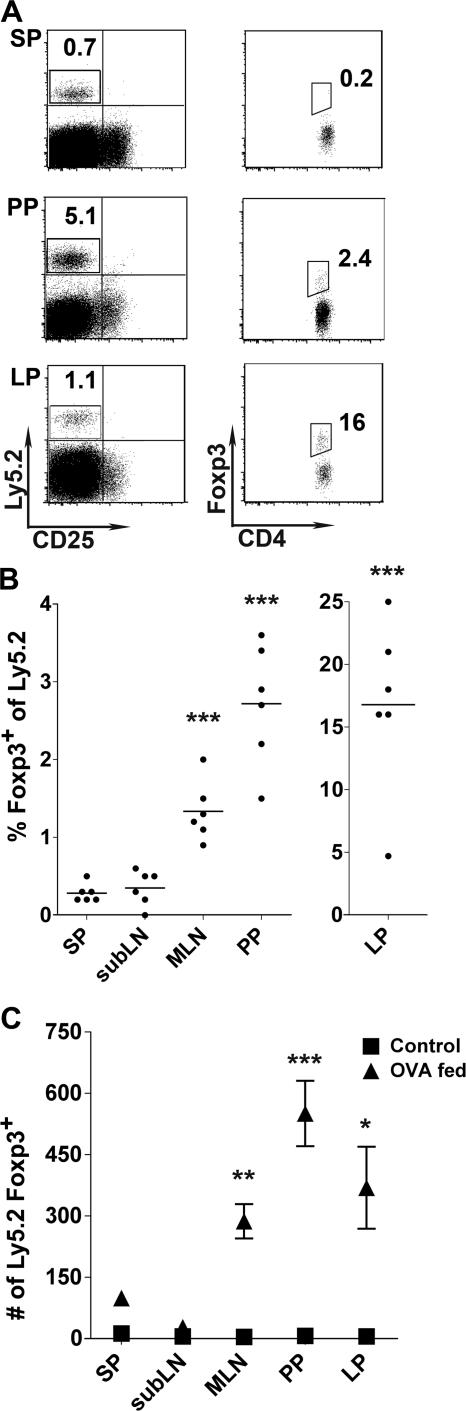
We next wanted to extend these findings to an immunologically complete setting. Therefore, we used another transfer model, in which we adoptively transferred Ly5.2+ T cells from RAG1−/− OT-II Tg mice, which are virtually devoid of Foxp3-expressing cells (<0.05%; unpublished data), into Ly5.1+- replete recipients. These recipients were then fed OVA antigen dissolved in drinking water for five consecutive days. This method allowed us not only to assess conversion in an immuno competent host but also to address the antigen-specific requirements for in vivo conversion. Moreover, this protocol was previously demonstrated to suppress airway inflammation in a monoclonal setting via Foxp3 up-regulation (21).
After 5 d of OVA administration, Ly5.2+ OVA-specific T cells had expanded and were readily detectable in the GALT, spleen, and in distal LNs, such as the subLN (Fig. 2 A and not depicted). However, in spite of the dissemination of transferred cells, Foxp3+-expressing T cells were only appreciably detected in the GALT, including the MLN, Lp, and Peyer's patches (PPs; Fig. 2 B). The highest frequency of Foxp3+-converted, OVA-specific T cells was consistently found in the Lp, with a mean frequency of 16.8 ± 6.5%. Translated into absolute numbers, the greatest number of Ly5.2+ Foxp3+ T cells was recovered from the PPs, which had the highest increase in cellularity during OVA feeding (Fig. 2 C). But in comparison to the spleen, statistically significant numbers of Ly5.2+ Foxp3+ T cells were also recovered from the MLN and Lp. Thus, after oral exposure to antigen, the GALT and the small intestine, particularly Lp and PP, favor the conversion and/or accumulation of antigen-specific Foxp3+ T cells.
Figure 2.
Foxp3 expression by RAG1−/− OT-II T cells in the GALT after oral administration of OVA protein. Ly5.2+ RAG1−/− OT-II T cells were transferred into Ly5.1+ recipient mice. Recipient mice were fed OVA antigen in drinking water for 5 d. On day 6, T reg cell conversion was assessed in various tissues by intracellular staining for Foxp3 and detected via flow cytometry. (A) After gating on CD4+ T cells, transferred T cells in OVA antigen-fed mice were identified by Ly5.2 expression. Ly5.2+ cells were then assessed for intracellular Foxp3 expression. Detection of Foxp3+ cells is illustrated for spleen (Sp), PP, and small intestinal Lp (bottom row). (B) Summary of the percentage of Ly5.2+ RAG1−/− OT-II T cells expressing Foxp3. Each dot represents a single mouse. Data were combined from two individual experiments (three mice each). (C) Absolute number of Ly5.2+Foxp3+ cells in OVA antigen-fed mice (▴) and nonfed control (▪). Error bars represent the SDs of the means of six individual samples from two combined experiments. Statistical comparisons were performed using the Student's t test, with Sp tissue serving as the baseline comparison for each tissue. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Characterization of LpDCs
