Abstract
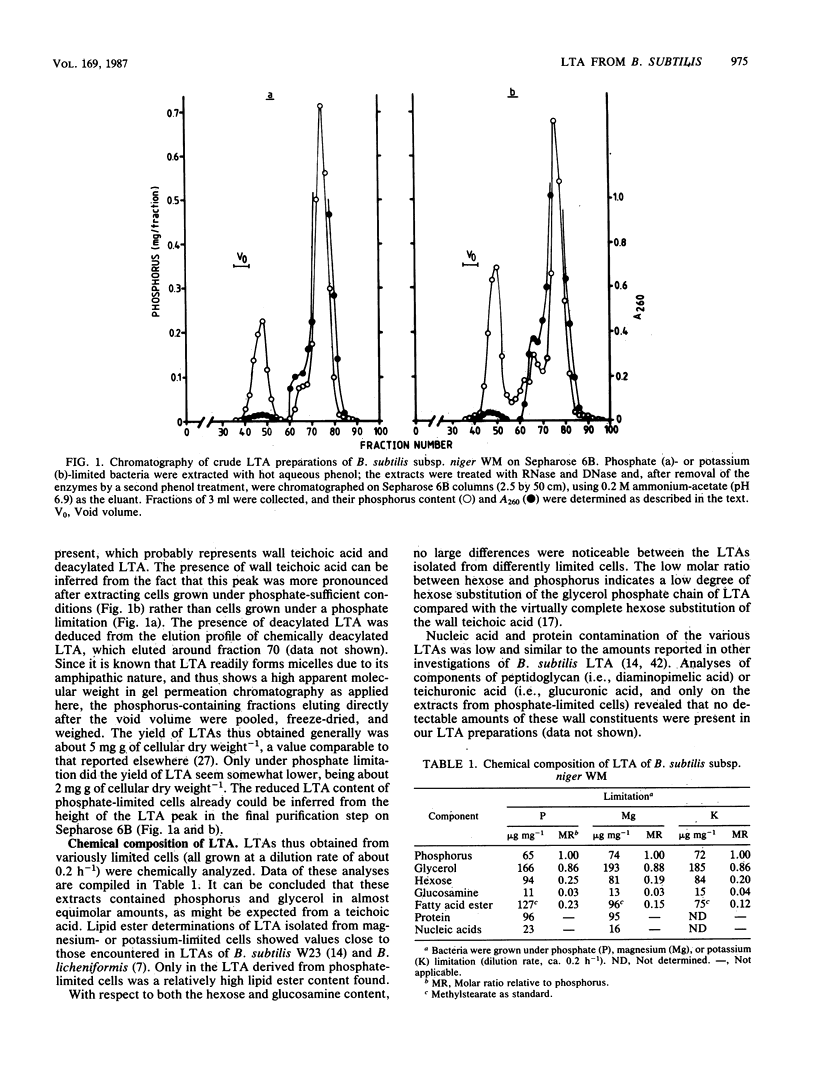
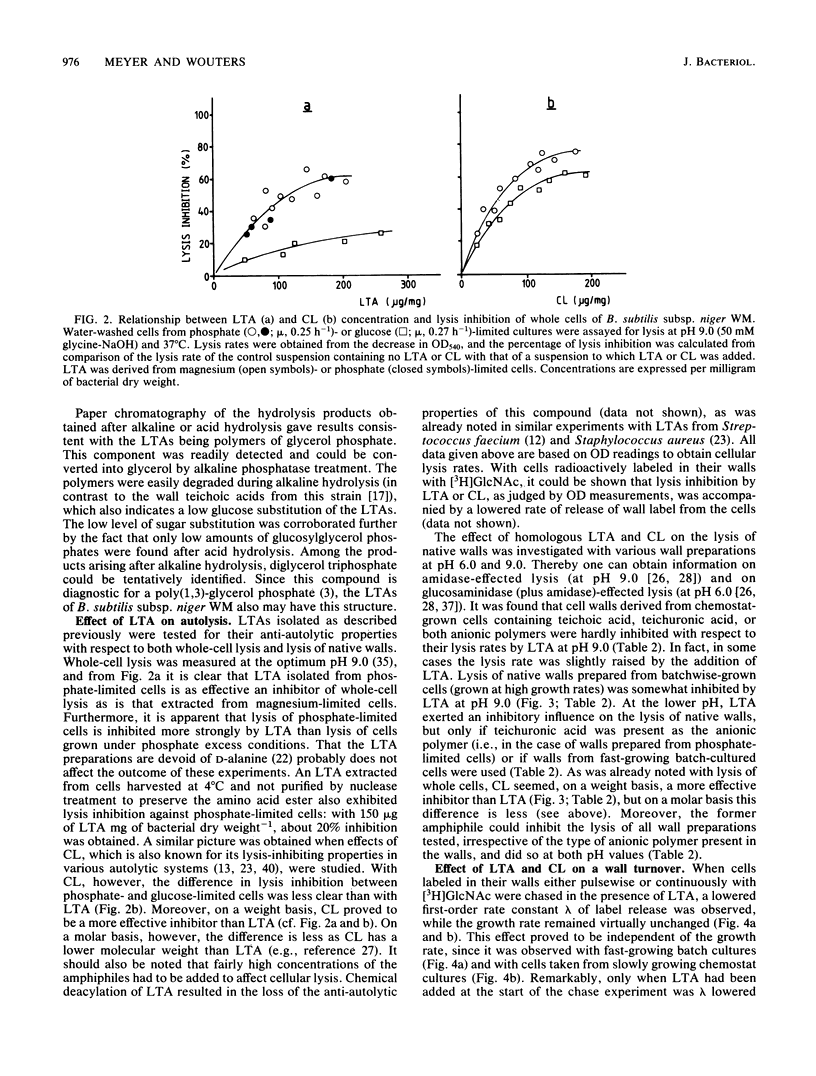
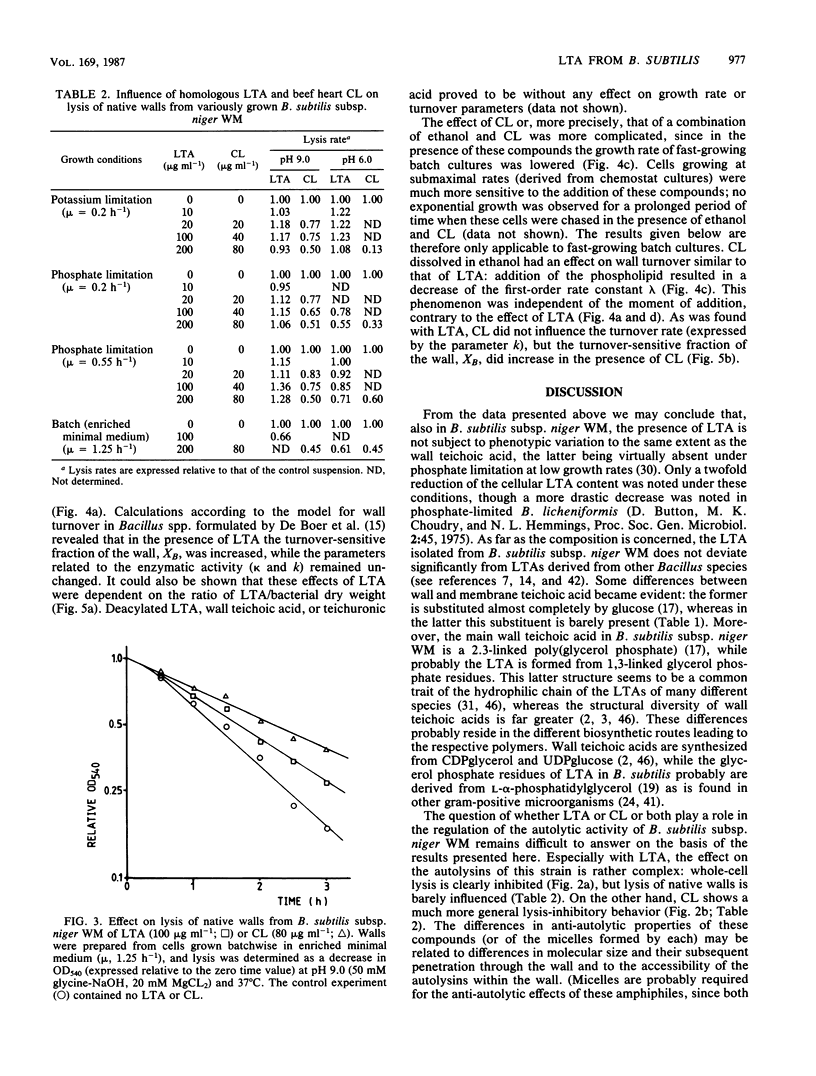
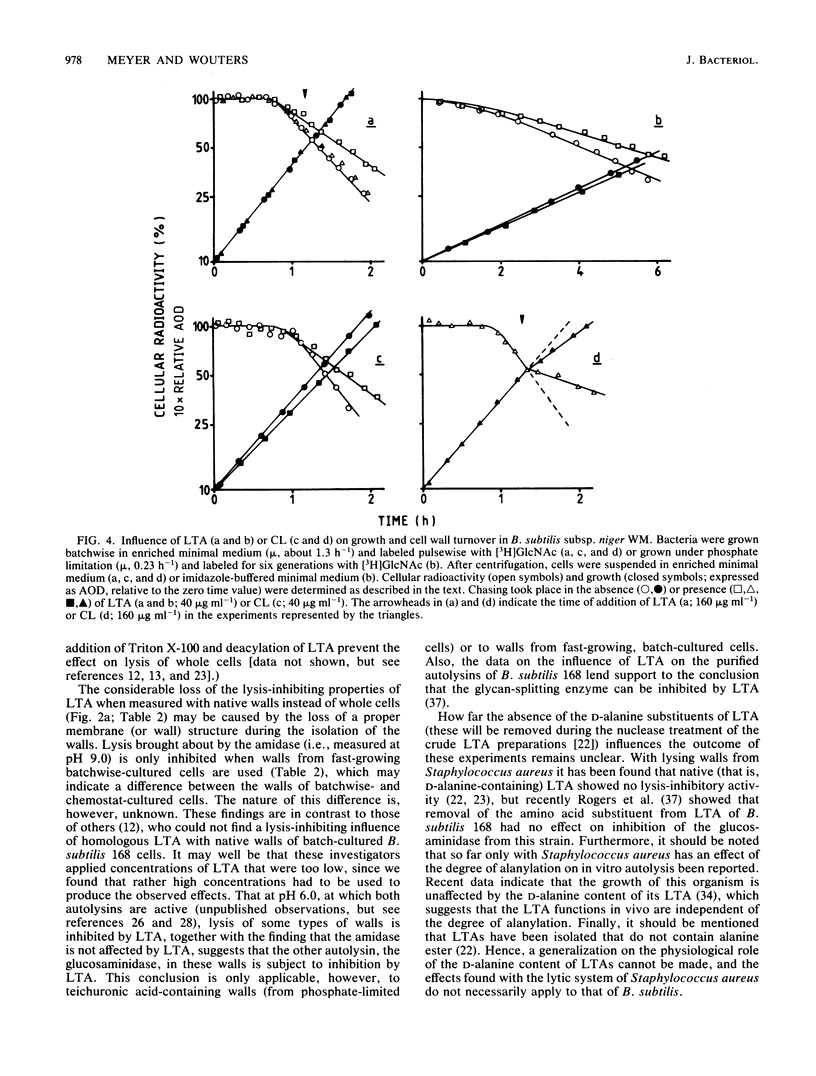
Lipoteichoic acid (LTA) was extracted by means of hot aqueous phenol from Bacillus subtilis subsp. niger WM cells grown under various conditions in chemostat culture. The extracts were partially purified by nuclease treatment and gel permeation chromatography. Chemical analyses revealed a composition consistent with a polyglycerol phosphate polymer. The influence on autolysis of the LTAs thus obtained was studied with both whole cells and autolysin-containing native walls of B. subtilis subsp. niger WM. Lysis rates of phosphate-limited cells could be reduced to about 40% of the control rate by the addition of LTA, whereas lysis of cells grown under phosphate-sufficient conditions was affected to a much lesser extent. The lysis of native walls prepared from variously grown cells proved to be fairly insensitive to the addition of LTA. The effect of LTA on wall turnover was studied by following the release of radioactively labeled wall material during exponential growth. The most obvious effect of LTA was a lowered first-order rate of release of labeled wall material; calculations according to the model for cell wall turnover in Bacillus spp. formulated by De Boer et al. (W. R. De Boer, F. J. Kruyssen, and J. T. M. Wouters, J. Bacteriol. 