Abstract
Peroxisome proliferator–activated receptor (PPAR)α is a nuclear receptor that mediates gender differences in lipid metabolism. PPARα also functions to control inflammatory responses by repressing the activity of nuclear factor κB (NF-κB) and c-jun in immune cells. Because PPARα is situated at the crossroads of gender and immune regulation, we hypothesized that this gene may mediate sex differences in the development of T cell–mediated autoimmune disease. We show that PPARα is more abundant in male as compared with female CD4+ cells and that its expression is sensitive to androgen levels. Genetic ablation of this gene selectively removed the brake on NF-κB and c-jun activity in male T lymphocytes, resulting in higher production of interferon γ and tumor necrosis factor (but not interleukin 17), and lower production of T helper (Th)2 cytokines. Upon induction of experimental autoimmune encephalomyelitis, male but not female PPARα−/− mice developed more severe clinical signs that were restricted to the acute phase of disease. These results suggest that males are less prone to develop Th1-mediated autoimmunity because they have higher T cell expression of PPARα.
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) characterized by recurrent episodes of immune-mediated attack on oligodendrocytes leading to myelin and axon damage and progressive motor and cognitive disability (1, 2). Examination of the gene expression profiles of MS plaques has revealed abundant evidence of Th1-, Th17-, and B cell–mediated immunological activity (3). Studies in experimental autoimmune encephalomyelitis (EAE) and in MS suggest that Th1 effector cells are important in initiating acute attacks (4, 5). Myelin-reactive Th1 cells comprise the majority of CNS-infiltrating cells during acute EAE (4), and treatment with IFN-γ causes relapses in MS (6). On the other hand, Th17 cells, although highly encephalitogenic (4), appear to be less relevant to acute EAE but important in mediating progression of this disease (5). Consistent with this, IL-17 is expressed at higher levels in chronic versus acute MS lesions (3). In contrast, Th2 cytokines such as IL-4 are associated with amelioration of EAE and remission in MS (7, 8).
An interesting feature of MS is that twice as many women as men develop the disease (9). Women are also more susceptible than men to develop a host of other autoimmune conditions, including rheumatoid arthritis (female to male ratio of 2:1), systemic lupus erythematosus (9:1), Sjogren's syndrome (9:1), and Hashimoto's thyroiditis (9:1) (9). The higher female prevalence of these diseases may be related to the fact that women develop more robust immune responses than men (10). Women have higher plasma levels of IgM (11) and higher CD4+ cell counts (12), and show stronger humoral- and cell-mediated responses to vaccination with a variety of antigens (10). Women are also reported to be more prone than male counterparts to develop Th1-polarized responses directed against myelin antigens during MS (13). Studies in mice have shown that this propensity to develop Th1 immunity also helps females clear certain viruses (i.e., Theilers murine encephalomyelitis and herpes simplex virus-1) more effectively (14–16). Whether gender differences also exist in the development of Th17 immunity is not known.
Although the molecular basis of the gender dichotomy in the development of Th1↔Th2 immunity is not understood, a large body of evidence suggests that sex hormones, particularly androgens, influence this process (10, 17). For instance, the castration of male mice, but not the ovariectomy of female mice, enhances Th1 autoimmunity and lowers Th2 cytokine production, leading to exacerbation of EAE (17–18) and acceleration of lupus (19) and diabetes (20) in animal models. In contrast, exogenous administration of testosterone or α-dihydroxytestosterone (DHT) promotes a Th2 bias in cytokine production that leads to suppression of these Th1-mediated autoimmune diseases (18, 21, 22). Akin to this, male MS and rheumatoid arthritis patients are reported to have diminished testosterone levels compared with age-matched controls (23, 24), and androgen treatment relieves clinical symptoms in hypogonadal patients with rheumatoid arthritis (25). Recent studies suggest that androgens act directly on CD4+ cells to increase Th2 cytokine production (22, 26). However, the genes in T cells that are sensitive to androgens and modulate Th responses have not been identified.
One potential gene candidate is peroxisome proliferator–activated receptor (PPAR)α, a member of the nuclear hormone receptor subfamily. This transcription factor, along with other PPAR family members, regulates whole body lipid and glucose homeostasis and controls inflammatory responses (27, 28). In metabolic tissues such as the liver, ligand activation of PPARα via either natural fatty acid intermediates (i.e., unsaturated fatty acids and leukotreine B4) or synthetic fibrate drugs (i.e., Gemfibrozil) leads to transcriptional activation of a subset of mitochondrial and peroxisomal enzymes that are involved in lipid mobilization and catabolism (28). However, in immune cells, PPARα primarily functions to inhibit inflammatory pathways through sequestration and repression of c-jun and NF-κB transcription factors (27, 29). Interestingly, studies of the hepatic function of PPARα have shown that this nuclear receptor is expressed at a higher level in male as compared with female rats (30) and can mediate gender dimorphism in lipid metabolism (31–33).
Here, we report that gender differences in PPARα action also extend to the antiinflammatory activities of this receptor. We show that PPARα is expressed at higher levels in male as compared with female T cells in mice and that this expression is reduced by castration and increased by α-DHT treatment. We also show that deficiency of the PPARα gene selectively affected the functioning of male T lymphocytes such that it resulted in higher IFN-γ and TNF (but not IL-17) production by these cells. Moreover, in comparison to WT counterparts, male but not female PPARα−/− mice developed more severe EAE that was restricted to the acute phase of disease. These findings thus provide a molecular basis for why males may be less prone to developing Th1-mediated autoimmunity.
RESULTS
PPARα−/− mice develop more severe EAE, but only in males
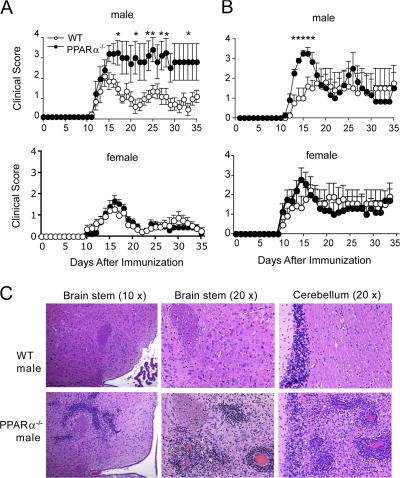
It has been reported that PPARα has a more prominent role in regulating lipid metabolism in male rodents (30–33). To investigate whether the gender dimorphism in PPARα function also extends to antiinflammatory activities of this receptor, we compared the clinical course of EAE in male and female WT versus PPARα−/− mice. EAE was induced in these mice (SV.129 strain, H2b) via immunization with a peptide encoding amino acids 35–55 of myelin oligodendrocyte glycoprotein (MOG p35-55) in CFA. We found in two separate experiments that male PPARα−/− mice displayed a more severe clinical course of EAE compared with WT counterparts, particularly in the acute phase of disease (Fig. 1, A and B, and Table I). Although disease appeared more chronic in male PPARα−/− mice in Fig. 1 A, it is because half of the mice died in the acute phase and were given a clinical score of 5 for the remaining days of the experiment. In contrast to findings for males, the severity of EAE in PPARα−/− females was almost indistinguishable from WT counterparts (Fig. 1, A and B, and Table I). Analysis of the histological features of EAE in male mice revealed that compared with WT, PPARα−/− mice showed higher numbers of parenchymal inflammatory foci in the cerebellum and brainstem in the acute phase of EAE (Fig. 1 C and Table I).