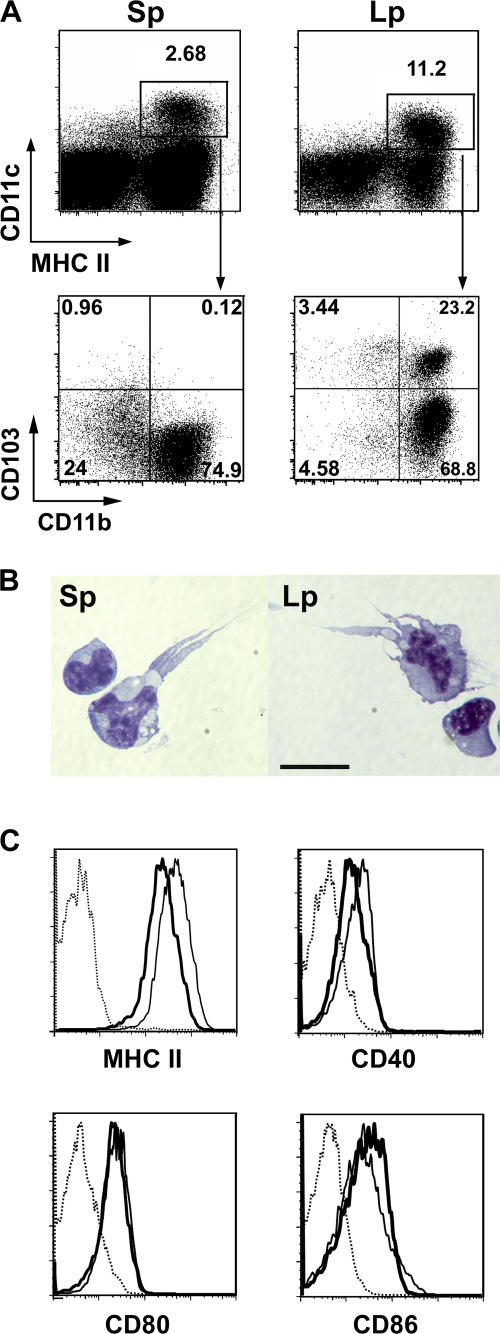
Our observations suggested that the GALT microenvironment was particularly well suited for peripheral conversion of T reg cells. We next determined whether GALT DCs could contribute to peripheral conversion of T reg cells. We confirmed previous findings that DCs are plentiful in the intestinal Lp (Fig. 3 A) (14, 22). In contrast to the spleen, where DCs accounted for <3% of total cells, MHCII+CD11c+ DCs comprised 10–15% of all cells in Lp (Fig. 3 A). As previously described (23), LpDCs express a highly homogenous phenotype in which >80% are CD11b+, and no CD4 or CD8α+ DCs are detectable (unpublished data). In SpDCs, no CD103 was detected on CD11b+ DCs and a low level of CD103 DCs were expressed on CD11b−DCs (Fig. 3 A). In contrast, CD103 was highly expressed on 25% of CD11b+ LpDCs (Fig. 3 A). A similar population has been previously described in the colon Lp (24).
Figure 3.
Phenotype of SpDCs and LpDCs. (A) Total spleen cells and Lp cells were stained with α-MHCII, α−CD11c, α−CD103 and α-CD11b mAb. (top) The percentages of CD11c+MHCII+ cells. (bottom) CD103 versus CD11b expression on CD11c+MHC II+ gated events. Numbers represent the percentage of events in each quadrant. (B) Giemsa staining of sorted spleen (left) and Lp (right) CD11c+MHCII+ DCs. Bar, 15 μm. (C) Expression of MHC II, CD40, CD80, and CD86 of spleen DC (thick line) and LpDC (thin line) were analyzed on CD11c+MHCII+ cells. Dotted line represents the corresponding isotype control. Data are from one of at least three independent experiments.
After cell sorting, LpDCs displayed the characteristic features of conventional DCs with a stellar shape comparable to freshly sorted SpDCs (Fig. 3 B). LpDCs could also prime naive T cells as efficiently as SpDCs (unpublished data). Furthermore, although their levels of CD80 and CD86 were comparable, LpDCs expressed higher levels of MHC II and CD40 than SpDCs (Fig. 3 C).
LpDCs convert Foxp3− T cells to Foxp3+ T cells
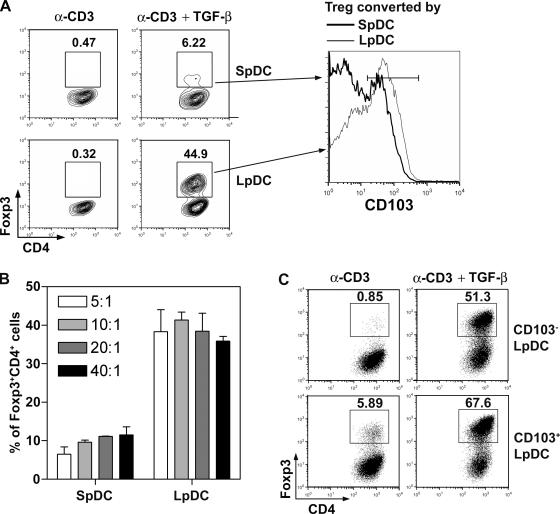
We next examined whether LpDCs could induce T reg cell conversion. To this end, DCs from the Lp or spleen were isolated, cocultured with purified CD4+eGFP− T cells, and stimulated with α-CD3 mAb. Although α-CD3 did not induce Foxp3 de novo, the addition of TGF-β resulted in T reg cell conversion in both cultures (Fig. 4 A). However, under the same conditions and at several T cell to DC ratios, the frequency of converted Foxp3+CD4+ T cells was up to 10-fold higher in LpDC cultures (Fig. 4 B). Notably, the level of expression of Foxp3 was higher when cells were converted in the presence of LpDCs than with SpDCs (mean fluorescence intensity [MFI] 90.9 vs. 57.4; Fig. 4 A). Converted cells with Lp or SpDCs expressed high levels of CD25 and GITR (unpublished data). LpDCs also induced moderate, but consistently higher, expression of the αE chain (CD103) of the αEβ7 integrin than SpDCs on converted T reg cells (59% [MFI 58.1] vs. 34.4% [MFI 43.2]), respectively (Fig. 4 A).
Figure 4.
