Abstract
We characterized several unusual phenotypes caused by stable insertion mutations in a gene that is located upstream in the same operon from hisT, which encodes the tRNA modification enzyme pseudouridine synthase I. Mutants containing kanamycin resistance (Kmr) cassettes in this upstream gene, which we temporarily designated usg-2, failed to grow on minimal plus glucose medium at 37 and 42 degrees C. However, usg-2::Kmr mutants did form oddly translucent, mucoid colonies at 30 degrees C or below. Microscopic examination revealed that cells from these translucent colonies were spherical and seemed to divide equatorially. Addition of D-alanine restored the shape of the mutant cells to rods and allowed the mutants to grow slowly at 37 degrees C and above. By contrast, addition of the common L-amino acids prevented growth of the usg-2::Kmr mutants, even at 30 degrees C. Furthermore, prolonged incubation of usg-2::Kmr mutants at 37 and 42 degrees C led to the appearance of several classes of temperature-resistant pseudorevertants. Other compounds also supported growth of usg-2::Kmr mutants at 37 and 42 degrees C, including glycolaldehyde and the B6 vitamers pyridoxine and pyridoxal. This observation suggested that usg-2 was pdxB, which had been mapped near hisT. Complementation experiments confirmed that usg-2 is indeed pdxB, and inspection of the pyridoxine biosynthetic pathway suggests explanations for the unusual phenotypes of pdxB::Kmr mutants. Finally, Southern hybridization experiments showed that pdxB and hisT are closely associated in several enterobacterial species. We consider reasons for grouping pdxB and hisT together in the same complex operon and speculate that these two genes play roles in the global regulation of amino acid metabolism.
Full text
PDF
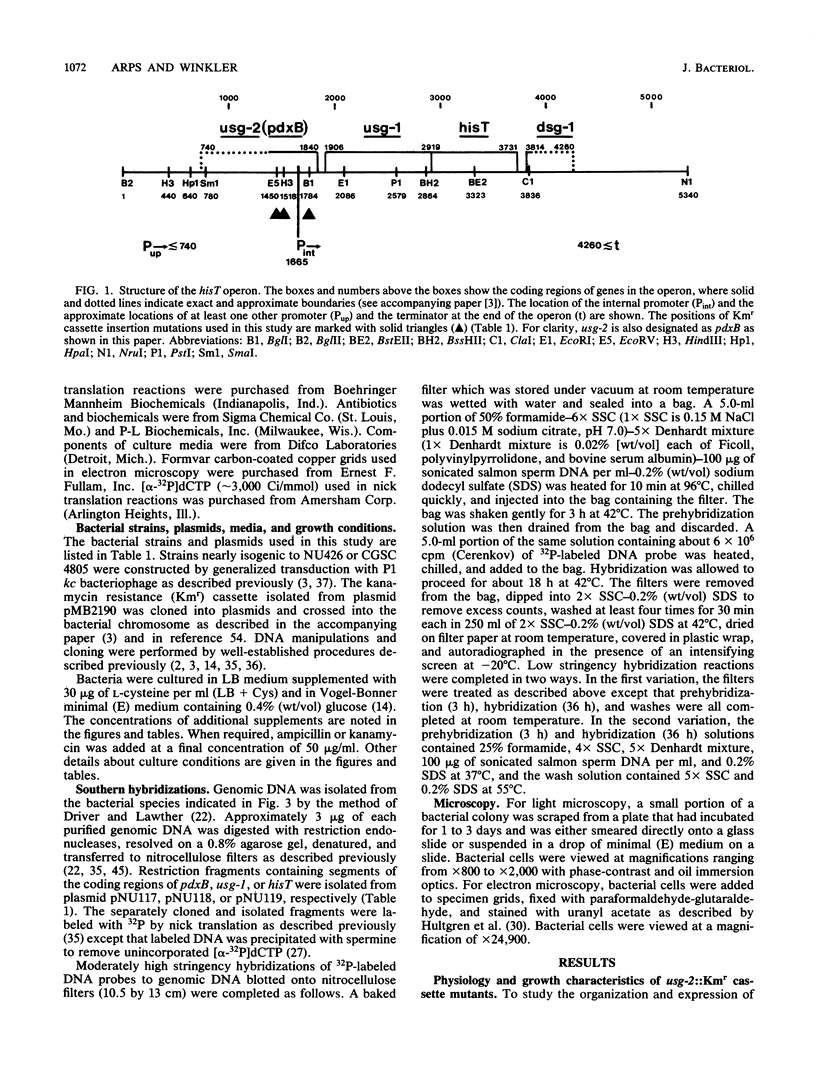

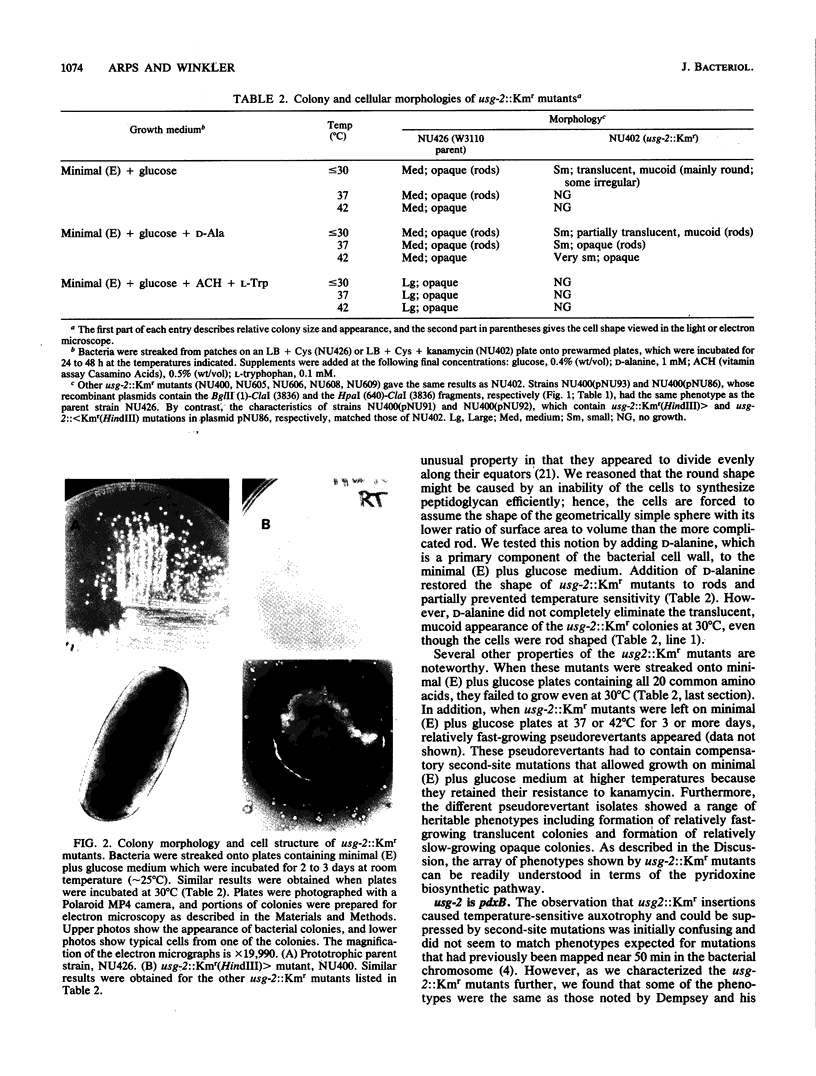


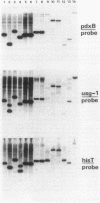
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrésson O. S., Davies J. E. Genetic organization and restriction enzyme cleavage map of the ksgA-pdxA region of the Escherichia coli chromosome. Mol Gen Genet. 1980;179(1):211–216. doi: 10.1007/BF00268465. [DOI] [PubMed] [Google Scholar]