Figure 1.
Male PPARα−/− mice developed more severe clinical signs and displayed increased numbers of inflammatory brain lesions in the acute phase of EAE. Male and female WT or PPARα−/− SV.129 mice (n = 8–10/group) were immunized with MOG p35-55 in CFA and given intravenous injections of pertussis toxin on days 0 and 2 after immunization. (A and B) Mean + SEM clinical scores of mice in the different groups at various times after immunization. Shown are results of two independent EAE experiments. * indicates a significant difference from WT group (P < 0.05) as determined using a Mann-Whitney U statistic. (C) Paraffin-embedded sections of brain stem and cerebellum from representative male WT and PPARα−/− mice (from experiment no. 2) during the acute phase of EAE stained with hematoxylin and eosin. Bar in the bottom right, 50 μM.
Table I.
Clinical and histological features of EAE in male and female WT and PPARα−/− mice
| WT male |
PPARα−/−
male |
WT female |
PPARα−/−
female |
|
|---|---|---|---|---|
| Clinical features | ||||
| Maximum score | ||||
| Experiment 1 | 2.5 (0.3) | 3.8 (0.4)a | 1.9 (0.2) | 1.9 (0.2) |
| Experiment 2 | 2.4 (0.5) | 3.5 (0.3)a | 2.7 (0.6) | 2.9 (0.3) |
| Death from disease | ||||
| Experiment 1 | 0/8 | 3/7 | 0/18 | 0/15 |
| Experiment 2 | 1/8 | 1/8 | 2/9 | 1/9 |
| Incidence | ||||
| Experiment 1 | 8/8 | 7/7 | 17/18 | 14/15 |
| Experiment 2 | 8/8 | 8/8 | 7/9 | 9/9 |
| Day of onset | ||||
| Experiment 1 | 14.2 (1.2) | 12.8 (0.3) | 13.9 (0.8) | 14.5 (1.0) |
| Experiment 2 | 16.4 (1.7) | 12.2 (0.2)a | 14.4 (1.8) | 11.1 (0.5) |
| Cumulative scoreb | ||||
| Experiment 1 | 12.5 (2.3) | 25.3 (5.2)a | 9.2 (1.4) | 10.3 (1.6) |
| Experiment 2 | 16.6 (6.2) | 23.3 (9.5) | 19.4 (7.0) | 22.8 (6.1) |
| Histological features (Experiment 2) | ||||
| Parenchymal inflammatory foci | ||||
| Cerebellum | 15 (8) | 31 (13) | 12 (2) | 12 (7) |
| Brain stem | 10 (4) | 23 (1)a | 22 (11) | 8 (4) |
| Cerebrum | 16 (6) | 39 (14) | 14 (4) | 2 (1)a |
| Spinal cord | 23 (15) | 49 (6) | 23 (9) | 32 (18) |
| Total | 64 (33) | 142 (27) | 71 (21) | 54 (31) |
| Meningeal inflammatory foci | ||||
| 139 (55) | 147 (7) | 123 (11) | 58 (12)a | |
Values are means (SEM).
A significant difference from WT counterpart; P < 0.05.
Up until day 22 (approximate time of remission after acute phase).
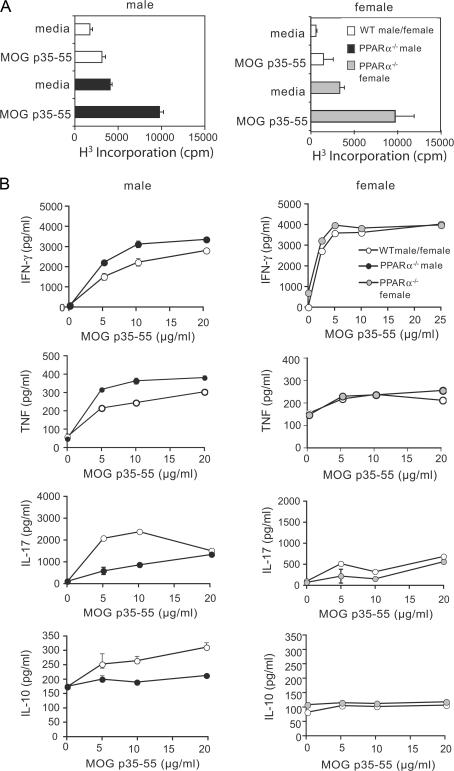
There appeared to be an immune basis for the more severe clinical phenotype in male PPARα−/− mice because MOG p35-55–reactive splenocytes from these mice proliferated more robustly (Fig. 2 A, left) and secreted higher levels of Th1 cytokines, such as IFN-γ and TNF, and lower levels of the antiinflammatory cytokine IL-10 (Fig. 2 B, left) compared with male WT splenocytes. Interestingly, the absence of PPARα in male splenocytes also resulted in lower production of IL-17 (Fig. 2 A, left). On the other hand, although female PPARα−/− MOG p35-55–reactive splenocytes displayed heightened proliferative responses (Fig. 2 A, right), no major differences in cytokine production were apparent between female WT and PPARα−/− groups (Fig. 2 B, right).
Figure 2.
Splenocytes from MOG-immunized male PPARα−/− mice secreted higher levels of IFN-γ and TNF compared with WT counterparts. Splenocytes from male and female SV.129 WT or PPARα−/− mice were harvested 10 d after EAE induction and stimulated with 0–20 μg/ml MOG p35-55. (A) Proliferation of cells in response to 5 μg/ml MOG peptide was measured by [3H]thymidine incorporation (cpm). Values are means ± SEM counts of radioactivity per minute (cpm) of triplicate culture wells. (B) Cytokine levels in culture supernatants were measured by ELISA at 48 (IL-17), 72 (IFN-γ and TNF), and 120 h (IL-10) after stimulation. Values are means ± SEM of cytokine levels (pg/ml) in triplicate culture wells. Note that IL-4 was not detected in culture supernatants. Results are representative of two to three independent experiments.
Analysis of splenic leukocyte distributions revealed a tendency (P = 0.1) for a higher T cell/B cell ratio in PPARα−/− mice of both genders (Table II), which may explain the higher proliferation, but not the sex differences in cytokine production of MOG p35-55–reactive PPARα−/− splenocytes. Collectively, these results suggest that PPARα selectively operates in male mice to suppress the development of Th1-mediated autoimmunity.
Table II.
