Abstract
CD8 T cells are nature's foremost defense in encephalitis and brain tumors. Antigen-specific CD8 T cells need to enter the brain to exert their beneficial effects. On the other hand, traffic of CD8 T cells specific for neural antigen may trigger autoimmune diseases like multiple sclerosis. T cell traffic into the central nervous system is thought to occur when activated T cells cross the blood-brain barrier (BBB) regardless of their antigen specificity, but studies have focused on CD4 T cells. Here, we show that selective traffic of antigen-specific CD8 T cells into the brain occurs in vivo and is dependent on luminal expression of major histocompatibility complex (MHC) class I by cerebral endothelium. After intracerebral antigen injection, using a minimally invasive technique, transgenic CD8 T cells only infiltrated the brain when and where their cognate antigen was present. This was independent of antigen presentation by perivascular macrophages. Marked reduction of antigen-specific CD8 T cell infiltration was observed after intravenous injection of blocking anti–MHC class I antibody. These results expose a hitherto unappreciated route by which CD8 T cells home onto their cognate antigen behind the BBB: luminal MHC class I antigen presentation by cerebral endothelium to circulating CD8 T cells. This has implications for a variety of diseases in which antigen-specific CD8 T cell traffic into the brain is a beneficial or deleterious feature.
The traffic of leucocytes into the central nervous system (CNS) is a highly regulated process. This protects the brain against the full ravages of the systemic inflammatory response that would otherwise compromise the delicate homeostasis required for neural activity. T cells, which initiate the adaptive immune response, traffic into the brain at a relatively low level compared with other organs (1). The question of whether antigen specificity is a prerequisite for T cell traffic into the brain has been previously addressed. Several investigators have transferred activated T cells reactive against neural or irrelevant antigens into naive animals and observed that both infiltrated the brain equally well (2–7). However, all these studies concentrated on CD4 T cells; although CD8 T cells were present among the transferred cells in some experiments (3, 4), no attempt was made to elucidate whether the antigen specificity of the CD8 T cells was influencing their infiltration into the brain.
There is reason to suspect that traffic of CD8 T cells recognizing antigens within the brain is favored over that of irrelevant CD8 T cells. In mice immunized with the myelin oligodendrocyte glycoprotein peptide MOG 35–55 that develop experimental autoimmune encephalomyelitis, 56% of brain-infiltrating CD8 T cells on day 10 were MOG specific (8). In humans with multiple sclerosis (MS), oligoclonal dominance of T cells in cerebrospinal fluid (CSF) (9) and brain parenchyma (10) are seen more commonly with CD8 than CD4 T cells. Although this has been interpreted as oligoclonal expansion within the CNS compartment, antigen-specific CD8 T cell infiltration could also contribute because the CD8 T cell clones were present in blood.
CD8 T cells are instrumental in the body's response to viral encephalitides and tumors. However, they are also responsible for various inflammatory neurological conditions such as MS, human T cell lymphotropic virus–associated myelopathy, and a whole host of neurological paraneoplastic syndromes (11). The crucial role of CD8 T cells in MS has only recently been recognized. It has been shown that CD8 T cells specific for myelin antigen can initiate severe experimental autoimmune encephalomyelitis disease when adoptively transferred (12). However, CD8 T cells are also important in disease maintenance because their number correlated with axon injury in MS plaques (13) and magnetic resonance imaging features of tissue destruction (14). CD8 T cell–mediated neuropathology may be mediated directly by CNS antigen-specific CD8 T cells or may occur indirectly as a result of bystander damage by co-infiltrating CD8 T cells with irrelevant antigen specificities. However, the overall contribution of bystander damage has been shown to be small (15). The factors governing antigen-specific infiltration of CD8 T cells into the brain are therefore important in both disease induction and maintenance.
To study antigen-specific CD8 T cell traffic into the brain, we injected antigen into the striatum of CL4 transgenic mice in which >95% of CD8 T cells express the Vα10 Vβ8.2 TCR (16). We show that CD8 T cell infiltration only occurred when the cognate antigen was present within the brain parenchyma. This proves that a mechanism capable of favoring antigen-specific CD8 T cell infiltration exists. To elucidate the origin of this antigen specificity, we depleted the brain of perivascular macrophages (PVMs), which are considered to be the foremost antigen-presenting cells at the blood-brain barrier (BBB), but this had no effect on CD8 T cell infiltration. We show that MHC class I expression by cerebral endothelium was luminal and, when blocked, resulted in a marked reduction of CD8 T cell infiltration.