145:50-60, 1981) revealed changes in wall geometry and not in turnover rate in the presence of LTA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson A. J., Green R. S., Sturman A. J., Archibald A. R. Cell wall assembly in Bacillus subtilis: location of wall material incorporated during pulsed release of phosphate limitation, its accessibility to bacteriophages and concanavalin A, and its susceptibility to turnover. J Bacteriol. 1978 Dec;136(3):886–899. doi: 10.1128/jb.136.3.886-899.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald A. R., Baddiley J. The teichoic acids. Adv Carbohydr Chem Biochem. 1966;21:323–375. doi: 10.1016/s0096-5332(08)60320-3. [DOI] [PubMed] [Google Scholar]
- Blümel P., Uecker W., Giesbrecht P. Zero order kinetics of cell wall turnover in Staphylococcus aureus. Arch Microbiol. 1979 May;121(2):103–110. doi: 10.1007/BF00689972. [DOI] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Button D., Hemmings N. L. Lipoteichoic acid from Bacillus licheniformis 6346 MH-1. Comparative studies on the lipid portion of the lipoteichoic acid and the membrane glycolipid. Biochemistry. 1976 Mar 9;15(5):989–995. doi: 10.1021/bi00650a007. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Characterization of degradation products of the cell wall released during growth and sporulation of Bacillus megaterium. Folia Microbiol (Praha) 1974;19(4):292–300. doi: 10.1007/BF02873221. [DOI] [PubMed] [Google Scholar]
- Chaloupka J., Krecková P. Turnover of mucopeptide during the life cycle of Bacillus megaterium. Folia Microbiol (Praha) 1971;16(5):372–382. doi: 10.1007/BF02875757. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley J., Duckworth M., Baddiley J. Extraction and purification of lipoteichoic acids from Gram-positive bacteria. Carbohydr Res. 1975 Mar;40(1):41–52. doi: 10.1016/s0008-6215(00)82667-1. [DOI] [PubMed] [Google Scholar]