Cellular composition of the spleen of male and female WT and PPARα−/− SV.129 mice
| Cell marker | WT male | PPARα−/− male | WT female | PPARα−/− female |
|---|---|---|---|---|
| CD3+ | 30.9 (1.9) | 35.2 (5.1) | 31.7 (2.0) | 36.1 (3.1) |
| CD8+ | 8.7 (0.5) | 9.1 (1.9) | 9.9 (0.4) | 11.3 (1.4) |
| CD4+ | 18.4 (0.8) | 20.2 (2.2) | 20.7 (0.8) | 22.7 (1.1) |
| CD4+CD25+ | 4.2 (2.2) | 3.5 (1.6) | 5.4 (1.4) | 4.7 (1.0) |
| CD11b+ | 3.6 (0.1) | 3.8 (0.8) | 5.4 (0.5) | 4.0 (1.4) |
| CD11c+ | 2.1 (0.5) | 1.5 (0.2) | 1.9 (0.3) | 2.0 (0.5) |
| B220+ | 65.5 (1.1) | 57.5 (6.3) | 55.8 (5.3) | 50.7 (4.0) |
| CD3+/B220 | 0.47 | 0.61 | 0.56 | 0.72 |
Values are means (SEM) of three experiments. There were no significant differences between groups.
T cells from male but not female PPARα−/− mice are hyperresponsive to TCR stimulation and trigger an earlier onset of EAE
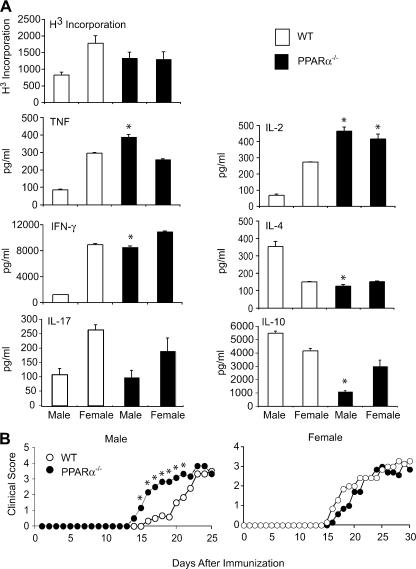
PPARα is expressed in T and B lymphocytes (27) and is detected at low levels in peritoneal macrophages and immature dendritic cells (34). To examine whether the enhanced Th1-mediated autoimmunity in male PPARα−/− mice was a result of intrinsic differences in these immune populations, we purified CD3+ T cells, B cells, and macrophages from male and female WT and PPARα−/− mice and compared the activation responses of these cells ex vivo. Consistent with previous reports (26), we found that female WT CD3+ cells proliferated more robustly and secreted higher levels of IFN-γ and TNF and lower amounts of Th2 cytokines (IL-4 and IL-10) as compared with male WT CD3+ cells in response to anti-CD3 and anti-CD28 costimulation (Fig. 3 A). Female CD3+ cells also produced higher levels of IL-17. On the other hand, male and female PPARα−/− T cells displayed similar activation characteristics that more closely resembled the Th1 profile of female WT cells (Fig. 3 A), with the exception that IL-17 production remained low in PPARα−/− male cells. Similar results were observed using CD4+-purified cells (not depicted). Notably, we did not observe major sex differences in the LPS- or IgM-elicited responses of purified B cells or LPS- or IFN-γ–elicited responses of peritoneal macrophages derived from WT or PPARα−/− mice (not depicted), thus indicating that intrinsic differences in T cells are likely responsible for the enhanced Th1 autoimmunity observed in male PPARα−/− mice.
Figure 3.
T cells from male PPARα−/− mice were hyperresponsive to TCR stimulation and caused an earlier onset of EAE. (A) CD3+ T cells from male and female SV.129 WT or PPARα−/− mice were stimulated with 1 μg/ml anti-CD3 and anti-CD28. The proliferation rate was determined by [3H]thymidine incorporation (cpms), and cytokine production was measured in culture supernatants by ELISA at 48 (IL-2), 72 (IFN-γ, TNF, and IL-17), and 120 h (IL-10 and IL-4) after stimulation. Values are means ± SEM of triplicate culture wells. * indicates a significant difference (P < 0.05) from WT counterpart. Results are representative of at least three independent experiments. (B) Naive CD4+ T cells from male and female WT and PPARα−/− mice were adoptively transferred into syngeneic male or female RAG2−/− mice by intravenous injection, and EAE was induced in recipient mice 2 d later via immunization with MOG p35-55 in CFA. Mean clinical scores of mice in the different groups at various times after immunization are shown. Results are representative of two independent experiments. * indicates a significant difference from WT (P < 0.05).
To test this notion, we reconstituted male and female RAG2−/− mice with gender-matched syngeneic PPARα−/− CD4+ T cells to generate chimeric mice that were PPARα sufficient except for their CD4+ T cell population. Upon induction of EAE, mice transferred with male PPARα−/− T cells showed a much earlier onset of clinical signs compared with male RAG2−/− mice reconstituted with male WT T cells (Fig. 3 B). In contrast, differences in disease onset were not apparent between these two groups in females (Fig. 3 B), confirming that intrinsic differences in T cells likely contribute to the more severe acute EAE developed in male PPARα−/− mice.
PPARα is more abundant in male as compared with female T cells and represses the DNA binding of NF-κB and c-jun
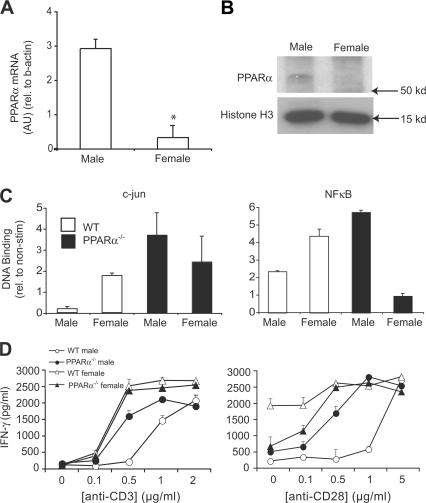
In light of reports of gender differences in hepatic PPARα expression (30), we investigated whether the selective influence of PPARα on the functioning of male T cells is due to higher levels of expression of this nuclear receptor. Consistent with this notion, we found that PPARα mRNA expression was higher in male as compared with female CD4+ cells derived from SV.129 (Fig. 4 A), C57/B6, or SJL/J mice (Fig. S1, A and B, available at http://www.jem.org/cgi/content/full/jem.20061839/DC1). This trend for higher male expression was also evident at the protein level as detected via Western blot analysis of nuclear extracts from naive T cells (Fig. 4 B). Compared with the situation in naive T cells, gender differences in PPARα mRNA expression were not as striking in CD4+ cells at 48 h after stimulation with anti-CD3 and anti-CD28 (Fig. S1 C). The latter finding suggests that PPARα may mediate the gender dichotomy in Th1↔Th2 differentiation by influencing T cell responses in the early period after antigen encounter.
Figure 4.