RESULTS AND DISCUSSION
Antigen-specific CD8 T cell traffic into the brain
In CL4 mice, CD8 T cells exhibit high avidity for the HA512-520 peptide (HA), which is Kd restricted (16). To investigate CD8 T cell traffic, we injected antigen dissolved in sterile PBS into the right striatum of CL4 mice. A small volume (0.5 μL) was delivered with a sterile, finely drawn glass micropipette, the tip of which measured 2–10 μm in diameter, to minimize tissue trauma and reflux into the periphery. Mice were killed on days 1, 3, 5, and 7 after injection (minimum n = 3 per time point). Because virtually all CD8 T cells in this mouse express the transgenic TCR, we used CD8 immunohistochemistry to track antigen-specific CD8 T cells in the brain parenchyma. Serial section immunohistochemistry had shown that all CD8+ cells were CD+ (unpublished data).
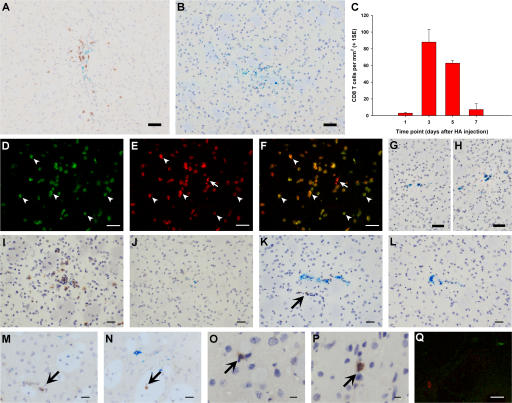
Intrastriatal injection of HA resulted in a focal CD8 T cell infiltrate, which was strictly limited to the area of antigen deposition (∼7.5 mm2) as shown by a co-injected inert blue dye (Fig. 1 A). This T cell infiltrate peaked at day 3, reaching a density of 88 cells/mm2, and had nearly disappeared by day 7 (Fig. 1 C). No CD8 T cells were seen in the contralateral hemisphere. Moreover, CD8 T cell infiltration did not occur at any time point after control intrastriatal injections of a Kd-restricted noncognate antigen, Cw3 peptide (Fig. 1 B). This showed that CD8 T cells were accumulating at the site of injection as a result of the presence of antigen.
Figure 1.
An antigen-specific model of CD8 T cell infiltration into the brain. (A and B) CD8 T cell recruitment 3 d after HA (A) or control Cw3 (B) peptide intrastriatal injection. (C) Kinetics of CD8 T cell recruitment after HA injection. (D–H) CD8 T cell infiltration 3 d after 3 million (D–G) or 30 million (H) in vitro–activated CL4 Thy1.1+ CD8 T cells were injected i.v. in Thy1.2-congenic wild-type BALB/c mice at the time of right intrastriatal HA (D–F) or Cw3 (G and H) injection. D–F show confocal micrographs of CD8 (red, E) and Thy1.1 (green, D) immunofluorescence, merged in F. G and H are light micrographs after CD8 immunohistochemistry (brown). (I–P) CD8 immunohistochemistry (brown) of striatum in CL4 mice after simultaneous HA and Cw3 injection in right (I and K) and left (J and L) striatum, on days 3 (I and J) and 1 (K and L); irrelevant adenovirus injection, days 1 (M) and 3 (N); stab lesion, days 1 (O) and 3 (P). Q is a representative merged confocal micrograph after double immunofluorescence of striatum for CD8 (red) and Ki67 (green) on day 1 after HA injection in CL4 mice. Bars: A and B, D–H, and Q, 50 μm; I–L, 30 μm; M–P, 20 μm.
The CD8 T cell infiltrate observed was not a peculiarity of the CL4 transgenic mouse. Using a different approach in a nontransgenic animal, we transferred 3 million in vitro–activated CL4 Thy1.1+ CD8 T cells into Th1.2-congenic wild-type BALB/c recipients at the time of intrastriatal HA injection (n = 3) and perfused them after 3 d. As expected, CD8 T cells were observed in the right striatum of HA-injected animals. The vast majority of parenchymal CD8 T cells (>95%) were Thy1.1+ (Fig. 1, D–F), indicating that they were donor HA-specific CD8 T cells. No T cells were seen infiltrating the brains of wild-type BALB/c mice receiving Cw3 in the right striatum and 3 or 30 million in vitro–activated CL4 CD8 T cells i.v. (n = 3 each dose) (Fig. 1, G and H).
The marked difference in CD8 T cell accumulation between the HA and Cw3 injections in the striatum of CL4 mice suggested that CD8 T cells were sensitive to the local presence of their cognate antigen in the CNS. HA is a soluble peptide and is likely to “leak” to the periphery (17). One possibility was that CD8 T cells were migrating into the striatum in a non-antigen–specific way at the site of intracerebral injection after being activated in the periphery by the leaking HA. To investigate this possibility, we injected HA into one hemisphere and Cw3 into the contralateral hemisphere of CL4 mice and killed them on day 3 after injection (n = 3). If peripherally activated transgenic T cells were migrating nonspecifically to the site of injection, we would have expected to see CD8 T cells in the Cw3-injected hemisphere. However, CD8 T cells only infiltrated the HA-injected hemisphere; none were present in the contralateral hemisphere (Fig. 1, I and J).