Intestinal DCs induce Foxp3 expression of effector T cells with higher efficiency than splenic DC. (A) 105 Foxp3− cells were cultured with 2 × 104 splenic or LpDCs for 5 d with α-CD3 mAb alone or combined with TGF-β. Plots are gated on CD4+ cells, and the percentages of Foxp3+ cells are shown. Phenotypic analysis of converted T reg cells is shown on the right. 5-d cultured cells from A were stained for CD4 and CD103. Histogram shows the CD103 expression of CD4+Foxp3+ cells cultured with SpDCs (thick line) or LpDCs (thin line). (B) 105 Foxp3−CD4+ T cells cultured with DCs and α-CD3 mAb and TGF-β as in (A) for 5 d, with the ratio of T/DC as 5/1, 10/1, 20/1 or 40/1. Data are the mean ± SD of triplicate wells. (C) Foxp3− cells were cultured as in A with CD103+LpDC or CD103−LpDC. Plots are gated on CD4+ cells and Foxp3 versus α4β7 stainings are shown. The numbers indicate percentage of events in each quadrant. Data are from one of at least three independent experiments.
To determine if these data were reproducible in an antigen-specific manner, we isolated naive T cells from OT-II TCR transgenic mice. Cocultures with OVAp323-339 peptide-pulsed DCs produced similar results; with the addition of TGF-β, LpDCs induced 10-fold higher Foxp3 expression in OT-II T cells than in SpDCs (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20070663/DC1). To determine if in vitro–converted T reg cells possessed suppressive activity, we isolated CD4+ T cells based on eGFP expression and measured their ability to suppress T cell proliferation. Suppression by converted T reg cells from both LpDCs and SpDCs cultures was at least as efficient as that of freshly isolated T reg cells (Fig. S3).
DCs expressing CD103 have been shown to display regulatory functions in a T cell transfer colitis model (24). As previously shown, 25% of LpDCs express CD103 (Fig. 3 A). To determine if this subset favored T reg cell conversion, we further separated LpDCs based on CD103 expression. LpDCs, particularly CD103+LpDCs, were able to induce Foxp3 expression in the absence of exogenous TGF-β (Fig. 4 C). Such conversion was dramatically enhanced in presence of TGF-β (Fig. 4 C). Although CD103−LpDCs failed to induce Foxp3 expression de novo in the absence of exogenous TGF-β, they remained much more potent than total SpDCs at inducing T reg cells in the presence of TGF-β (Fig. 4, A and C).
Stability of converted Foxp3+ T reg cells
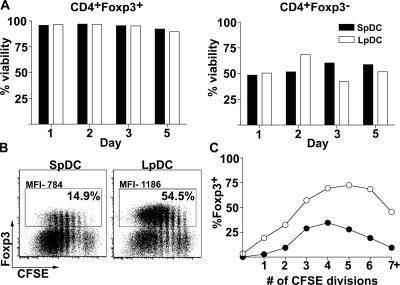
The relative paucity of converted T reg cells in SpDC cocultures could be caused by a lower viability or reduced proliferative capacity of converted cells. To determine whether these elements were contributing to the outcome of conversion in our assays, we examined the survival of in vitro–activated T cells. In both SpDC and LpDC cocultures, converted T reg cells remained significantly more viable than unconverted Foxp3− cells. Thus, the lower yield of T reg cells in SpDC cocultures was not the result of increased cell mortality (Fig. 5 A). We next explored the effects of proliferation on converted T reg cells. Because eGFP and CFSE share overlapping emission spectra, we isolated naive CD4+ T cells (Foxp3 contamination was <0.5%) from wt mice. Consistent with findings using CD4+ T cells from reporter mice, the proportion of converted T reg cells continued to dominate in LpDC cocultures (54.5 vs. 14.9% for SpDC; Fig. 5 B). Moreover, Foxp3 expression, as assessed by anti-Foxp3 antibody staining, was more intense when differentiated in the presence of LpDCs (1,186 vs. 784 MFI; Fig. 5 B). CFSE dilution profiles indicated that CD4+ T cells proliferated vigorously, regardless of the origin of DCs during the length of incubation. Yet, even at late division cycles, the proportion of converted T reg cells remained high in LpDC cocultures (Fig. 5 C). These data suggested an initiation of a more stable Foxp3+ T reg cell program in the presence of LpDCs.
Figure 5.
Maintenance of Foxp3 contributes to higher frequency of Foxp3+CD4+ T cells in the presence of LpDC. (A) eGFP−CD4+ T cells were cocultured with purified DC at a 5:1 ratio with α-CD3 and TGF-β. At indicated time points, cells were harvested and stained for CD4, Class II, and 7-AAD. Foxp3 expression was determined based on eGFP fluorescence. The percentage of viable cells is depicted by open bars for LpDC and filled bars for SpDC. Data are from one of two independent experiments. (B) CFSE-labeled naive CD4+ T cells were cocultured with purified DCs as described in A. On day 5, cells were harvested and stained for CD4 and intracellular Foxp3. Illustrated are dot plots of Foxp3 versus CFSE. The percentage of Foxp3+ cells and the MFI was defined as the bordered population. (C) In cultures containing LpDC (○) or SpDC (•) the proportion of Foxp3+ cells among CD4 T cells is plotted as a function of the number of cell divisions. Data are representative of three independent experiments.
RA produced by LpDC is responsible for conversion of Foxp3+ T cells
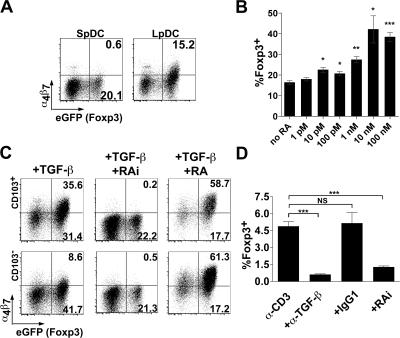
We further explored the mechanism underlying enhanced conversion in LpDC cocultures. Several reports have demonstrated that lymphocytes activated in the presence of GALT LpDCs selectively up-regulate the gut-homing surface molecules CCR9 and α4β7, resulting in their preferential migration into intestinal tissue (15, 16, 18, 25). This up-regulation was recently shown to depend on RA (18, 26). Consistent with these reports, we observed up-regulated α4β7 expression on converted Foxp3+ T reg cells in LpDC, but not in SpDC, cocultures (Fig. 6 A). Next, we asked whether RA was able to exert an enhancing effect on T reg cell conversion. To test this, we incubated SpDCs with α-CD3 and TGF-β in the presence of increasing doses of the synthetic RA, all-trans RA. Although RA alone did not induce Foxp3 expression, RA in the presence of TGF-β enhanced T reg cell conversion and up-regulated α4β7 (Fig. 6 B and not depicted). At low RA concentrations (1 nM), T reg cell conversion increased by >50% in SpDC cocultures (from 16.4 to 27.4%). The enhanced conversion by RA plateaued above concentrations of 10 nM, with the percentage of Foxp3+ cells reaching 40–55% (Fig. 6 B).
