Abstract
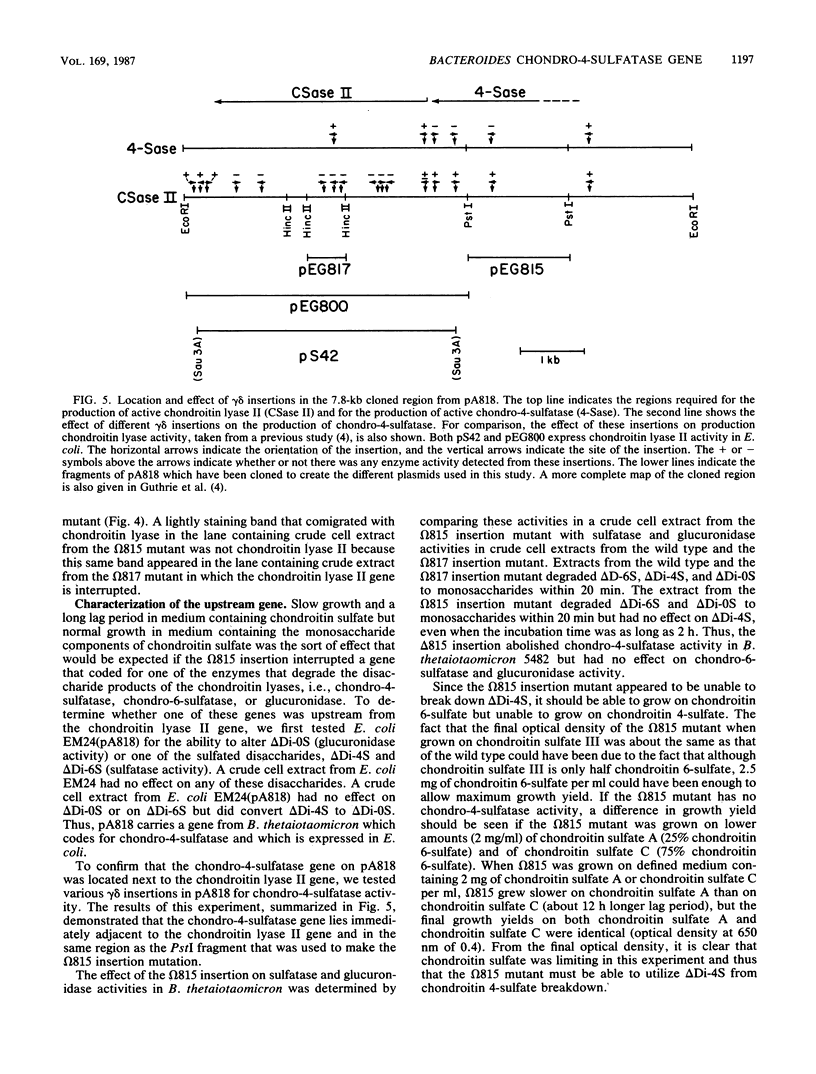
The chondroitin lyase II gene from Bacteroides thetaiotaomicron has previously been cloned in Escherichia coli on a 7.8-kilobase (kb) fragment (pA818). In E. coli, the chondroitin lyase II gene appeared to be expressed from a promoter that was about 0.5 kb from the beginning of the gene. However, when a subcloned 5-kb fragment from pA818 which contained the chondroitin lyase II gene and the promoter from which the gene is expressed in E. coli was introduced into B. thetaiotaomicron on a multicopy plasmid (pEG800), the chondroitin lyase specific activity of B. thetaiotaomicron was not altered. Further evidence that the promoter that is recognized in E. coli may not be the promoter from which the chondroitin lyase II gene is transcribed in B. thetaiotaomicron was obtained by making an insertion in the B. thetaiotaomicron chromosome at a point which is 1 kb upstream from the chondroitin lyase II gene. This insertion stopped synthesis of the chondroitin lyase II gene product, as would be predicted if the gene was part of an operon and was transcribed in B. thetaiotaomicron from a promoter that was at least 1 kb upstream from the chondroitin lyase II gene. A region of pA818 which was adjacent to the chondroitin lyase II gene and which included the region used to make the insertional mutation was found to code for chondro-4-sulfatase, an enzyme that breaks down one of the products of the chondroitin lyase reaction. The upstream insertion mutant of B. thetaiotaomicron which stopped synthesis of chondroitin lyase II had no detectable chondro-4-sulfatase activity. This mutant was still able to grow on chondroitin sulfate, although the rate of growth was slower than that of the wild type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erickson P. F., Minier L. N., Lasher R. S. Quantitative electrophoretic transfer of polypeptides from SDS polyacrylamide gels to nitrocellulose sheets: a method for their re-use in immunoautoradiographic detection of antigens. J Immunol Methods. 1982 Jun 11;51(2):241–249. doi: 10.1016/0022-1759(82)90263-0. [DOI] [PubMed] [Google Scholar]
- Faltynek C. R., Silbert J. E. Copolymers of chondroitin 4-sulfate and chondroitin 6-sulfate in chick embryo epiphyses and other cartilage. J Biol Chem. 1978 Nov 10;253(21):7646–7649. [PubMed] [Google Scholar]
- Guthrie E. P., Salyers A. A. Use of targeted insertional mutagenesis to determine whether chondroitin lyase II is essential for chondroitin sulfate utilization by Bacteroides thetaiotaomicron. J Bacteriol. 1986 Jun;166(3):966–971. doi: 10.1128/jb.166.3.966-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E. P., Shoemaker N. B., Salyers A. A. Cloning and expression in Escherichia coli of a gene coding for a chondroitin lyase from Bacteroides thetaiotaomicron. J Bacteriol. 1985 Nov;164(2):510–515. doi: 10.1128/jb.164.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Linz J., Braun D. M., Salyers A. A. Analysis of outer membrane proteins which are associated with growth of Bacteroides thetaiotaomicron on chondroitin sulfate. J Bacteriol. 1985 Sep;163(3):1080–1086. doi: 10.1128/jb.163.3.1080-1086.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn S., Chan T., Lipeski L., Salyers A. A. Isolation and characterization of two chondroitin lyases from Bacteroides thetaiotaomicron. J Bacteriol. 1983 Nov;156(2):859–866. doi: 10.1128/jb.156.2.859-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatin D. E., Voss E. W., Jr Anti-lysergyl antibody: measurement of binding parameters in IgG fractions. Immunochemistry. 1974 Jun;11(6):285–293. doi: 10.1016/0019-2791(74)90364-4. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Kotarski S. F. Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):781–788. doi: 10.1128/jb.143.2.781-788.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., O'Brien M. Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J Bacteriol. 1980 Aug;143(2):772–780. doi: 10.1128/jb.143.2.772-780.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986 Mar;165(3):929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker N. B., Guthrie E. P., Salyers A. A., Gardner J. F. Evidence that the clindamycin-erythromycin resistance gene of Bacteroides plasmid pBF4 is on a transposable element. J Bacteriol. 1985 May;162(2):626–632. doi: 10.1128/jb.162.2.626-632.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]