- Arps P. J., Marvel C. C., Rubin B. C., Tolan D. A., Penhoet E. E., Winkler M. E. Structural features of the hisT operon of Escherichia coli K-12. Nucleic Acids Res. 1985 Jul 25;13(14):5297–5315. doi: 10.1093/nar/13.14.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arps P. J., Winkler M. E. Structural analysis of the Escherichia coli K-12 hisT operon by using a kanamycin resistance cassette. J Bacteriol. 1987 Mar;169(3):1061–1070. doi: 10.1128/jb.169.3.1061-1070.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R. A novel link between the biosynthesis of aromatic amino acids and transfer RNA modification in Escherichia coli. J Mol Biol. 1980 Jul 5;140(3):391–410. doi: 10.1016/0022-2836(80)90391-5. [DOI] [PubMed] [Google Scholar]
- Björk G. R. E. coli ribosomal protein operons: the case of the misplaced genes. Cell. 1985 Aug;42(1):7–8. doi: 10.1016/s0092-8674(85)80093-3. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Hjalmarsson K. J., Wikström P. M., Björk G. R. The nucleotide sequence of an Escherichia coli operon containing genes for the tRNA(m1G)methyltransferase, the ribosomal proteins S16 and L19 and a 21-K polypeptide. EMBO J. 1983;2(6):899–905. doi: 10.1002/j.1460-2075.1983.tb01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMPSEY W. B. CONTROL OF PYRIDOXINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:431–437. doi: 10.1128/jb.90.2.431-437.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs E. R. Order of ribosomal protein genes in the Rif cluster of Bacillus subtilis is identical to that of Escherichia coli. J Bacteriol. 1984 Aug;159(2):770–772. doi: 10.1128/jb.159.2.770-772.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Characterization of pyridoxine auxotrophs of Escherichia coli: P1 transduction. J Bacteriol. 1969 Mar;97(3):1403–1410. doi: 10.1128/jb.97.3.1403-1410.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Characterization of pyridoxine auxotrophs of Escherichia coli: chromosomal position of linkage group I. J Bacteriol. 1969 Oct;100(1):295–300. doi: 10.1128/jb.100.1.295-300.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B., Pachler P. F. Isolation and characterization of pyridoxine auxotrophs of Escherichia coli. J Bacteriol. 1966 Feb;91(2):642–645. doi: 10.1128/jb.91.2.642-645.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Synthesis of Pyridoxine by a Pyridoxal Auxotroph of Escherichia coli. J Bacteriol. 1966 Aug;92(2):333–337. doi: 10.1128/jb.92.2.333-337.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey W. B. Threonine prevents derepression of pyridoxine synthesis in Escherichia coli B. J Bacteriol. 