PPARα was more abundant in male as compared with female T cells and was associated with decreased NF-κB and c-jun activity and increased IFN-γ production. (A) Total RNA was obtained from naive CD4+ T cells that were pooled from the spleens of male and female SV.129 mice (n = 4 mice/group). The expression of PPARα mRNA in these cells was measured using real-time RT-PCR, and abundance was expressed relative to β-actin mRNA. Values are means ± SEM of PPARα/β-actin product abundance in triplicate reactions expressed in arbitrary units (AU). (B) Western blot analysis of PPARα in nuclear extracts (250 μg) prepared from male and female T cells. Histone H3 was used as a loading control. (C) c-jun (left) and NF-κB (right) DNA binding was measured in nuclear extracts from male and female SV.129 WT or PPARα−/− CD3+ T cells using an ELISA-based assay. Nuclear extracts were prepared from T cells at 16 h after stimulation with 5 μg/ml anti-CD3 and 5 μg/ml anti-CD28. Values are means ± SEM of absorbance units (duplicate culture wells) of stimulated wells expressed relative to absorbance in nonstimulated control wells. (D) CD3+ T cells from male and female SV.129 WT or PPARα−/− mice were stimulated with 0–2 μg/ml anti-CD3 (left) and 0–5 μg/ml anti-CD28 (right). IFN-γ production in culture supernatants was measured by ELISA at 72 h after stimulation. Values are means ± SEM of triplicate culture wells. Note that anti-CD28 and anti-CD3 were held constant at 0.5 μg/ml in the left and right panels, respectively. Results in A–D are representative of two independent experiments.
It has been reported that upon ligand activation, PPARα binds to and represses NF-κB or c-jun, preventing these transcription factors from binding DNA (29). Both NF-κB and c-jun convey signals downstream of the TCR, IL-18R, and CD28 to transactivate the IFN-γ promoter and promote Th1 differentiation (35–37). Consistent with our finding of higher male expression of PPARα, we found that the DNA binding of NF-κB or c-jun to target DNA consensus elements was lower in male as compared with female WT T cells, and that it was selectively increased in male T cells in the absence of PPARα (Fig. 4 C). This higher NF-κB and c-jun activity in male PPARα−/− T cells was also associated with a lower threshold of activation of these cells such that they produced IFN-γ at much lower concentrations of anti-CD3 and anti-CD28 as compared with male WT counterparts (Fig. 4 D). In contrast to findings of male T cells, NF-κB activity was lower in female PPARα−/− as compared with female WT cells (Fig. 4, C and D).
In addition to PPARα, T cells also express lower levels of PPARγ and PPARδ, which can also transrepress NF-κB activity (27). We therefore investigated whether the gender dimorphism in T cell activation was related to expression of these other PPAR isoforms. Real-time RT-PCR analysis showed that PPARγ had an opposite pattern of expression than PPARα, with relatively higher mRNA levels in female as compared with male CD4+ T cells, whereas sex differences in PPARδ mRNA expression were not apparent in these cells (Fig. S1 D). Interestingly, the mRNA levels of PPARγ and PPARδ were both elevated in a compensatory fashion in PPARα−/− cells. This finding that male PPARα−/− cells were hyperresponsive and displayed higher NF-κB activity in the face of elevations in PPARγ and PPARδ highlights the special regulatory role of PPARα in male T cells. On the other hand, the higher expression of PPARγ and PPARδ in female PPARα−/− T cells (Fig. S1 D) did appear to have a functional impact because it was associated with lowered NF-κB activity in these cells (Fig. 4 C).
PPARα expression is sensitive to testosterone and mediates androgen sensitivity of Th responses
Given the male bias in PPARα expression in T cells, we next investigated whether this gene is sensitive to androgen levels. We found that castration of male mice, which lowered serum testosterone (0.05 ± 0.01 ng/dL in castrated vs. 6.21 ± 2.91 ng/dL in sham-operated mice), decreased the mRNA levels of PPARα in CD4+ T cells pooled from these mice (Fig. 5 A). On the other hand, implantation of female mice with pellets containing the testosterone metabolite 5α-DHT (5 mg) greatly enhanced the expression of PPARα in CD4+ T cells over levels observed in the placebo-treated group (Fig. 5 B). We also investigated the association between PPARα in T cells and serum testosterone levels in individual male mice (n = 10 mice in two cages). As is typical of group-housed males, several males in the cohort had serum testosterone levels that were ∼10-fold higher than cage mates (Fig. 5 C). Interestingly, these dominant males, as defined by testosterone levels, also displayed the highest transcript levels of PPARα in their T cells (Fig. 5 C). Collectively, these findings suggest that the expression of PPARα in T cells correlates with circulating androgen levels.
Figure 5.
PPARα mediates androgen sensitivity of Th responses. (A and B) Total RNA was obtained from naive CD4+ T cells that were pooled from the spleens of castrated- or sham-operated male (n = 4 mice/group) (A) or placebo or α-DHT pellet-implanted female (n = 4 mice/group) (B) mice. The expression of PPARα mRNA in these cells was measured using real-time RT-PCR, and abundance was expressed relative to β-actin mRNA. Values are means ± SEM of PCR product abundance in arbitrary units (AU). * indicates a significant difference (P < 0.05) from either sham (A) or placebo (B). (C) Correlation of serum testosterone (ng/dL) and T cell PPARα mRNA levels in 10 individual mice that were group housed in two cages. (D) CD3+ T cells from sham or castrated male WT and PPARα−/− mice were isolated at 4 wk after surgery and stimulated with 1 μg/ml anti-CD3 and 0.5 μg/ml anti-CD28. Cytokine secretion by T cells was assessed by ELISA analysis of culture supernatants at 48 (IL-2), 72 (IFN-γ, TNF, and IL-17), and 120 h (IL-10) after stimulation. Values are means ± SEM of cytokine levels in triplicate culture wells. * indicates a significant difference (P < 0.05) from sham counterpart.
It is reported that reduction in androgen levels with castration enhances the development of Th1 responses and suppresses the development of Th2 responses in male mice (22). To determine the extent to which changes in PPARα expression contribute to the androgen sensitivity of Th responses, we contrasted the effects of castration on the cytokine production of male WT versus PPARα−/− T cells. Consistent with previous findings, castration enhanced production of Th1 cytokines (IFN-γ, IL-2, and TNF) and reduced the production of the Th2 cytokine IL-4 by WT T cells cultured ex vivo with anti-CD3 and anti-CD28 (Fig. 5 D). Castration also enhanced the production of IL-17 and IL-10 by T cells (Fig. 5 D). Interestingly, this effect of castration on cytokine production was blunted in T cells derived from PPARα−/− mice (Fig. 5 D), confirming that changes in PPARα expression mediate a major part of the effects of androgens on T cell cytokine responses.
Treatment of mice with the PPARα agonist Gemfibrozil reduces the incidence of EAE only in male mice
Previously, it was reported that oral administration of 25 mg/kg Gemfibrozil, a potent agonist of PPARα, ameliorates EAE in female B10.PL mice in part by inducing a Th2 bias of myelin-reactive T cells (38). To investigate whether males, in having higher expression of PPARα in naive T cells, are more responsive to the effects of fibrates, we induced EAE in male and female C57BL/6 mice and administered either Gemfibrozil (25 or 50 mg/kg) or vehicle once daily via gavage. 25 and 50 mg/kg treatment groups were not different (P > 0.05) and were pooled for analysis. Although Gemfibrozil treatment tended to decrease the maximum clinical scores of both male (1.3 ± 0.5 in Gemfibrozil vs. 2.4 ± 0.7 in vehicle) and female mice (2.4 ± 0.4 in Gemfibrozil vs. 3.3 ± 0.6 in vehicle) as compared with the vehicle control group, it lowered the incidence of disease only in males (43% in Gemfibrozil vs. 71% in vehicle group in males; 79% in Gemfibrozil vs. 82% in vehicle group in females). These results provide further support for a role for PPARα in modulating the threshold of activation during the initial priming of autoreactive T cells.