Figure 6.
RA production by LpDC is requisite for optimal Foxp3+ T reg cell conversion. Foxp3− CD4 T cells were cocultured with DC at a 10:1 ratio as described in Fig 4. (A) Cells were stained for α4β7 and assessed for eGFP fluorescence (Foxp3) by flow cytometry. Results are representative of three independent experiments. (B) The dose responsiveness of all-trans RA on eGFP (Foxp3) expression by CD4 T cells cocultured with SpDC was determined by flow cytometry. Error bars represent the SDs of the means of three individual samples from one experiment. Statistical significance was determined using the Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Results are representative of three independent experiments. (C) Foxp3− CD4+ T cells were cocultured with CD103+ or CD103− DC at a 10:1 ratio as described in Fig 4. Cells were stained for α4β7 and the effect of 100 nM RA, or the RA receptor inhibitors LE540 and LE135 on (eGFP) Foxp3 expression was determined by flow cytometry. Results are representative of three independent experiments. (D) Foxp3− CD4 T cells were cocultured with CD103+ DC in the presence of α-CD3 or α-CD3 in combination with TGF-β neutralizing antibody (isotype IgG1) or LE540 and LE135. eGFP (Foxp3) expression was determined by flow cytometry. NS. Error bars represent the SD from the means of three independent experiments. ***, P < 0.001.
The addition of RA in the presence of TGF-β to LpDC cocultures also increased the proportion of converted cells and diminished the intrinsic differences in each subset's ability to induce T reg cell conversion (Fig. 6 C). Interestingly, in cultures in which conversion is already high (as with CD103+ LpDC) RA has a more dramatic effect on α4β7 than on Foxp3 expression. This suggests that the amount of RA required for Foxp3 expression may be lower than that required for up-regulation of α4β7. Because CD103+ LpDCs consistently induced low levels of T reg cell conversion in cocultures containing α-CD3 alone, we tested whether exogenous RA would improve conversion. However, exogenous RA, in the absence of TGF-β, failed to increase the frequency of Foxp3+ T reg cells (unpublished data).
Together, our data suggested that RA from LpDCs might be, in part, responsible for the enhanced T reg cell conversion observed in LpDC coculture. To address this hypothesis, we added the synthetic RA receptor antagonists LE540 and LE135 (RAi) to block RA signaling in T cells. Remarkably, the addition of 1 μM each of these inhibitors inhibited T reg cell conversion by 67 and 57% in CD103+ and CD103− LpDC cocultures, respectively (Fig. 6 C). Collectively, these data suggest that RA signaling by LpDCs synergize with TGF-β to favor T reg cell conversion. In support of this, α–TGF-β and RAi significantly decreased spontaneous α-CD3–induced conversion by CD103+ LpDCs (87%, P < 0.0005, and 73%, P < 0.001, respectively; Fig. 6 D).
DISCUSSION
In this study, we provide evidence that the GALT environment favors de novo Foxp3+ T reg cell conversion. We also reveal that DCs from the Lp of the small intestine have the unique ability to generate T reg cells in vitro via a mechanism that, in addition to TGF-β, is dependent on the vitamin A metabolite RA.
Our hypothesis that LpDCs could mediate Foxp3+ T reg cell differentiation is supported by our adoptive transfer studies, which indicate that peripheral conversion occurs preferentially in the GALT and/or associated tissue. We first observed T reg cell conversion in a lymphopenic transfer setting via eGFP expression in T cells possessing a Foxp3 bicistronic eGFP reporter construct. This system allowed us to isolate and transfer pure T cell populations devoid of Foxp3. Though our findings differ from another study that showed that transferred Foxp3− reporter cells into lymphopenic hosts did not express Foxp3 de novo (9), on several counts our findings were similar. We also found that Foxp3+ T reg cell conversion was marginal in the pLN and spleen (9). The fact that the authors observed no conversion in the MLN may reflect the transfer model used, where CD4+Foxp3− T cells were transferred into lymphopenic hosts without the cotransfer of T reg cells. Consequently, the development of massive inflammation in the large bowel under these conditions may have led to a redistribution of converted T reg cells away from the MLN. Remarkably, the intestinal immune system tolerates repeated exposure to resident microflora and food antigens, while maintaining the capacity to mount powerful immune responses against pathogens. This property, referred to as oral tolerance, can be associated with the induction of TGF-β–producing Th2-like cells (Th3 cells), peripheral deletion, or Ag-specific T reg cells (21, 27). Studies have demonstrated that repeated Ag feeding induced Ag-specific Foxp3+ T reg cells in the absence of natural T reg cells (21). We confirmed these findings in complete mice that received Foxp3− OT-II T cells and were placed on a similar OVA feeding regimen. In our model, converted cells were rapidly found in the MLNs, PPs, and particularly in the small intestine Lp, in which 17% of antigen-specific T cells were Foxp3+. Considering the amount of antigens persistently present in the lumen of the intestine, these data suggest that peripheral conversion may represent a significant pathway for the generation of Foxp3+ T cells. Although some PPDC subsets possess unique immunoregulatory properties, we have yet to examine the conversion capacity of DCs from the PPs (12). Nevertheless, our findings indicate that LpDCs are quite competent inducers of T reg cells in vitro. Previous work demonstrated that small intestinal LpDCs not only presented orally administered Ag, but also suppressed delayed type hypersensitivity responses (14). Further supporting a role for LpDCs in T reg cell conversion are findings that the MLNs are crucial for oral tolerance induction, and that a significant proportion of CD103+ DCs, which we found potently induced T reg cell conversion, enter the MLN from the Lp (16, 28).