1982 Jun;150(3):1476–1478. doi: 10.1128/jb.150.3.1476-1478.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver R. P., Lawther R. P. Physical analysis of deletion mutations in the ilvGEDA operon of Escherichia coli K-12. J Bacteriol. 1985 May;162(2):598–606. doi: 10.1128/jb.162.2.598-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K., Coggins J. R. The serC-aro A operon of Escherichia coli. A mixed function operon encoding enzymes from two different amino acid biosynthetic pathways. Biochem J. 1986 Feb 15;234(1):49–57. doi: 10.1042/bj2340049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J. U., Björk G. R. Pleiotropic effects induced by modification deficiency next to the anticodon of tRNA from Salmonella typhimurium LT2. J Bacteriol. 1986 Jun;166(3):1013–1021. doi: 10.1128/jb.166.3.1013-1021.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. E., Rowell F. J., Gupta R. N., Spenser I. D. Biosynthesis of vitamin B 6 . J Biol Chem. 1972 Mar 25;247(6):1869–1882. [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Genes aroA and serC of Salmonella typhimurium constitute an operon. J Bacteriol. 1985 Jul;163(1):355–361. doi: 10.1128/jb.163.1.355-361.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Cookson B. T., Ladika D., Olivera B. M., Roth J. R. 6-Aminonicotinamide-resistant mutants of Salmonella typhimurium. J Bacteriol. 1983 Jun;154(3):1126–1136. doi: 10.1128/jb.154.3.1126-1136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultgren S. J., Porter T. N., Schaeffer A. J., Duncan J. L. Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun. 1985 Nov;50(2):370–377. doi: 10.1128/iai.50.2.370-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten H. On the biological significance of modified nucleosides in tRNA. Prog Nucleic Acid Res Mol Biol. 1984;31:59–114. doi: 10.1016/s0079-6603(08)60375-x. [DOI] [PubMed] [Google Scholar]
- Kitchingman G. R., Fournier M. J. Modification-deficient transfer ribonucleic acids from relaxed control Escherichia coli: structures of the major undermodified phenylalanine and leucine transfer RNAs produced during leucine starvation. Biochemistry. 1977 May 17;16(10):2213–2220. doi: 10.1021/bi00629a027. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Godson G. N. The rpsU-dnaG-rpoD macromolecular synthesis operon of E. coli. Cell. 1984 Dec;39(2 Pt 1):251–252. doi: 10.1016/0092-8674(84)90001-1. [DOI] [PubMed] [Google Scholar]