- De Boer W. R., Kruyssen F. J., Wouters J. T. The structure of teichoic acid from Bacillus subtilis var, niger WM as determined by C nuclear-magnetic-resonance spectroscopy. Eur J Biochem. 1976 Feb 2;62(1):1–6. doi: 10.1111/j.1432-1033.1976.tb10090.x. [DOI] [PubMed] [Google Scholar]
- Deutsch R. M., Engel R., Tropp B. E. Effect of 3,4-dihydroxybutyl-1-phosphonate on phosphoglyceride and lipoteichoic acid synthesis in Bacillus subtilis. J Biol Chem. 1980 Feb 25;255(4):1521–1525. [PubMed] [Google Scholar]
- Fischer W., Rösel P., Koch H. U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981 May;146(2):467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. The biosynthesis of nascent membrane lipoteichoic acid of Streptococcus faecium (S. faecalis ATCC 9790) from phosphatidylkojibiosyl diacylglycerol and phosphatidylglycerol. J Biol Chem. 1980 Jun 10;255(11):5164–5169. [PubMed] [Google Scholar]
- Greenway D. L., Perkins H. R. Turnover of the cell wall peptidoglycan during growth of Neisseria gonorrhoeae and Escherichia coli. Relative stability of newly synthesized material. J Gen Microbiol. 1985 Feb;131(2):253–263. doi: 10.1099/00221287-131-2-253. [DOI] [PubMed] [Google Scholar]
- Herbold D. R., Glaser L. Bacillus subtilis N-acetylmuramic acid L-alanine amidase. J Biol Chem. 1975 Mar 10;250(5):1676–1682. [PubMed] [Google Scholar]