Apart from their T reg cell–inducing capacity, DCs from the small intestinal Lp retain several immunoregulatory features, e.g., constitutive expression of IL-10 (14). Some of these features may have been influenced by conditioning signals received from noninflammatory cytokines constitutively produced by the intestinal epithelia. These cytokines include TGF-β and the Th2 response driving thymic stromal lymphopoietin (TSLP) (19, 29). Intriguingly, a recent study demonstrated that TSLP from human epithelial cells was involved in the thymic induction of Foxp3+ T reg cells (30). However, our examination of TSLP receptor–deficient mice (TSLPR−/−) revealed no alterations in the frequency and absolute number of T reg cells across several lymphoid and nonlymphoid tissues (not depicted). Moreover, we noted no intrinsic differences between LpDCs from TSLPR−/− and wt mice to induce T reg cell conversion in vitro (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20070663/DC1). Conversely, TGF-β exerted a strong influence on the induction of T reg cell conversion in our system. Virtually every work detailing the extrathymic generation of CD25+ or Foxp3+ T reg cells thus far, both in vitro and in vivo, has noted the critical involvement of TGF-β in this process (4, 6, 7, 9).
TGF-β is also known to play a positive role in the induction of the integrin CD103 (31). Importantly, a large proportion of DCs present in the colon and MLN express CD103 and were found to be crucial for protection from pathology in a mouse transfer model of colitis (24). Another study found that the majority of LpDCs express CD103 (16). In untreated mice, we found a smaller, but still appreciable (∼25%) population of CD103+ LpDCs. These CD103+ induced Foxp3+ T reg cell conversion in the absence of exogenously added TGF-β. Endogenous TGF-β was responsible for T reg cell conversion, as neutralizing antibodies to TGF-β largely ablated T reg cell conversion. Cumulatively, these findings suggest a critical role for CD103+ DCs in the maintenance of gut homeostasis. How these DCs respond to intestinal inflammation in response to infection and whether their ability to induce conversion can be altered remains to be addressed. Yet, TGF-β cannot explain, in full, how these populations of DCs induce Foxp3+ because the addition of a relatively high dose of exogenous TGF-β to SpDC cocultures still induced significantly less conversion compared with LpDC cocultures.
It is becoming clear that nutrient status can impact an individual's susceptibility to intestinal pathologies (32). In the case of vitamin A, and in particular, its transcriptionally active metabolite, RA, prolonged insufficiency not only disrupts the integrity of the intestinal epithelial barrier but also prevents the proper deployment of effector lymphocytes into the GALT after priming (18, 26, 32). Indeed, the GALT is a significant producer of RA in the body, based on the abundance of RA-synthesizing enzymes present in the epithelia and certain resident DC populations (26). The precise factors that govern the activation of some of these enzymes have yet to be clarified. More recently, it was demonstrated that RA and cytokines produced by DCs in the Peyer's patches synergized to promote IgA secretion by gut-activated B cells (18). Importantly, a recent study demonstrated that the addition of RA to naturally occurring T reg cells in vitro can promote their expression of gut tropism receptors and, subsequently, favor their migration to the GALT (17). Consistent with this observation, we present in vitro results that suggest that RA from LpDCs synergizes with TGF-β to induce strong extrathymic T reg cell conversion. First, LpDCs, but not SpDCs, induced the integrin heterodimer α4β7, which is a hallmark of RA signaling (26), on converted T reg cells. Second, the levels of α4β7, which were highest in cocultures containing CD103+ LpDCs, correlated strongly with the degree of conversion. But perhaps most compelling was the dramatic increase in T reg cell conversion upon addition of synthetic RA to SpDC cocultures, and the decrease of converted T reg cells when nuclear RA signaling was blocked in LpDC cocultures.
Although RAi significantly inhibited α-CD3–induced conversion by CD103+ LpDCs, RA alone is not sufficient for Foxp3+ T reg cell conversion. In the absence of exogenous TGF-β, RA failed to induce T reg cells in SpDC cocultures (unpublished data). Moreover, RA also failed to enhance the basal level of conversion observed in CD103+ LpDC cocultures. These findings support the idea that RA acts to enhance signals delivered by TGF-β and that TGF-β, but not RA, is the limiting factor in CD103+ LpDC-induced conversion. Notably, we did not observe a reduction in T reg cell priming by LpDCs upon treatment with citral, which is an inhibitor of RALDH that is involved in the oxidation of retinal to RA (unpublished data). We therefore favor the hypothesis that RA is being released from prestored pools. Previous studies demonstrated that RA can also manipulate DC functions (14). Thus, Foxp3 induction by RA may be the consequence of a direct action on T cells or via an autocrine conditioning of LpDC functions.
The cooperation observed between GALT-derived TGF-β and RA in the optimal production of T reg cells in our in vitro system, also fits particularly well with our in vivo findings, in which peripherally converted cells are detected predominantly in the LP and MLN. From these data, we postulate that the intestinal immune system, through the simultaneous production of TGF-β and RA, has evolved a self-contained strategy to promote neoconversion. This dual requirement would prevent spontaneous de novo conversion in other peripheral compartments and further explains the unique capacity of the gut to induce tolerogenic outcomes. Enhanced TGF-β–mediated conversion in the presence of RA also suggests that the use of vitamin A and associated precursors and metabolites in chemoprevention clinical trials may need to be reevaluated depending on the tissue of target. In several clinical trials, β-carotene– and retinol (vitamin A)-based therapies resulted in the exacerbation of lung cancer (33). Nevertheless, these results do not necessarily negate the potential for treating discrete sites of acute or chronic inflammation with infusions of RA.