The above experiments showed that the transgenic CD8 T cells were recruited only to the site where cognate antigen was present in the brain parenchyma. It is currently thought that a basal low level of immunosurveillance by T cells occurs in the brain (18). Therefore, the CD8 T cells could, as part of normal surveillance, have infiltrated the parenchyma in a non-antigen–specific fashion only to be retained or to proliferate if their cognate antigen was present (antigen-specific retention and/or proliferation, respectively). We therefore asked whether CD8 T cells recruited into the brain in a non-antigen–specific fashion persist for any length of time in the absence of their cognate antigen. In separate experiments, CL4 mice received an injection of an irrelevant adenovirus (Ad70-3) or a sterile stab lesion in the right striatum (n = 3 each time point). Both manipulations recruited CD8 T cells, which were seen at day 1 and persisted until day 3 (Fig. 1, M–P). This means that had CD8 T cells been recruited in a non-antigen–specific fashion after Cw3 injection, they would have been seen on days 1 or 3. To further test this hypothesis, CL4 mice injected with HA in one hemisphere and Cw3 in the contralateral hemisphere (n = 3) were perfused at an earlier time point (day 1 after injection) to see whether T cells were failing to persist until day 3 after injection in the contralateral hemisphere. No CD8 T cells were seen in the Cw3-injected hemisphere (Fig. 1 L), but CD8 T cells were observed in the HA-injected side, with some in a perivascular location representing the initial phase of CD8 T cell migration (Fig. 1 K). Confocal microscopy showed that CD8 T cells infiltrating the striatum on day 1 after cognate antigen injection did not express the nuclear proliferation marker Ki67 and therefore were not proliferating (Fig. 1 Q). Therefore, initial CD8 T cell accumulation within the parenchyma was the result of antigen-specific infiltration rather than non-antigen–specific infiltration followed by antigen-specific retention or proliferation.
The role of cerebral PVMs
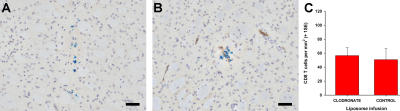
We next addressed whether antigen presentation at the BBB is a potential mechanism whereby CD8 T cells specifically home in on their target behind the BBB. Cerebral PVMs are strategically located at the BBB between the endothelial basement membrane and the glia limitans, and are considered to be the brain's foremost antigen-presenting cells (19). We therefore investigated whether PVMs play a role in antigen-specific CD8 T cell traffic. CL4 mice were depleted of cerebral PVMs with an intracerebroventricular infusion of clodronate-loaded liposomes as described previously (20) (n = 6) (Fig. 2 A). These liposomes are selectively phagocytosed by cerebral PVMs, leading to progressive intracellular accumulation of sodium clodronate. This kills all PVMs by day 5 as a result of adenosine triphosphate depletion and apoptosis (20). Control CL4 mice (n = 4) received an intracerebroventricular infusion of empty liposomes (Fig. 2 B). On day 5, both groups of mice received an intrastriatal injection of cognate antigen, and they were perfused 3 d later. Similar numbers of CD8 T cells had infiltrated the parenchyma in both the PVM-depleted and control groups (Fig. 2 C), indicating that antigen presentation by PVMs was not directing CD8 T cell traffic into the brain.
Figure 2.
Cerebral PVM depletion does not affect antigen-specific CD8 T cell infiltration into the brain. (A and B) Mannose receptor immunohistochemistry (brown) of striatum after clodronate (A) and control (B) liposome intracerebroventricular infusion in CL4 mice. Bar, 50 μm. (C) Quantification of CD8 T cell infiltration 3 d after intrastriatal HA injection in CL4 mice pretreated with clodronate or control liposomes (two-tailed Student's t test, P = 0.767).