DISCUSSION
Compared with males, females mount heightened Th1 immunity in response to immunization with a variety of antigens. This propensity to develop Th1 immune responses also correlates with the greater likelihood of females to develop MS and other autoimmune diseases (9). Studies in EAE have demonstrated that gender differences in immunity are shaped by the levels of androgens that are present in the lymphoid microenvironment during the initial development of the encephalitogenic T cell responses (22, 26). In the present study, we identify PPARα as a gene in CD4+ T cells that is sensitive to androgen levels and through expression changes can influence the development of Th fate. We show that PPARα is expressed at higher levels in male as compared with female naive T cells, which correlates with lowered TCR-induced NF-κB and c-jun activity and higher Th2 cytokine production by these cells. Genetic ablation of PPARα selectively removed the brake on NF-κB and c-jun in male T lymphocytes, resulting in an increased production of IFN-γ and TNF, but not IL-17. These hyper-Th1 male PPARα−/− CD4+ cells caused more severe paralysis during EAE that was restricted to the acute phase of disease.
Our finding that the sex differences in PPARα expression were more prominent in naive than in activated T cells suggests that this nuclear receptor may mediate gender dimorphism in encephalitogenic T cell responses in the early period after antigen priming. This window of T cell activation is a time when Th fate is influenced by the strength of signaling downstream of the TCR and CD28 (39, 40). For instance, antigens that bind the TCR weakly induce transient activation of downstream pathways (i.e., MAPK) that favors Th2 differentiation, whereas antigens that bind to the TCR with strong avidity trigger more sustained MAPK signaling events that lead to Th1 differentiation (39, 40). Taken in this light, our finding that elevated PPARα expression in male T cells was associated with decreased DNA binding of transcription factors (NF-κB and c-jun) that transactivate the IFN-γ promoter downstream of the TCR-MAPK and CD28 signaling (35, 37) explains why male naive T cells may be less likely to differentiate along the Th1 path. The findings that PPARα did not influence TCR- and CD28-induced IL-17 production by T cells and that the absence of this receptor was associated with lowered IL-17 production by male PPARα−/− MOG-reactive splenocytes suggest that NF-κB and c-jun transcription factors do not mediate the production of this cytokine downstream of antigen signals. These results thus align with emerging data that Th17 cells develop via a lineage that is separate from Th1 cells (41–44).
It is reported that PPARα must bind either endogenous or synthetic lipid ligands to repress NF-κB and c-jun activity (27). Our observation that PPARα expression in male T cells was associated with lowered DNA binding of these transcription factors indicates that PPARα may bind endogenous ligands to effect changes in T cell activation. The identity of these natural ligands is not known but may include monounsaturated and polyunsaturated fatty acids (C18-C22), the lipid mediator oleoylethanolamide, or various eicosanoids (5S-hydroxy-6,8,11,14-eicosatetraenoic acid, or leukotriene B4) that are generated during inflammatory responses (45–47). In addition to these effects of ligand-bound PPARα on NF-κB and c-jun, it has also been reported that ligand-free PPARα may inhibit the phosphorylation of p38 (48), a kinase that also promotes IFN-γ transcription downstream of TCR stimulation (49). Future studies should distinguish whether PPARα binds endogenous lipids or acts in a ligand-free fashion to mediate its suppressive effects on IFN-γ and TNF production.
We found that male RAG2−/− mice reconstituted with male PPARα−/− T cells developed an earlier onset of EAE as compared with mice provided with WT T cells. Similarly, male PPARα−/−mice developed more severe paralysis compared with WT counterparts in the acute phase of EAE, but not in subsequent relapses of disease. Collectively, these results indicate that the effect of PPARα in dampening CNS inflammation in male mice is restricted to the early stages of EAE development. These findings therefore align with recent reports that suggest that Th1 cells (the T cell subset influenced by PPARα) are more important in the initiation of EAE, whereas Th17 cells are more critical for disease progression (4, 5). An alternative explanation for the limited influence of PPARα on disease progression is that once EAE is initiated, proinflammatory cytokines present in the circulation may neutralize any suppressive effects of the testosterone–PPARα axis on immune responses. In support of this notion, serum testosterone levels drop in the acute phase of EAE (24), and PPARα mRNA levels are suppressed in the liver by inflammation (50). Nonetheless, our findings that PPARα plays a role in the initiation of EAE and alters the threshold of T cell activation, collectively with our observation that Gemfibrozil altered EAE incidence in males but not females, strongly support a role for this molecule in regulating gender susceptibility to autoimmune disease.
Our finding that the proinflammatory effect of castration was severely blunted in purified PPARα−/− T cells suggests that changes in the expression of PPARα in T cells explain the bulk of the effects of androgens on Th fate. However, our results do not preclude that sex hormones or chromosomes influence the development of Th autoimmunity via their action on other genes in T cells or other immune cell populations. Indeed, APCs also exhibit gender dimorphism in cytokine production (51). Female APCs secrete more of the Th1-promoting cytokine IL-12, whereas male APCs secrete more IL-10 upon T cell–mediated activation (51). A recent study also suggests that male T regulatory cells may be more competent at suppressing the activation of autoreactive CD4+ T cells (52). Moreover, our results do not take into account the influences of the adipokine leptin (53) or the Y chromosome (54) on Th fate. Leptin is present in the circulation at higher levels in females and has been shown to enhance the Th1 differentiation of myelin-reactive cells during EAE (53), whereas Y chromosome–encoded genes appear to antagonize the suppressive actions of androgens on Th1 responses (54). Finally, the fact that female SV.129 mice (this study) and female mice of other H2b- or H2u-restricted strains (17, 18) fail to develop more severe EAE, despite having more robust T cell–mediated autoimmunity than male counterparts (this study and reference 22), highlights the role of T cell–independent factors in the development of CNS inflammation.
In conclusion, our findings suggest that males tend to develop protective Th2 immune responses because they have higher levels of androgens that drive T cell expression of PPARα. Our results may help to explain why females are more susceptible to develop MS and other autoimmune diseases. Future studies should investigate whether PPARα also exhibits a sexual dimorphic pattern of expression in humans and influences the development of MS. This knowledge that androgens act through PPARα to dampen inflammation should also be considered in the design of future clinical trials testing the efficacy of fibrate agonists of this receptor and androgens in the treatment of T cell–mediated autoimmune diseases.
MATERIALS AND METHODS
Mice.
SV.129 WT (129S1/SvImJ), PPARα−/− (129S4/SvJae-Pparatm1Gonz), SJL/J, and C57/BL6 mice were from The Jackson Laboratory. RAG2−/− mice (129S6/SvEvTac-Rag2tim1Fwa) were from Taconic Farms. Colonies of WT and PPARα−/− mice were maintained in our animal facility. Genotyping of mice was performed by amplifying a portion of exon 8 of the ligand-binding domain of PPARα from tail DNA (isolated using a Dneasy kit; QIAGEN). Cycling conditions were as follows: 1 min at 94°C, 1 min at 60°C, and 1 min at 72°C. Primer sequences were: PPARα (sense): 5′-CAGAAGTTGCAGGAGGGGATTGTG-3′ and PPARα (antisense): 5′-CAGAAGTTGCAGGAGGGGATTGTG-3′.