- Huff E. Lipoteichoic acid, a major amphiphile of Gram-positive bacteria that is not readily extractable. J Bacteriol. 1982 Jan;149(1):399–402. doi: 10.1128/jb.149.1.399-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe L. K., Langemeier S. O., Doyle R. J. Hydrogen ion control of autolysin-dependent functions in Bacillus subtilis. Microbios. 1983;38(153-154):187–194. [PubMed] [Google Scholar]
- Koch H. U., Fischer W., Fiedler F. Influence of alanine ester and glycosyl substitution on the lipoteichoic acid carrier activity of lipoteichoic acids. J Biol Chem. 1982 Aug 25;257(16):9473–9479. [PubMed] [Google Scholar]
- Kruyssen F. J., de Boer W. R., Wouters J. T. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger. J Bacteriol. 1980 Oct;144(1):238–246. doi: 10.1128/jb.144.1.238-246.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. Occurrence and function of membrane teichoic acids. Biochim Biophys Acta. 1977 May 31;472(1):1–12. doi: 10.1016/0304-4157(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Lion F., Rotmans J. P., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XXVII. Stereospecificity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta. 1975 Apr 19;384(2):283–292. doi: 10.1016/0005-2744(75)90030-3. [DOI] [PubMed] [Google Scholar]
- MacArthur A. E., Archibald A. R. Effect of culture pH on the D-alanine ester content of lipoteichoic acid in Staphylococcus aureus. J Bacteriol. 1984 Nov;160(2):792–793. doi: 10.1128/jb.160.2.792-793.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Glaser L. Turnover of the cell wall of Bacillus subtilis W-23 during logarithmic growth. Biochem Biophys Res Commun. 