The role of cerebral endothelium
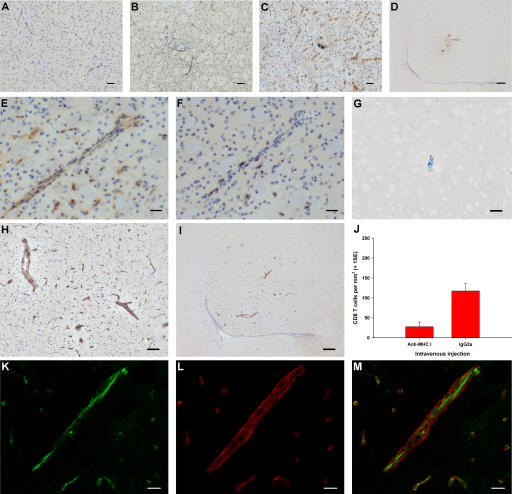
Because an antigen-presenting process was suspected to play an important role in CD8 T cell infiltration, we studied the expression of MHC class I by immunohistochemistry on days 0, 0.5, 1, 1.5, 2, 2.5, 3, 5, and 7 after intrastriatal injection of HA in CL4 mice (at least n = 3 for each time point). In naive uninjected animals, a very low basal level of MHC class I expression by endothelium was noted (Fig. 3 A). Constitutive MHC class I expression was slightly up-regulated upon injection of Cw3 (Fig. 3 B). After injection of HA, there was dramatic up-regulation of MHC class I by endothelial cells, peaking at day 3 (Fig. 3 C). This up-regulation was largely limited to the site of antigen deposition (Fig. 3 D) and coinciding temporally and spatially with peak CD8 T cell infiltration. CD8 T cells were seen in association with MHC class I+ blood vessels at various stages of infiltration (Fig. 3, E and F). This raised the possibility that CD8 T cell traffic into the brain was facilitated by recognition of the cognate antigen presented by cerebral endothelial cells. For this to occur, however, antigen presentation by cerebral endothelial MHC class I would have to be luminal.
Figure 3.
The role of endothelial MHC class I in antigen-specific CD8 T cell infiltration into the brain. (A–F) Immunohistochemistry (brown) for MHC class I (A–E) and CD8 (F) in naive striatum (A) and striatum from CL4 mice injected with Cw3 (B) or HA (C–F). E and F show serial sections. (G–I) Immunohistochemistry (brown) for biotinylated IgG 3 d after intrastriatal HA injection (day 0) in CL4 mice receiving i.v. biotinylated anti–MHC class I antibody (H and I) or control biotinylated IgG (G) on day 2. (J) CL4 mice injected with HA intrastriatally received an i.v. bolus of blocking anti–MHC class I antibody or control IgG on day 2 and were perfused on day 3. There was a 76% reduction (95% CI = −139.5 to −40.0) in CD8 T cell infiltration (two-tailed Student's t test, P = 0.002). (K–M) High power confocal micrographs after double immunofluorescence on striatum for biotin (green in K) and γ1-laminin (red in L) (merged in M) 3 d after intrastriatal HA injection (day 0) in CL4 mice receiving i.v. biotinylated anti–MHC class I antibody on day 2. Bars: K–M, 10 μm; E and F, 30 μm; A–C, 50 μm; G and H, 100 μm; D and I, 200 μm.
To investigate whether the up-regulated endothelial MHC class I was luminally expressed, CL4 mice received an intracerebral injection of HA peptide followed 3 d later by an i.v. injection of 200 μg of biotinylated anti-Kd antibody (n = 3) or biotinylated control IgG (n = 3) in 200 μL of sterile PBS. Mice were perfused 3 h later, and the biotinylated antibody was detected on tissue sections. Luminal expression of MHC class I was observed in the striatum and was restricted to the site of CD8 T cell infiltration in animals receiving biotinylated anti-Kd (Fig. 3, H and I); it was below the limit of detection in the contralateral hemisphere. BBB breakdown was excluded by the lack of staining in sections from mice receiving the biotinylated control IgG (Fig. 3 G).
To directly address the issue of whether luminal endothelial MHC class I was responsible for antigen-specific CD8 T cell migration, CL4 mice received an intrastriatal injection of HA peptide on day 0, followed by an i.v. injection of 200 μg anti-Kd blocking antibody (n = 6) or control IgG (n = 6) in 200 μL of sterile PBS on day 2, 1 d before peak CD8 T cell recruitment. Blocking of luminal MHC class I resulted in a 76% reduction in CD8 T cell recruitment (Fig. 3 J), indicating that circulating CD8 T cells use luminal endothelial MHC class I as a molecular address to target their cognate antigen within the brain parenchyma. The critical interaction between circulating CD8 T cells and MHC I occurred on the luminal aspect of the endothelium because confocal microscopy showed that i.v. injected biotinylated anti-Kd blocking antibody binding was limited to the luminal aspect of the endothelium (Fig. 3, K–M).