Adoptive transfer.
CD4+ T cells were purified from the spleens of male and female SV.129 WT or PPARα−/− mice using negative selection columns (R&D Systems), and cells were suspended in sterile PBS. 7 × 106 T cells were injected intravenously into naive male and female RAG2−/− recipients 48 h before EAE induction.
EAE induction.
EAE was induced in mice (8–12 wk of age) via subcutaneous immunization with 100 μg MOG p35-55 in an emulsion (vol ratio of 1:1) with CFA (containing 4 mg/ml of heat-killed Mycobacterium tuberculosis H37Ra; Difco Laboratories). Mice were also injected intravenously with Bordetella pertussis toxin (Difco Laboratories) in PBS at the time of and 2 d after immunization. MOG p35-55 peptide was synthesized by the Stanford Pan Facility and purified by HPLC. Mice (n = 7–10 per treatment group) were examined daily for clinical signs of EAE and were scored as followed: 0, no clinical disease; 1, limp tail; 2, hindlimb weakness; 3, complete hindlimb paralysis; 4, hindlimb paralysis plus some forelimb paralysis; 5, moribund or dead. All animal protocols were approved by the Division of Comparative Medicine at Stanford University, and animals were maintained in accordance with the guidelines of the National Institutes of Health. Splenocytes were harvested from representative mice (n = 2–3/group) at 10 d after immunization.
Histopathology.
Brains and spinal cords were dissected from mice, fixed in 10% formalin in PBS, and embedded in paraffin. 10-μm-thick sections were stained with hematoxylin and eosin. Inflammatory lesions in selected brain, thoracic, and lumbar spinal cord sections were counted by an examiner blinded to the clinical and treatment status of the animal.
Castration and hormone pellet implantation.
All surgeries were approved by the Division of Comparative Medicine at Stanford University. Mice were anaesthetized intraperitoneally with 80 mg/kg ketamine hydrochloride/16 mg/kg xylazine. For castration surgery, the skin on the abdomen of each mouse was shaved and disinfected, and a mid-ventral incision (1–1.5 cm) was made just above the penis to expose the abdominal fat pads. Left and right fat pads were each pulled outwards to expose underlying testes. Testes were removed by severing each vas deferens using a Bovie cautery stick, and the abdominal incision was closed using surgical silk and staples. For sham surgeries, the abdominal fat pads were pulled outwards, but the vas deferens was not severed.
90-d release pellets of 5α-DHT (5 mg dose) or placebo pellets that contained carrier binder alone (cholesterol-methyl cellulose-a-lactulose) were purchased from Innovative Research of America. To implant pellets, a midline incision was made in the scapular area of the neck. Hormone or placebo pellets were implanted subcutaneously, and the incision was closed with surgical staples.
Serum testosterone measurement.
Testosterone was measured in the serum (from blood drawn at 10 a.m.) by the Department of Comparative Medicine at Stanford University.
Activation assays and cytokine analysis.
Male or female splenocytes (0.5 × 106 cells/well) or CD3+ or CD4+ T cells (5 × 104 cells/well) purified by negative selection (columns from R&D Systems) were cultured in flat-bottomed 96-well plates in media (RPMI 1640 supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.5 μM 2-mercaptoethanol, and 10% FCS) with 1–25 μg/ml MOG p35-55 peptide or with 0.1–5 μg/ml each of anti-CD3 (clone 145-2C11; BD Biosciences) and anti-CD28 (clone 37.51; BD Biosciences).
To assess proliferation rate, cultures were pulsed with [3H]thymidine (1 μCi/well) after 24–72 h of culture and 18 h later were harvested onto filter paper. The counts per minute of incorporated [3H] thymidine were read using a β counter. Cytokines were measured in the supernatants of cultured cells using anti–mouse OPTEIA ELISA kits (BD Biosciences). Supernatants were taken at the time of peak production for each cytokine (48 h, IL-2; 72 h, IFN-γ, TNF, and IL-17; 120 h, IL-4 and IL-10).
FACS analysis of leukocyte subsets.
Spleens were isolated from male and female naive SV.129 WT and PPARα−/− mice (n = 3/group). A single cell suspension was prepared, and red blood cells were lysed using hypotonic buffer. 106 splenocytes were washed in 1× PBS, centrifuged at 1,200 rpm, and incubated with various antibodies from BD Biosciences (1:100 dilution in 2% FCS in PBS) in the dark for 20 min at room temperature. Cells were then washed three times with 2% FCS in PBS. Propidium iodide was used to stain dead cells. Three-color fluorescence-activating cell sorting was performed using a FACScan (BD Biosciences). Data were analyzed using FloJo software (version 6.3.3).
Real-time RT-PCR.
CD4+ and CD3+ T cells were purified by negative selection (columns from R&D Systems). Total RNA was isolated from these T cells using the Absolutely RNA RT-PCR Miniprep kit (Statagene). 2 μg RNA was reverse-transcribed, and PPARα, PPARδ, PPARγ, and β-actin cDNAs were amplified according to methods described previously (55) using a Lightcycler (Roche). Primer sequences are as follows: PPARα (same as used for genotyping); PPARδ (sense): GCCTCGGGCTTCACTAC; PPARδ (antisense): AGATCCGATCGCACTTCTCA; PPARγ (sense): CCAGAGTCTGCTGATCTGCG-3′; PPARγ (antisense): GCCACCTCTTTGCTCTGATC-3′; β-actin (sense): 5′-TGTCCCTGTATGCCTCTGGT-3′; and β-actin (antisense): 5′-CACGCACGATTTCCCTCTC-3′. Amplification conditions for PPARδ, PPARγ, and β-actin were 94°C for 5 min, followed by various cycles of 20 s at 94°C, 30 s at 55°C, and 30 s at 72°C.
Western blot analysis.
250 μg of nuclear extract was suspended in 2 vol of 2× SDS Sample Buffer (Bio-Rad Laboratories) and subjected to SDS-PAGE electrophoresis using 10% Tris-HCl Ready Gels (Bio-Rad Laboratories). Proteins were transferred to PVDF membranes, and immunoblotting was performed using conventional methods (Santa Cruz Biotechnology, Inc.) using a rabbit polyclonal PPARα antibody (catalog no. 101710; Cayman Chemical).
DNA binding assays.
Spleens from five to six mice were pooled, and CD3+ cells were purified using negative selection columns (R&D Systems). T cells were suspended in media and cultured in flat-bottomed six-well plates precoated with 5 μg/ml anti-CD3 and anti-CD28. After 16 h, T cells were harvested and nuclear extracts were prepared from these cells using the BD Transfactor Extraction kit (BD Clontech) according to the manufacturer's directions. The concentration of protein in each sample was determined using a BCA assay kit (Pierce Chemical Co.). DNA binding assays for NF-κB and c-jun were performed using BD Transfactor kits (BD Clontech).