Using our in vitro system, we further found that T reg cells generated in the presence of LpDCs possessed higher Foxp3 expression and up-regulated the tissue-homing integrins CD103 and α4β7 in comparison to T reg cells generated in the presence of SpDCs. These findings may be harnessed to generate or expand T reg cells with specific homing features to target tissue pathology that occurs in diseases where the epithelia or intestine are affected, while preventing global suppression.
In summary, we propose that our in vivo and in vitro data are highly relevant to a variety of situations in which de novo T reg cell conversion may be necessary. First, the GALT and its associated tissues are often considered to be in a state of physiological activation, and thus poised to control pathogenic microbial invasion (34). A system to generate T reg cells in this region ensures their rapid deployment when intestinal homeostasis is threatened. Moreover, as the thymus and gut likely maintain a degree of nonoverlapping antigen repertoires, it is plausible that T reg cells conversion in the GALT potentially expands the T reg cell repertoire.
In some instances, T reg cell levels have to be limited to favor efficient immune responses, whereas in other cases, such levels must be increased to limit immunopathology. Manipulation of T reg cell numbers or functions offers promising therapeutic avenues. Together, our results suggest that manipulation of RA metabolism or the use of RA could become part of a rational strategy to manipulate the ratio between T reg cells and effector cells.
MATERIALS AND METHODS
Mice.
C57BL/6, B6.SJL, OT-II TCR transgenic mice Ly5.2+, and OT-II transgenic RAG-1−/− mice were purchased from Taconic Farms. Foxp3 eGFP reporter mice (Foxp3GFP) were originally obtained from M. Oukka (Brigham and Women's Hospital, Cambridge, MA) (5). All mice were maintained at an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at the National Institute for Allergy and Infectious Diseases (NIAID) and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee.
In vivo cell transfer.
Purified CD4+eGFP− (3 × 105) and CD4+CD25hi (105) from peripheral LNs were transferred intravenously into RAG-1−/− mice, and the ability of CD4+GFP− cells to convert into CD4+eGFP+ cells was assessed 4–17 wk later. Specifically, single-cell suspensions of peripheral LNs from either Foxp3eGFP or congenic B6.SJL mice were enriched for CD4+ T cells by a negative selection kit using an autoMACs (Miltenyi Biotec). CD4+Foxp3eGFP T cells or congenic CD4+ T cells were further labeled with fluorescent dye–conjugated mAb (CD4, RM4-5; CD25, 7D4; eBioscience) and sorted by flow cytometry on a FACSVantage or FACSAria (BD Biosciences). 4–17 wk after transfer, the ear dermis, subLN, ingLN, MLN, and Lp were removed from RAG-1−/− hosts to assess cell conversion. LN single-cell suspensions were prepared by passing tissue through a 70-μm cell strainer. Ear dermis cells were prepared as previously described (35). For Lp cells, small intestinal segments were treated with medium containing 3% FCS and 20 mM Hepes (HyClone) for 20 min at 37°C with constant stirring. Tissue was further digested with 250 mg/ml liberase CI (Roche) and 500 mg/ml DNase I (Sigma-Aldrich), with continuous stirring at 37°C for 30 min. Digested tissue was forced through a Cellector tissue sieve, (Bellco Glass, Inc.) and passed through 70- and 40-μm cell strainers. Lymphocytes were further enriched by centrifugation at room temperature at 500 g for 20 min in 30% Percoll (GE Healthcare) in RPMI-1640. All cells were incubated with antibodies to Ly5.2 (104), CD4, CD25, CD103 (2E7; all from eBioscience) and assessed for the expression of these markers in addition to eGFP by flow cytometry on an LSRII (BD Biosciences). In some experiments, in vivo–converted CD4+eGFP+ cells pooled from all peripheral LNs were purified based on eGFP expression by flow cytometry on a FACSVantage or FACSAria and assessed for their suppressive capacity in vitro.
Oral antigen administration.
T lymphocytes were extracted from the secondary LNs (excluding the spleen) of RAG1−/− OT-II Tg mice (Ly5.2) and adoptively transferred into B6.SJL recipient mice (Ly5.1). Each mouse received 106 cells. Recipient mice were then divided into two groups, with one group receiving a 1.5% OVA solution dissolved in drinking water that was replaced every 48 h (grade V; Sigma-Aldrich) for 5 consecutive days, and another receiving normal drinking water. On day 6, subLNs, MLNs, PPs, and Lps were collected from B6.SJL hosts, and Foxp3 expression was assessed in transferred cells. LN and LP single-cell suspensions were prepared as described in the previous section.
Suppression assays.
T reg cell ability to suppress T cell proliferation was determined as previously reported (36). In brief, CD4+GFP+ T reg cells were cultured in 96-well flat-bottom plates (Costar) with 5 × 104 freshly isolated CD4+GFP− T cells used as effectors. For antigen-presenting cells, splenocytes were depleted of CD90+ cells by isolation kit and autoMACs (Miltenyi Biotec), irradiated, and cultured at 105 per well. Cells were stimulated with 0.5 μg/ml α-CD3 (145-2C11) mAb (BD Biosciences) for 72 h at 37°C/5% CO2. Cultures were pulsed with [3H]TdR (MP Biomedicals) at 1 μCi/well for the last 8 h. All experiments were performed in duplicate or triplicate, cell yield permitting.
DC purification.
For LpDCs, total Lp cells after digestion with Liberase CI were passed through 70- and 40-μm cell strainers. Cells were resuspended in 1.077 g/cm3 iso-osmotic NycoPrep medium (Accurate Chemical & Scientific Corp.), and the low-density fraction was collected after centrifugation at 1,650 g for 15 min. Nycodenz gradient excludes debris and red blood cells and decreases lymphocyte numbers without changing the composition of the different subsets of LpDC (Fig. S5, available at http://www.jem.org/cgi/content/full/jem.20070663/DC1). Cells were washed and incubated with a mixture of mAb containing α−CD11c (HL-3), α−MHC II (AF6-120.1), α−CD16/32 (2.4G2), and α−CD103 mAb (2E7), as well as the non-DC components α−DX5 (DX5), α-NK1.1 (PK136), and α−B220 (RA3-6B2, all from eBioscience). DCs were defined as CD11c+MHCII+ cells and non-DCs were excluded when sorted by flow cytometry on a FACSVantage or FACSAria. In some experiments, CD103+ and CD103− DCs were separated. Purity was verified by flow cytometry on a FACSCalibur. I-Ab+CD11c+ cells were >90%.