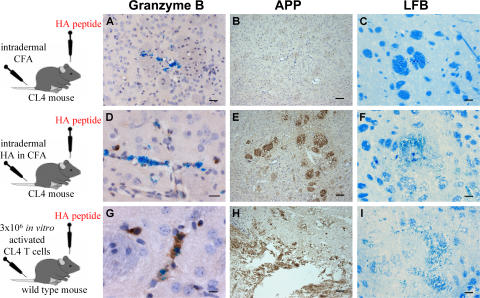
CD8 T cells infiltrating the brain after intrastriatal injections of HA in CL4 mice were not fully differentiated at any time point, as shown by the lack of granzyme B expression (Fig. 4 A) and lack of tissue damage, as assessed by amyloid precursor protein expression to detect damaged axons (Fig. 4 B) and Luxol Fast Blue histochemistry to detect myelin damage (Fig. 4 C). In contrast, granzyme B expression and axon/myelin damage were seen when CD8 T cells were peripherally activated by immunization with HA in CFA (Fig. 4, D–F) or after in vitro activation (Fig. 4, G–I). CD8 T cells infiltrating the brain after HA injection alone did not proliferate on day 1 (Fig. 1 Q) but started expressing Ki67 at later time points (Fig. 5, A and B). Expression of Ki67 is an indicator of commitment to the cell cycle, and the Ki67 index is the percentage of CD8 T cells expressing this marker; the Ki67 index was 21% on day 2 and peaked at 52% on day 3.
Figure 4.
Brain-infiltrating CD8 T cells were not fully activated in this model of antigen-specific CD8 T cell traffic. (A–I) Representative sections 3 d after intrastriatal injection of HA in CL4 mice immunized intradermally with CFA alone (A–C) or HA in CFA (D–F) 5 d previously, and in wild-type littermates receiving 3 million in vitro−activated CL4 CD8 T cells i.v. (G–I). Sections were submitted to immunohistochemistry (brown) for granzyme B (A, D, and G), amyloid precursor protein (B, E, and H), or Luxol Fast Blue histochemistry (C, F, and I). Bars are 60 μm except for the following: A, 30 μm; D, 20 μm; G, 10 μm.
Figure 5.
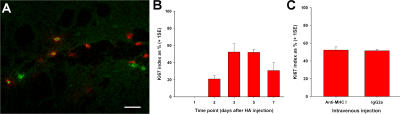
Intravenously administered anti–MHC class I antibody does not affect proliferation of brain-infiltrating CD8 T cells in this model of antigen-specific CD8 T cell traffic. (A) Merged confocal micrograph after double immunofluorescence for CD8 (red) and Ki67 (green) on striatum from CL4 mice 3 d after HA injection. (B) Ki67 index of brain-infiltrating CD8 T cells at several time points after intrastriatal HA injection in CL4 mice. (C) Ki67 index of brain-infiltrating CD8 T cells 3 d after intrastriatal HA injection (day 0) in CL4 mice receiving i.v. blocking anti–MHC class I antibody or control IgG on day 2 (two-tailed Student's t test, P = 0.844). Bar, 20 μm.
Therefore, the number of CD8 T cells within the brain parenchyma at time points beyond day 1 can be considered to be a result of two processes: infiltration and proliferation. This raised the question of whether the i.v. injected blocking anti-Kd antibody, which resulted in a 76% reduction in parenchymal CD8 T cell numbers after cognate antigen injection in the striatum, could have affected proliferation rather than infiltration. To address this issue, we studied the CD8 T cell Ki67 index in CL4 mice receiving HA in the striatum, followed by blocking anti-Kd antibody or control isotype i.v. 2 d later. No difference in Ki67 index was observed (Fig. 5 C). This provides definitive evidence that the blocking anti-Kd antibody acted by inhibiting infiltration rather than proliferation of CD8 T cells in the brain.
Antigen-specific CD8 T cell traffic: concluding remarks
The study of T cell traffic into the brain has been dominated by CD4 T cells in view of their perceived importance in neuroinflammatory disease. This is now changing, and CD8 T cells are increasingly recognized as major players (11), but relatively little is known about CD8 T cell traffic into the brain. Here, we show that unlike CD4 T cells, antigen specificity is a factor that governs CD8 T cell infiltration into the brain. We also demonstrate that the underlying mechanism favoring antigen-specific CD8 T cell traffic is luminal expression of MHC class I by cerebral endothelium, which acts as a molecular address at the BBB. This crucial difference between CD4 and CD8 T cell traffic into the brain is a reflection of cerebral endothelial cell biology. Low level MHC class I, but not class II, expression by cerebral endothelium occurs constitutively (21). Therefore, the mechanism for initiating transendothelial antigen-specific T cell traffic into the naive brain only exists for CD8 T cells. This situation might have evolved as a result of selective pressure in favor of enhanced antiviral immunosurveillance of the CNS (18).