- Marvel C. C., Arps P. J., Rubin B. C., Kammen H. O., Penhoet E. E., Winkler M. E. hisT is part of a multigene operon in Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):60–71. doi: 10.1128/jb.161.1.60-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. H., Snell E. E. Transport and metabolism of vitamin B6 in Salmonella typhimurium LT2. J Biol Chem. 1976 Feb 25;251(4):1052–1056. [PubMed] [Google Scholar]
- Prentki P., Karch F., Iida S., Meyer J. The plasmid cloning vector pBR325 contains a 482 base-pair-long inverted duplication. Gene. 1981 Sep;14(4):289–299. doi: 10.1016/0378-1119(81)90161-x. [DOI] [PubMed] [Google Scholar]
- Searles L. L., Jones J. W., Fournier M. J., Grambow N., Tyler B., Calvo J. M. Escherichia coli B/r leuK mutant lacking pseudouridine synthase I activity. J Bacteriol. 1986 Apr;166(1):341–345. doi: 10.1128/jb.166.1.341-345.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S., Dempsey W. B. Genetic map position of the pdxH gene in Escherichia coli. J Bacteriol. 1976 Sep;127(3):1593–1594. doi: 10.1128/jb.127.3.1593-1594.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tani Y., Dempsey W. B. Glycolaldehyde is a precursor of pyridoxal phosphate in Escherichia coli B. J Bacteriol. 1973 Oct;116(1):341–345. doi: 10.1128/jb.116.1.341-345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittawella I. P. Evidence for clustering of RNA polymerase and ribosomal protein genes in six species of Enterobacteria. Mol Gen Genet. 1984;195(1-2):215–218. doi: 10.1007/BF00332749. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Vella G. J., Hill R. E., Mootoo B. S., Spenser I. D. The status of glycolaldehyde in the biosynthesis of vitamin B6. J Biol Chem. 1980 Apr 10;255(7):3042–8. [PubMed] [Google Scholar]
- Vella G. J., Hill R. E., Spenser I. D. Biosynthesis of pyridoxol. The origin of the C2-unit, C-2',-2. J Biol Chem. 1981 Oct 25;256(20):10469–10474. [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- White R. S., Dempsey W. B. Purification and properties of vitamin B6 kinase from Escherichia coli B. Biochemistry. 1970 Oct 13;9(21):4057–4064. doi: 10.1021/bi00823a005. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buul C. P., van Knippenberg P. H. Nucleotide sequence of the ksgA gene of Escherichia coli: comparison of methyltransferases effecting dimethylation of adenosine in ribosomal RNA. Gene. 1985;38(1-3):65–72. doi: 10.1016/0378-1119(85)90204-5. [DOI] [PubMed] [Google Scholar]