For SpDCs, spleens were cut into fragments and digested by 100 mg/ml Liberase CI and 150 mg/ml DNase I, and then dissociated in Ca2+-free medium in the presence of EDTA. IAb+CD11c+ SpDCs were further purified for LpDCs, resulting in >98% purity. For phenotypic analysis, I-Ab+CD11c+ DCs were stained with fluorescent dye-conjugated α-CD11b (M1/70), α-CD103 (2E7; all from eBioscience), α-CD40 (3/23), α-CD80 (16-10A) (1), and α-CD86 (GL-1; all from BD Biosciences) mAb and analyzed by flow cytometry on a FACSCalibur or LSR II (BD Biosciences).
In vitro conversion assay.
Our conversion protocol was performed as previously described (5), with the following modifications. 105 CD4+eGFP− T cells or CD4+CD25−CD62L+CD44− naive T cells from wt or OT-II mice were cultured with 2.5 × 103–2 × 104 purified Lp or SpDCs in 250 μl of complete medium (RPMI-1640 containing 10% FBS, 2 × 10−5 M of 2ME, 100 μg/ml of penicillin, and 100 μg/ml of streptomycin) with soluble 1 μg/ml α−CD3 mAb (BD Biosciences) and human 3 ng/ml rTGF-β (Cell Science, Canton, MA) for 5 d. The precise ratio of T cells to DCs used is provided in the text. In some experiments, the following were included in the coculture conditions: (a) all-trans RA (Sigma-Aldrich) at varying concentrations; (b) RA receptor β inhibitors LE540 (Wako Chemicals USA) and LE135 (Tocris Bioscience), each at 1 μM; and (c) α-TGF-β mAb (1D11.16.8) or isotype control mAb MOPC (31C; both from American Type Culture Collection) at 10 μg/ml. 5 ng/ml human rIL-2 (Peprotech) was added to cultures wells every other day beginning on day 2. On day 5, cells were stained with PE-Cy7–conjugated α-CD4 mAb (RM4-5), and Foxp3+ cells were detected either by eGFP expression and/or FITC/PB-conjugated α-Foxp3 intracellular mAb (FJK-16; both from eBioscience) in accordance with the manufacturer's protocol on a FACSCalibur or LSRII. For viability assays, cultured cells were additionally stained with 7-amino-actinomycin D (7-AAD; BD Biosciences) to detect dead cells. For phenotypic analysis, eGFP+CD4+ cells were further stained for α-CD103 (2E7) or α-α4β7 (DATK32; BD Biosciences) mAb, α−CD25 (PC61.5; eBioscience), and 7-AAD. Live cells were analyzed by flow cytometry on a FACSCalibur or LSR II (BD Biosciences).
In vitro proliferation assay.
FACS-purified CD4+CD25−CD44− T cells from wt mice were labeled with 1.25 μM CFSE (Invitrogen) in PBS for 5 min at room temperature. Cells were washed twice in media containing 3% FBS and cultured in 2 × 104 purified Lp or SpDCs.
Statistical analysis.
Groups were compared with Prism software (GraphPad) using the unpaired or paired Student's t test.
Online supplemental material.
Fig. S1 shows that the number of converted cells increases in the MLN and Lp and that all converted cells express CD103. Fig. S2 shows that LpDC can convert OTII CD4+Foxp3− T cells into Foxp3+ T cells in the presence of peptide and TGF-β. Fig. S3 shows that converted T reg cells are as suppressive as naturally occurring T reg cells in vitro. Fig. S4 shows that Nycodenz gradient does not change LpDC subsets. Fig. S5 shows that LpDCs do not require TSLP conditioning signals to induce Foxp3 T reg cell conversion.
Supplemental Material
Acknowledgments
We would like to thank Dr. Angela Thornton for technical advice. We would like to thank Jakson Egen for helpful discussions. We would also like to thank Dr. A. Sher for critical reading of the manuscript.
This work was supported by the Division of Intramural Research, NIAID, National Institutes of Health. We thank the NIAID sorting facility and Kim Beacht for technical assistance.
The authors have no conflicting financial interests.
Abbreviations used: AAD, amino-actinomycin D; eGFP, enhanced GFP; IEL, intraepithelial lymphocyte; ingLN, inguinal LN; Lp, lamina propria; GALT, gut-associated lymphoid tissue; MFI, mean fluorescence intensity; MLN, mesenteric LN; NIAID, National Institute for Allergy and Infectious Diseases; pLN, peripheral LN; PP, Peyer's patch; RA, retinoic acid; RAG, recombination-activating gene; subLN, submandibular LN; SpDC, spleen DC.
C.-M. Sun, J. Hall, and R.B. Blank contributed equally to this paper.
