Abstract
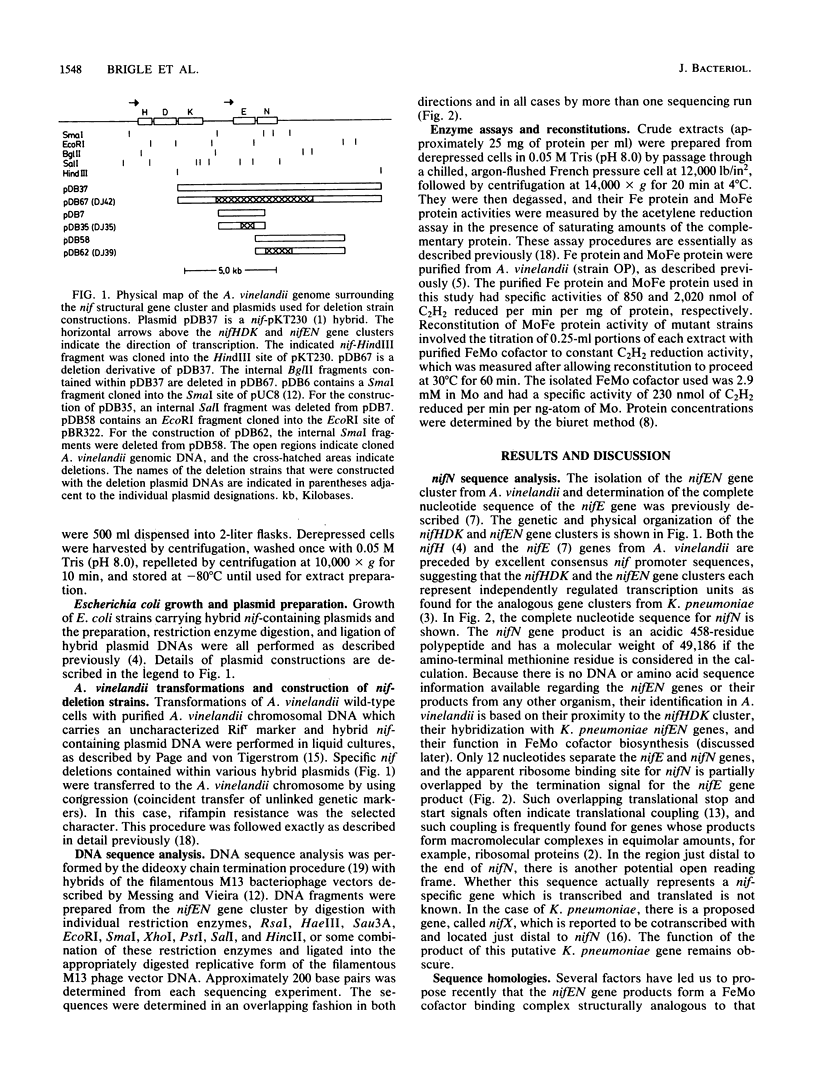