The additional molecular requirements for the MHC-dependent transendothelial T cell migration we describe here are known to be present at the cerebral endothelium. As elsewhere in the body, T cell infiltration into the brain is a three-step process: rolling, adhesion, and diapedesis (22). Strong adhesion is a requirement for subsequent diapedesis, and this was classically thought to be mediated by interaction between integrins on the T cell surface and cellular adhesion molecules on the endothelium. An example of such a pair is LFA-1 and intercellular adhesion molecule (ICAM)-1, and the strength of this interaction is potentiated by chemokine receptor signaling. Arrest of T cell rolling suggesting adhesion, similar to that seen with chemokine ligand–chemokine receptor binding, has been observed after cognate MHC-TCR interaction (23). Also, MHC-peptide–TCR interaction gives rise to a similar increase in avidity of LFA-1 to ICAM-1 (24) as happens after chemokine receptor ligation. The affinity of a typical MHCI-peptide–TCR interaction is less than that of LFA-1–ICAM-1 (KD of 10 μM [25] and 500 nM [26], respectively), but it is strengthened by the accompanying MHCI–CD8 interaction. Moreover, cognate MHCI-peptide–TCR interaction results in the formation of a supramolecular activation cluster that recruits LFA-1, thereby strengthening overall adhesion.
An integral requirement of MHC-dependent CD8 T cell traffic into the brain is the presentation of processed exogenous antigen by MHC class I on the luminal surface of cerebral endothelium. Interestingly, presentation of endogenous antigen by endothelial cells in peritoneum and cremasteric venules facilitates diapedesis of T cells (27). We used HA peptide in our experiments to simulate the availability of processed antigen within the extracellular milieu of brain parenchyma under inflammatory conditions. Various CNS proteins are released into the CSF in the course of neuropathology that may be degraded into smaller proteins or peptides by interstitial enzymes. Cross-presentation by endothelial cells has been reported to occur in the pancreas (28). It remains to be shown in brain endothelium.
In this study, we used an antigen-naive system. CL4 mice were housed in individually filtered cages throughout and were not allowed to come into contact with sources of influenza infection. HA was stereotaxically injected into the striatum using very fine glass micropipettes; great care was taken to avoid reflux to the periphery and minimize trauma to surrounding tissue. Indeed brain-infiltrating CD8 T cells were not activated on day 1 after such injections in CL4 mice. In agreement with a recent study (29), our results suggest that activation is not an absolute prerequisite for CNS infiltration by CD8 T cells. CD8 T cells entered the cell cycle after infiltrating the brain, but there was no detectable granzyme B expression or tissue damage at any time point, indicating that they were not fully activated to achieve cytotoxic potential. Importantly, such a dampened system allowed us to use a blocking anti–MHC class I antibody to study the function of luminal endothelial MHC class I in CD8 T cell traffic into the brain without confounding effects on T cell proliferation.
It is likely that the novel pathway we describe in mice for antigen-specific CD8 T cell traffic into the brain also occurs in humans. Human endothelial cells constitutively express MHC class I (30), supporting a role in disease initiation by CD8 T cells reactive against antigens present behind the BBB. Also, oligoclonal dominance of CD8 T cells was observed within the CSF (9) and brain parenchyma (10) from patients with MS. This has profound therapeutic consequences for neurological diseases mediated by CD8 T cell entry into the CNS such as MS, human T cell lymphotropic virus–associated myelopathy, and various paraneoplastic CNS syndromes, as well as encephalitis and brain tumors. Our description of an antigen-specific pathway for CD8 T cells across the BBB means that therapies aimed at blocking/augmenting the MHC-dependent migration of antigen-specific CD8 T cells, as opposed to the whole T cell repertoire, are possible.
MATERIALS AND METHODS
Mice.
CL4 mice (16) were bred and experiments were performed under Home Office Licence and in accordance with the Animals (Scientific Procedures) Act, 1986, UK.
Liposomes.
Multilamellar mannosylated liposomes were prepared as described previously (20). Sodium clodronate was a gift of Roche Diagnostics.
Reagents.
Haemagglutinin peptide (IYSTVASSL) and control Kd-binding peptide (Cw3 peptide, RYLKNGKETL) were purchased from Alta Bioscience. Replication-defective human type 5 adenovirus vector (Ad70-3) was provided by J. Gauldie (McMaster University, Ontario, Canada). The anti-Kd antibody SF1-1.1.10 was provided by P. Kourilsky (Pasteur Institute, Paris, France). IgG2aκ was used as control IgG (Sigma-Aldrich). Biotinylation of SF1-1.1.10 and IgG2aκ was performed using the EZ-Link Sulfo-NHS-LC-Biotinylation kit (Pierce Biotechnologies).
Surgery.
Intracerebral antigen injections and intracerebroventricular liposome infusions were performed stereotaxically using a minimally invasive technique, as described previously (20). Great care was taken to minimize tissue trauma and reflux to the periphery. A sterile, finely drawn glass micropipette, the tip of which measured 2–10 μm in diameter, was used to deliver antigen (0.5 μg HA or Cw3, or 5 × 104 PFU Ad70-3) in a volume of 0.5 μL of PBS over a period of 1 min. To minimize reflux along the injection tract, the needle was left in place for 1 min before being slowly withdrawn over another minute. Injections contained a trace of autoclaved Colanyl Blue (Clariant) to help with localization of the focal antigen deposit during subsequent tissue processing. Striatal stab lesions were performed using a sterile pointed scalpel blade.