1970 May 22;39(4):699–706. doi: 10.1016/0006-291x(70)90261-5. [DOI] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Taylor C., Rayter S., Ward J. B. Purification and properties of autolytic endo-beta-N-acetylglucosaminidase and the N-acetylmuramyl-L-alanine amidase from Bacillus subtilis strain 168. J Gen Microbiol. 1984 Sep;130(9):2395–2402. doi: 10.1099/00221287-130-9-2395. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Shimatani M., Ogawa M., Kotani S. Prevention of penicillin-induced lysis of Staphylococcus aureus by cellular lipoteichoic acid. J Antibiot (Tokyo) 1979 Jan;32(1):73–77. doi: 10.7164/antibiotics.32.73. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Taron D. J., Childs W. C., 3rd, Neuhaus F. C. Biosynthesis of D-alanyl-lipoteichoic acid: role of diglyceride kinase in the synthesis of phosphatidylglycerol for chain elongation. J Bacteriol. 1983 Jun;154(3):1110–1116. doi: 10.1128/jb.154.3.1110-1116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B. Teichoic and teichuronic acids: biosynthesis, assembly, and location. Microbiol Rev. 1981 Jun;45(2):211–243. doi: 10.1128/mr.45.2.211-243.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer W. R., Meyer P. D., Jordens C. G., Kruyssen F. J., Wouters J. T. Cell wall turnover in growing and nongrowing cultures of Bacillus subtilis. J Bacteriol. 1982 Mar;149(3):977–984. doi: 10.1128/jb.149.3.977-984.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer W., Kruyssen F. J., Wouters J. T. Cell wall metabolism in Bacillus subtilis subsp. niger: accumulation of wall polymers in the supernatant of chemostat cultures. J Bacteriol. 1981 Jun;146(3):877–884. doi: 10.1128/jb.146.3.877-884.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