Statistical analysis.
Data are presented as means + SEM. When data were parametric (kurtosis and skewness <2) and group variances were homogenous (Bartlett homogeneity test), a one-way analysis of variance and Scheffé post-hoc test (for more than two groups) or a t test (n = 2 groups) were used to detect between-group differences. When data were nonparametric, ranks were compared among groups using a Kruskal-Wallis test and nonparametric test for multiple comparisons (for more than two groups) or a Mann-Whitney U test (n = 2 groups). A value of P < 0.05 was considered significant.
Online supplemental material.
Fig. S1 shows real-time PCR analysis of PPARα, PPARγ, and PPARδ mRNA expression in mouse CD4+ cells. A and B show that the expression of PPARα is higher in WT male versus female CD4+ cells derived from C57BL/6 and SJL/J strains. C shows that PPARα expression is altered in CD4+ cells at 48 h after stimulation with 5 μg/ml anti-CD3 and 5 μg/ml anti-CD28. D shows that PPARδ and PPARγ mRNAs are up-regulated in a compensatory fashion in CD4+ cells in the absence of PPARα. Fig. S1 is available at http://www.jem.org/cgi/content/full/jem.20062839/DC1.
Supplemental Material
Acknowledgments
The authors wish to acknowledge Annette Langer-Gould for insightful comments.
This work was supported by National Institutes of Health and National Multiple Sclerosis Society (NMSS) grants to L. Steinman and fellowships to S.E. Dunn and S.S. Ousman from the NMSS and Multiple Sclerosis Society of Canada (MSSC).
The authors have no conflicting financial interests.
Abbreviations used: CNS, central nervous system; DHT, 5α-dihydroxytestosterone; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PPAR, peroxisome proliferator–activated receptor.
S.E. Dunn and S.S. Ousman contributed equally to this work.
References
- 1.Steinman, L. 1996. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 85:299–302. [DOI] [PubMed] [Google Scholar]
- 2.Noseworthy, J.H., C. Lucchinetti, M. Rodriguez, and B.G. Weinshenker. 2000. Multiple sclerosis. N. Engl. J. Med. 343:938–952. [DOI] [PubMed] [Google Scholar]
- 3.Lock, C., G. Hermans, R. Pedotti, A. Brendolan, E. Schadt, H. Garren, A. Langer-Gould, S. Strober, B. Cannella, J. Allard, et al. 2002. Gene microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8:500–508. [DOI] [PubMed] [Google Scholar]
- 4.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Komiyama, Y., S. Nakae, T. Matsuki, A. Nambu, H. Ishigame, S. Kakuta, K. Sudo, and Y. Iwakura. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573. [DOI] [PubMed] [Google Scholar]
- 6.Panitch, H.S., R.L. Hirsch, J. Schindler, and K.P. Johnson. 1987. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 37:1097–1102. [DOI] [PubMed] [Google Scholar]
- 7.Racke, M.K., A. Bonomo, D.E. Scott, B. Cannella, A. Levine, C.S. Raine, E.M. Shevach, and M. Rocken. 1994. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 180:1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navikas, V., and H. Link. 1996. Review: cytokines and the pathogenesis of multiple sclerosis. J. Neurosci. Res. 45:322–333. [DOI] [PubMed] [Google Scholar]
- 9.Beeson, P.B. 1994. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 96:457–462. [DOI] [PubMed] [Google Scholar]
- 10.Whitacre, C.C., S.C. Reingold, and P.A. O'Looney. 1999. A gender gap in autoimmunity. Science. 283:1277–1278. [DOI] [PubMed] [Google Scholar]
- 11.Lichtman, M.A., J.H. Vaughan, and C.G. Hames. 1967. The distribution of serum immunoglobulins, anti-gamma-G globulins (“rheumatoid factors”) and antinuclear antibodies in White and Negro subjects in Evans County, Georgia. Arthritis Rheum. 10:204–215. [DOI] [PubMed] [Google Scholar]
- 12.Amadori, A., R. Zamarchi, G. De Silvestro, G. Forza, G. Cavatton, G.A. Danieli, M. Clementi, and L. Cheico-Bianchi. 1995. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1:1279–1283. [DOI] [PubMed] [Google Scholar]
- 13.Pelfrey, C., A. Cotleur, J.-C. Lee, and R.A. Rudick. 2002. Sex differences in cytokine responses to myein peptides in multiple sclerosis. J. Neuroimmunol. 130:211–213. [DOI] [PubMed] [Google Scholar]
- 14.Barna, M., T. Komatsu, Z. Bi, and C.S. Reiss. 1996. Sex differences in susceptibility to viral infection of the central nervous system. J. Neuroimmunol. 67:31–39. [DOI] [PubMed] [Google Scholar]
- 15.Fuller, A.C., B. Kang, H.K. Kang, H. Yahikozowa, M.C. Dal Canto, and B.S. Kim. 2005. Gender bias in Theiler's virus-induced demyelinating disease correlates with the level of anti-viral immune responses. J. Immunol. 175:3955–3963. [DOI] [PubMed] [Google Scholar]
- 16.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and interferon-mutant mice. J. Virol. 75:3048–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voskuhl, R.R., and K. Palszynski. 2001. Sex hormones in experimental autoimmune encephomyelitis: implications for multiple sclerosis. Neuroscientist. 7:258–270. [DOI] [PubMed] [Google Scholar]
- 18.Palaszynski, K.M., K.K. Loo, J.F. Ashouri, H.B. Lu, and R.R. Voskuhl. 2004. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 146:144–152. [DOI] [PubMed] [Google Scholar]
- 19.Roubinian, J.R., N. Talal, J.S. Greenspan, J.R. Goodman, and R.P. Siiteri. 1978. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J. Exp. Med. 147:1568–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox, H.S. 1992. Androgen treatment prevents diabetes in nonobese diabetic mice. J. Exp. Med. 175:1409–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalal, M., S. Kim, and R. Voskuhl. 1997. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J. Immunol. 159:3–6. [PubMed] [Google Scholar]
- 22.Bebo, B.F., Jr., J.C. Schuster, A.A. Vandenbark, and H. Offner. 1999. Androgens alter the cytokine profile and reduce encephalogenicity of myelin-reactive T cells. J. Immunol. 162:35–40. [PubMed] [Google Scholar]
- 23.Wei, T., and S.L. Lightman. 1997. The neuroendocrine axis in patients with multiple sclerosis. Brain. 120:1067–1076. [DOI] [PubMed] [Google Scholar]
- 24.Foster, S.C., C. Daniels, D.N. Bourdette, and B.F. Bebo Jr. 2003. Dysregulation of the hypothalamic-pituitary-gonadal axis in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neuroimmunol. 140:78–87. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo, M., E. Balleari, M. Giusti, E. Intra, and S. Accardo. 1991. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 34:1–5. [DOI] [PubMed] [Google Scholar]
- 26.Liva, S.M., and R.R. Voskuhl. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 167:2060–2067. [DOI] [PubMed] [Google Scholar]