Immunohistochemistry.
After perfusion and tissue processing, 10-μm-thick coronal sections were used for indirect immunohistochemistry or double immunofluorescence as described previously for frozen and wax-embedded sections (20). Primary antibodies were purchased from Serotec (CD3, CD4, CD8, CD68, MHCI, mannose receptor, and Thy1.1), Abcam (granzyme B), Novocastra (Ki67), Neomarkers (γ1-laminin), Chemicon (MBP and NeuN), Sigma-Aldrich (CNPase and MAP2), Zymed Laboratories (APP), and DakoCytomation (GFAP). For antibodies raised in mouse, the MOM kit was used (Vector Laboratories). Bright field and fluorescence images were captured on a Leica light microscope using LeicaQwin software and a Leica TCS SP2 AOBS confocal system, respectively.
Cell culture.
CL4 CD8 T cells were purified by positive selection as described previously (18).
Immunization.
CL4 mice were immunized with an intradermal injection of 100 μL PBS/CFA (1:1) with or without 5 μg HA. On day 5, the animals received an intrastriatal injection of HA followed by perfusion 5 d later.
Adoptive transfer.
Wild-type BALB/c mice received an intrastriatal injection of HA or Cw3, and 4 h later they were injected i.v. with 3 or 30 million in vitro–activated CL4 CD8 T cells in 200 μL DMEM. They were perfused 3 d later.
Quantification.
This was performed manually under light or fluorescence microscopy. The operator was blinded to the identity of the slides counted. Cells were counted using a graticule under a high power objective (×25), and the density of cells was converted to a value per mm2. In all cases, at least four lesion-center sections (as shown by the co-injected blue dye) from the same animal and several animals from each experimental group (as denoted by number n) were taken, and counts were averaged.
Statistics.
Data were analyzed using SPSS version 12. All the data were parametric, and therefore the two-tailed Student's t test for two independent samples was used throughout. The confidence level was 95%.
Acknowledgments
We thank the following people for advice, technical assistance, and reagents: J. Cabarrocas, J. Zappulla, and E. Piaggio (Institut National de la Santé et de la Recherche Médicale U563); P. Kourilsky (Pasteur Institute); J. Gauldie (McMaster University); and M. Glennie, S. Quaratino, T. Elliott, A. Williams, S. Waters, A. Pringle, J. Mepham, H. Schuppe, and N. Platt (University of Southampton).
This work was supported by EU grants QLG3-CT-2002-00612 (to I. Galea, R.S. Liblau, and V.H. Perry) and LSHM-CT-2005–018637 (to R.S. Liblau and V.H. Perry) and Wellcome Trust grant 066801/Z/02/Z (to M. Bernardes-Silva, P.A. Forse, and V.H. Perry).
The authors have no conflicting financial interests.
References
- 1.Yeager, M.P., J.A. DeLeo, P.J. Hoopes, A. Hartov, L. Hildebrandt, and W.F. Hickey. 2000. Trauma and inflammation modulate lymphocyte localization in vivo: quantitation of tissue entry and retention using indium-111-labeled lymphocytes. Crit. Care Med. 28:1477–1482. [DOI] [PubMed] [Google Scholar]
- 2.Carrithers, M.D., I. Visintin, S.J. Kang, and C.A. Janeway Jr. 2000. Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 123:1092–1101. [DOI] [PubMed] [Google Scholar]
- 3.Hickey, W.F., B.L. Hsu, and H. Kimura. 1991. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28:254–260. [DOI] [PubMed] [Google Scholar]
- 4.Irani, D.N., and D.E. Griffin. 1996. Regulation of lymphocyte homing into the brain during viral encephalitis at various stages of infection. J. Immunol. 156:3850–3857. [PubMed] [Google Scholar]
- 5.Krakowski, M.L., and T. Owens. 2000. Naive T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur. J. Immunol. 30:1002–1009. [DOI] [PubMed] [Google Scholar]
- 6.Ludowyk, P.A., D.O. Willenborg, and C.R. Parish. 1992. Selective localisation of neuro-specific T lymphocytes in the central nervous system. J. Neuroimmunol. 37:237–250. [DOI] [PubMed] [Google Scholar]
- 7.Wekerle, H., C. Linington, H. Lassmann, and R. Meyermann. 1986. Cellular immune reactivity within the CNS. Trends Neurosci. 9:271–277. [Google Scholar]
- 8.Ford, M.L., and B.D. Evavold. 2005. Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur. J. Immunol. 35:76–85. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen, M., S. Cepok, E. Quak, M. Happel, R. Gaber, A. Ziegler, S. Schock, W.H. Oertel, N. Sommer, and B. Hemmer. 2002. Oligoclonal expansion of memory CD8+ T cells in cerebrospinal fluid from multiple sclerosis patients. Brain. 125:538–550. [DOI] [PubMed] [Google Scholar]