References
- 1.Fontenot, J.D., J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202:901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensinger, S.J., A. Bandeira, M.S. Jordan, A.J. Caton, and T.M. Laufer. 2001. Major histocompatibility complex class II–positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 194:427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan, M.S., A. Boesteanu, A.J. Reed, A.L. Petrone, A.E. Holenbeck, M.A. Lerman, A. Naji, and A.J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301–306. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., W. Jin, N. Hardegen, K.J. Lei, L. Li, N. Marinos, G. McGrady, and S.M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 6.Fantini, M.C., C. Becker, G. Monteleone, F. Pallone, P.R. Galle, and M.F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149–5153. [DOI] [PubMed] [Google Scholar]
- 7.Kretschmer, K., I. Apostolou, D. Hawiger, K. Khazaie, M.C. Nussenzweig, and H. von Boehmer. 2005. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 6:1219–1227. [DOI] [PubMed] [Google Scholar]
- 8.Knoechel, B., J. Lohr, E. Kahn, J.A. Bluestone, and A.K. Abbas. 2005. Sequential development of interleukin 2–dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 202:1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan, Y.Y., and R.A. Flavell. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 102:5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. [DOI] [PubMed] [Google Scholar]
- 11.Powrie, F., S. Read, C. Mottet, H. Uhlig, and K. Maloy. 2003. Control of immune pathology by regulatory T cells. Novartis Found. Symp. 252:92–114. [PubMed] [Google Scholar]
- 12.Iwasaki, A., and B.L. Kelsall. 2001. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J. Immunol. 166:4884–4890. [DOI] [PubMed] [Google Scholar]
- 13.Mowat, A.M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 3:331–341. [DOI] [PubMed] [Google Scholar]
- 14.Chirdo, F.G., O.R. Millington, H. Beacock-Sharp, and A.M. Mowat. 2005. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 35:1831–1840. [DOI] [PubMed] [Google Scholar]
- 15.Johansson-Lindbom, B., M. Svensson, M.A. Wurbel, B. Malissen, G. Marquez, and W. Agace. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson-Lindbom, B., M. Svensson, O. Pabst, C. Palmqvist, G. Marquez, R. Forster, and W.W. Agace. 2005. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202:1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siewert, C., A. Menning, J. Dudda, K. Siegmund, U. Lauer, S. Floess, D.J. Campbell, A. Hamann, and J. Huehn. 2007. Induction of organ-selective CD4(+) regulatory T cell homing. Eur. J. Immunol. 37:978–989. [DOI] [PubMed] [Google Scholar]
- 18.Mora, J.R., M. Iwata, B. Eksteen, S.Y. Song, T. Junt, B. Senman, K.L. Otipoby, A. Yokota, H. Takeuchi, P. Ricciardi-Castagnoli, et al. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 314:1157–1160. [DOI] [PubMed] [Google Scholar]
- 19.Barnard, J.A., G.J. Warwick, and L.I. Gold. 1993. Localization of transforming growth factor beta isoforms in the normal murine small intestine and colon. Gastroenterology. 105:67–73. [DOI] [PubMed] [Google Scholar]
- 20.Agace, W.W., J.M. Higgins, B. Sadasivan, M.B. Brenner, and C.M. Parker. 2000. T-lymphocyte-epithelial-cell interactions: integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 12:563–568. [DOI] [PubMed] [Google Scholar]
- 21.Mucida, D., N. Kutchukhidze, A. Erazo, M. Russo, J.J. Lafaille, and M.A. Curotto de Lafaille. 2005. Oral tolerance in the absence of naturally occurring Tregs. J. Clin. Invest. 115:1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pavli, P., C.E. Woodhams, W.F. Doe, and D.A. Hume. 1990. Isolation and characterization of antigen-presenting dendritic cells from the mouse intestinal lamina propria. Immunology. 70:40–47. [PMC free article] [PubMed] [Google Scholar]
- 23.Niess, J.H., and H.C. Reinecker. 2005. Lamina propria dendritic cells in the physiology and pathology of the gastrointestinal tract. Curr. Opin. Gastroenterol. 21:687–691. [DOI] [PubMed] [Google Scholar]
- 24.Annacker, O., J.L. Coombes, V. Malmstrom, H.H. Uhlig, T. Bourne, B. Johansson-Lindbom, W.W. Agace, C.M. Parker, and F. Powrie. 2005. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 202:1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora, J.R., M.R. Bono, N. Manjunath, W. Weninger, L.L. Cavanagh, M. Rosemblatt, and U.H. Von Andrian. 2003. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 424:88–93. [DOI] [PubMed] [Google Scholar]
- 26.Iwata, M., A. Hirakiyama, Y. Eshima, H. Kagechika, C. Kato, and S.Y. Song. 2004. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 21:527–538. [DOI] [PubMed] [Google Scholar]
- 27.Chen, Y., V.K. Kuchroo, J. Inobe, D.A. Hafler, and H.L. Weiner. 1994. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 265:1237–1240. [DOI] [PubMed] [Google Scholar]
- 28.Worbs, T., U. Bode, S. Yan, M.W. Hoffmann, G. Hintzen, G. Bernhardt, R. Forster, and O. Pabst. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J. Exp. Med. 203:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimoldi, M., M. Chieppa, V. Salucci, F. Avogadri, A. Sonzogni, G.M. Sampietro, A. Nespoli, G. Viale, P. Allavena, and M. Rescigno. 2005. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat. Immunol. 6:507–514. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe, N., Y.H. Wang, H.K. Lee, T. Ito, Y.H. Wang, W. Cao, and Y.J. Liu. 2005. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 436:1181–1185. [DOI] [PubMed] [Google Scholar]
- 31.Smith, T.J., L.A. Ducharme, S.K. Shaw, C.M. Parker, M.B. Brenner, P.J. Kilshaw, and J.H. Weis. 1994. Murine M290 integrin expression modulated by mast cell activation. Immunity. 1:393–403. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler, T.R., M.E. Evans, C. Fernandez-Estivariz, and D.P. Jones. 2003. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu. Rev. Nutr. 23:229–261. [DOI] [PubMed] [Google Scholar]
- 33.Paolini, M., G. Cantelli-Forti, P. Perocco, G.F. Pedulli, S.Z. Abdel-Rahman, and M.S. Legator. 1999. Co-carcinogenic effect of beta- carotene. Nature. 398:760–761. [DOI] [PubMed] [Google Scholar]
- 34.Sansonetti, P.J., and J.P. Di Santo. 2007. Debugging how bacteria manipulate the immune response. Immunity. 26:149–161. [DOI] [PubMed] [Google Scholar]
- 35.Mendez, S., S.K. Reckling, C.A. Piccirillo, D. Sacks, and Y. Belkaid. 2004. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent Leishmaniasis and control of concomitant immunity. J. Exp. Med. 200:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregu latory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.