- 27.Daynes, R.A., and D.C. Jones. 2002. Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2:748–759. [DOI] [PubMed] [Google Scholar]
- 28.Barbier, O., I.P. Torra, Y. Duguay, C. Blanquart, J.C. Fruchart, C. Glineur, and B. Staels. 2002. Pleiotropic actions of peroxisome proliferators-activated receptors in lipid metabolism and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 22:717–726. [DOI] [PubMed] [Google Scholar]
- 29.Delerive, P., K. De Bosscher, S. Besnard, W. Vanden Berghe, J.M. Peters, F.J. Gonzalez, J.C. Fruchart, A. Tedgui, G. Haegeman, and B. Staels. 1999. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 274:32048–32054. [DOI] [PubMed] [Google Scholar]
- 30.Jalouli, M., L. Carlsson, C. Ameen, D. Linden, A. Ljungberg, L. Michalik, S. Eden, W. Wahli, and J. Oscarsson. 2003. Sex difference in hepatic peroxisome proliferator-activated receptor alpha expression: influence of pituitary and gonadal hormones. Endocrinology. 144:101–109. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins, J.M., W.E. Jones, F.W. Bonner, and G.G. Gibson. 1987. The effect of peroxisome proliferators on microsomal, peroxisomal, and mitochondrial enzyme activities in the liver and kidney. Drug Metab. Rev. 18:441–515. [DOI] [PubMed] [Google Scholar]
- 32.Kawashima, Y., N. Uy-Yu, and H. Kozuka. 1989. Sex-related differences in the enhancing effects of perfluoro-octanoic acid on stearoyl-CoA desaturase and its influence on the acyl composition of phospholipid in rat liver. Comparison with clofibric acid and tiadenol. Biochem. J. 263:897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Djouadi, F., C.J. Weinheimer, J.E. Saffitz, C. Pitchford, J. Bastin, F.J. Gonzalez, and D.P. Kelly. 1998. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator–activated receptor alpha-deficient mice. J. Clin. Invest. 102:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schachtrup, C., T.E. Scholzen, V. Grau, T.A. Luger, C. Sorg, F. Spener, and C. Kerkhoff. 2004. L-FABP is exclusively expressed in alveolar macrophages within the myeloid lineage: evidence for a PPARalpha-independent mechanism. Int. J. Biochem. Cell Biol. 36:2042–2053. [DOI] [PubMed] [Google Scholar]
- 35.Dong, C., R.J. Davis, and R.A. Flavell. 2001. Signaling by the JNK group of MAP kinases. c-jun N-terminal Kinase. J. Clin. Immunol. 21:253–257. [DOI] [PubMed] [Google Scholar]
- 36.Nakahira, M., H.J. Ahn, W.R. Park, P. Gao, M. Tomura, C.S. Park, T. Hamaoka, T. Ohta, M. Kurimoto, and H. Fujiwara. 2002. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J. Immunol. 168:1146–1153. [DOI] [PubMed] [Google Scholar]
- 37.Kane, L.P., J. Lin, and A. Weiss. 2002. It's all rel-ative: NF-kappaB and CD28 costimulation in T-cell activation. Trends Immunol. 23:413–420. [DOI] [PubMed] [Google Scholar]
- 38.Lovett-Racke, A.E., R.Z. Hussain, S. Northrop, J. Choy, A. Rocchini, L. Matthes, J.A. Chavis, A. Diab, P.D. Drew, and M.K. Racke. 2004. Peroxisome proliferators-activated receptor α agonists as therapy for autoimmune disease. J. Immunol. 172:5790–5798. [DOI] [PubMed] [Google Scholar]
- 39.Badou, A., M. Savignac, M. Moreau, C. Leclerc, G. Foucras, G. Cassar, P. Paulet, D. Lagrange, P. Druet, J.C. Guery, and L. Pelletier. 2001. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-γ production. Eur. J. Immunol. 31:2487–2496. [DOI] [PubMed] [Google Scholar]
- 40.Jorritsma, P.J., J.L. Brogdon, and K. Bottomly. 2003. Role of TCR-induced extracellular signal-related kinase activation in the regulation of early IL-4 expression in naïve CD4+ cells. J. Immunol. 170:2427–2434. [DOI] [PubMed] [Google Scholar]
- 41.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 43.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadorkoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 44.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, H.L. Weiner, and V.K. Kuchroo. 2006. Reciprical developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238. [DOI] [PubMed] [Google Scholar]
- 45.Forman, B.M., J. Chen, and R.M. Evans. 1997. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA. 94:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desvergne, B., and W. Wahli. 1999. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr. Rev. 20:649–688. [DOI] [PubMed] [Google Scholar]
- 47.Guzman, M., J. Lo Verme, J. Fu, F. Oveisi, C. Blazquez, and D. Piomelli. 2004. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 279:27849–27854. [DOI] [PubMed] [Google Scholar]
- 48.Jones, D.C., X. Ding, T.Y. Zhang, and R.A. Daynes. 2003. Peroxisome proliferators-activated receptor negatively regulates T-bet transcription through suppression of p38 mitogen-activated protein kinase activation. J. Immunol. 171:196–203. [DOI] [PubMed] [Google Scholar]
- 49.Rincon, M., and R.A. Flavell. 1999. Reprogramming transcription during the differentiation of precursor CD4+ cells into effector Th1 and Th2 cells. Microbes Infect. 1:43–50. [DOI] [PubMed] [Google Scholar]
- 50.Tai, E.S., A. bin Ali, Q. Zhang, L.M. Loh, C.E. Tan, L. Retnam, R.M. El Oakley, and S.K. Lim. 2003. Hepatic expression of PPARalpha, a molecular target of fibrates, is regulated during inflammation in a gender-specific manner. FEBS Lett. 546:237–240. [DOI] [PubMed] [Google Scholar]
- 51.Wilcoxen, S.C., E. Kirkman, K.C. Dowdell, and S.A. Stohlman. 2000. Gender-dependent IL-12 secretion by APC is regulated by IL-10. J. Immunol. 164:6237–6243. [DOI] [PubMed] [Google Scholar]
- 52.Reddy, J., H. Waldner, Z. Illes, K.W. Wucherpfennig, R.A. Sobel, and V.K. Kuchroo. 2005. Cutting edge: CD4+CD25+ regulatory T cells contribute to gender differences in susceptibility to experimental autoimmune encephalomyelitis. J. Immunol. 175:5591–5595. [DOI] [PubMed] [Google Scholar]
- 53.Sanna, V., A. Di Giacoma, A. La Cava, R.I. Lechler, S. Fontana, S. Zappacosta, and G. Matarese. 2003. Leptin surge precedes onset of experimental autoimmune encephalomyelitis and correlates with development of pathogenic T cell responses. J. Clin. Invest. 111:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palaszynski, K.M., D.L. Smith, S. Kamrava, P.S. Burgoyne, A.P. Arnold, and R.R. Voskuhl. 2005. A ying-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 146:3280–3285. [DOI] [PubMed] [Google Scholar]
- 55.Pedotti, R., J.J. DeVoss, S. Youssef, D. Mitchell, J. Wedemeyer, R. Madanat, H. Garren, P. Fontoura, M. Tsai, S.J. Galli, et al. 2003. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc. Natl. Acad. Sci. USA. 100:1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.