- 10.Babbe, H., A. Roers, A. Waisman, H. Lassmann, N. Goebels, R. Hohlfeld, M. Friese, R. Schroder, M. Deckert, S. Schmidt, et al. 2000. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann, H., I.M. Medana, J. Bauer, and H. Lassmann. 2002. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 25:313–319. [DOI] [PubMed] [Google Scholar]
- 12.Huseby, E.S., D. Liggitt, T. Brabb, B. Schnabel, C. Ohlen, and J. Goverman. 2001. A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J. Exp. Med. 194:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitsch, A., J. Schuchardt, S. Bunkowski, T. Kuhlmann, and W. Bruck. 2000. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 123:1174–1183. [DOI] [PubMed] [Google Scholar]
- 14.Killestein, J., M.J. Eikelenboom, T. Izeboud, N.F. Kalkers, H.J. Ader, F. Barkhof, R.A. Van Lier, B.M. Uitdehaag, and C.H. Polman. 2003. Cytokine producing CD8+ T cells are correlated to MRI features of tissue destruction in MS. J. Neuroimmunol. 142:141–148. [DOI] [PubMed] [Google Scholar]
- 15.McGavern, D.B., and P. Truong. 2004. Rebuilding an immune-mediated central nervous system disease: weighing the pathogenicity of antigen-specific versus bystander T cells. J. Immunol. 173:4779–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan, D.J., R. Liblau, B. Scott, S. Fleck, H.O. McDevitt, N. Sarvetnick, D. Lo, and L.A. Sherman. 1996. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J. Immunol. 157:978–983. [PubMed] [Google Scholar]
- 17.Cserr, H.F., B. Harling, and P.M. Knopf. 1992. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 2:269–276. [DOI] [PubMed] [Google Scholar]
- 18.Cabarrocas, J., J. Bauer, E. Piaggio, R. Liblau, and H. Lassmann. 2003. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur. J. Immunol. 33:1174–1182. [DOI] [PubMed] [Google Scholar]
- 19.Fabriek, B.O., I. Galea, V.H. Perry, and C.D. Dijkstra. 2005. Cerebral perivascular macrophages and the blood brain barrier. In The Blood-Brain Barrier and Its Microenvironment: Basic Physiology to Neurological Disease. H.E. de Vries and A. Prat, editors. Taylor and Francis, New York. 295–316.
- 20.Galea, I., K. Palin, T.A. Newman, N. van Rooijen, V.H. Perry, and D. Boche. 2005. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 49:375–384. [DOI] [PubMed] [Google Scholar]
- 21.Vass, K., and H. Lassmann. 1990. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am. J. Pathol. 137:789–800. [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhardt, B., and R.M. Ransohoff. 2005. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 26:485–495. [DOI] [PubMed] [Google Scholar]
- 23.Dustin, M.L., S.K. Bromley, Z. Kan, D.A. Peterson, and E.R. Unanue. 1997. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA. 94:3909–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dustin, M.L., and T.A. Springer. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 341:619–624. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson, S.L., L. Wooldridge, S. Tafuro, B. Laugel, M. Glick, J.M. Boulter, B.K. Jakobsen, D.A. Price, and A.K. Sewell. 2003. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J. Biol. Chem. 278:24285–24293. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga, Y., Y. Kita, A. Satoh, S. Asai, K. Kato, K. Ishikawa, T. Horiuchi, and T. Takashi. 1998. Affinity and kinetic analysis of the molecular interaction of ICAM-1 and leukocyte function-associated antigen-1. J. Immunol. 161:4016–4022. [PubMed] [Google Scholar]
- 27.Marelli-Berg, F.M., M.J. James, J. Dangerfield, J. Dyson, M. Millrain, D. Scott, E. Simpson, S. Nourshargh, and R.I. Lechler. 2004. Cognate recognition of the endothelium induces HY-specific CD8+ T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood. 103:3111–3116. [DOI] [PubMed] [Google Scholar]
- 28.Savinov, A.Y., F.S. Wong, A.C. Stonebraker, and A.V. Chervonsky. 2003. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J. Exp. Med. 197:643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabb, T., P. von Dassow, N. Ordonez, B. Schnabel, B. Duke, and J. Goverman. 2000. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. J. Exp. Med. 192:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobel, R.A., and M.B. Ames. 1988. Major histocompatibility complex molecule expression in the human central nervous system: immunohistochemical analysis of 40 patients. J. Neuropathol. Exp. Neurol. 47:19–28. [DOI] [PubMed] [Google Scholar]