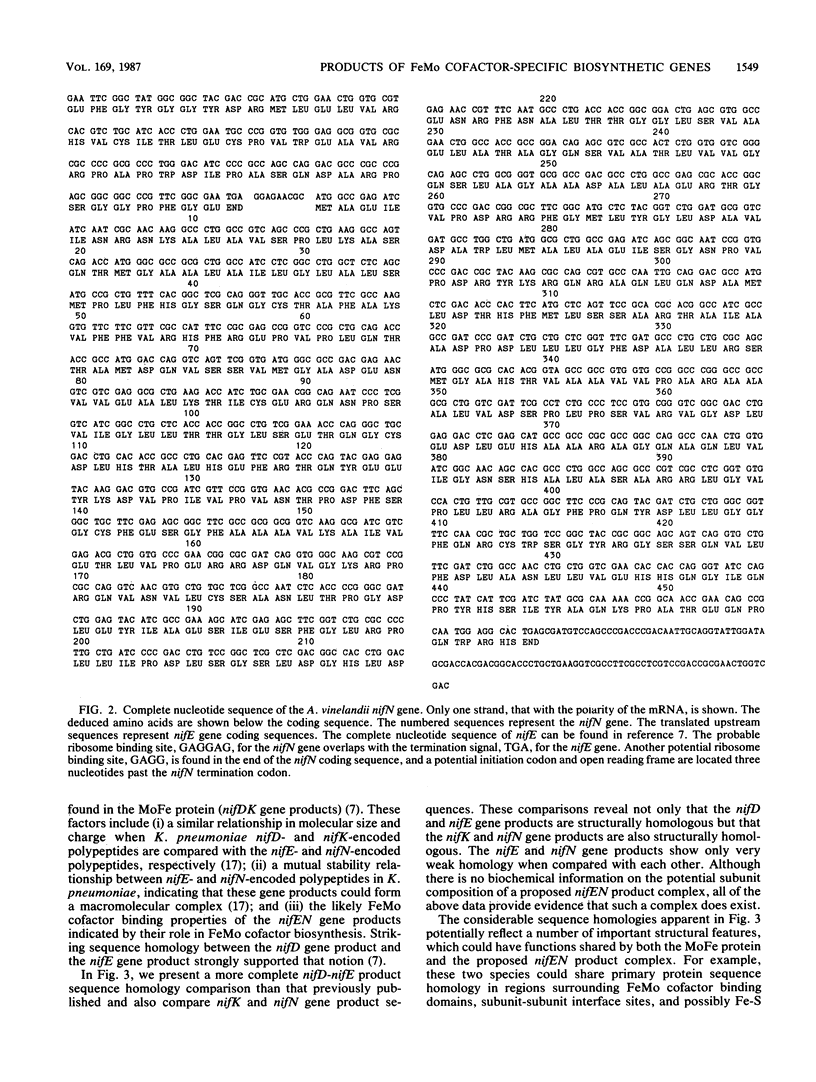
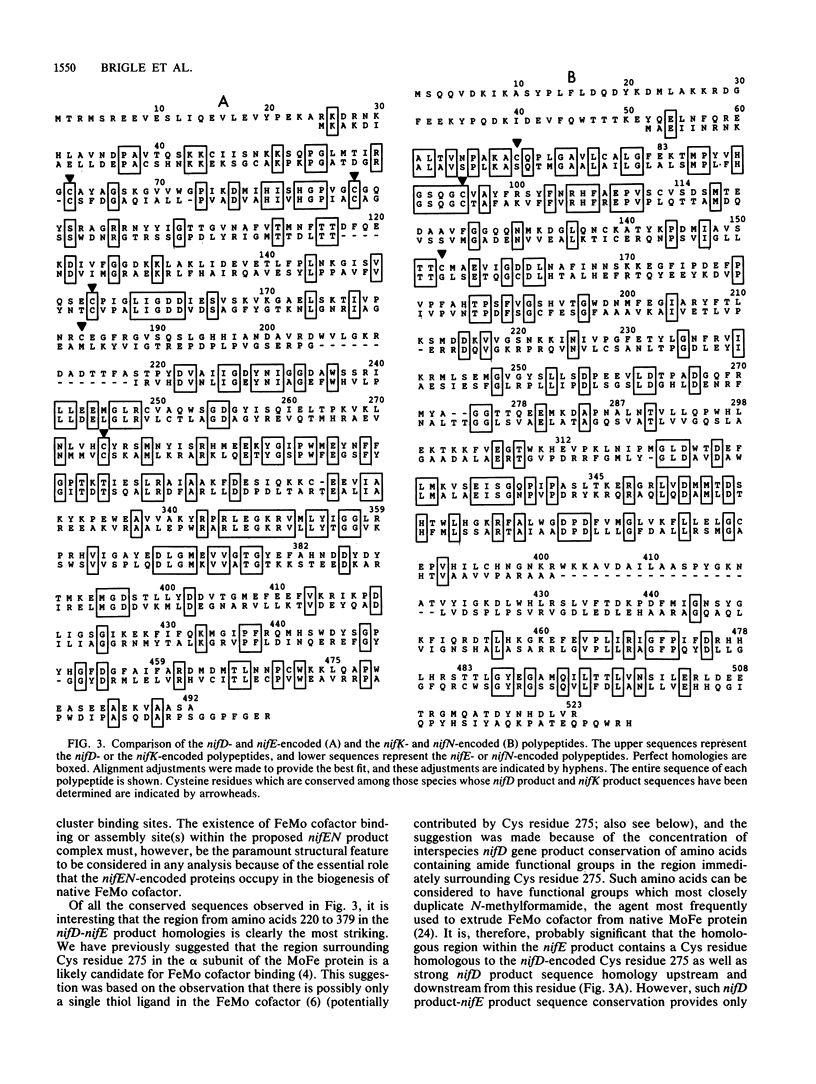
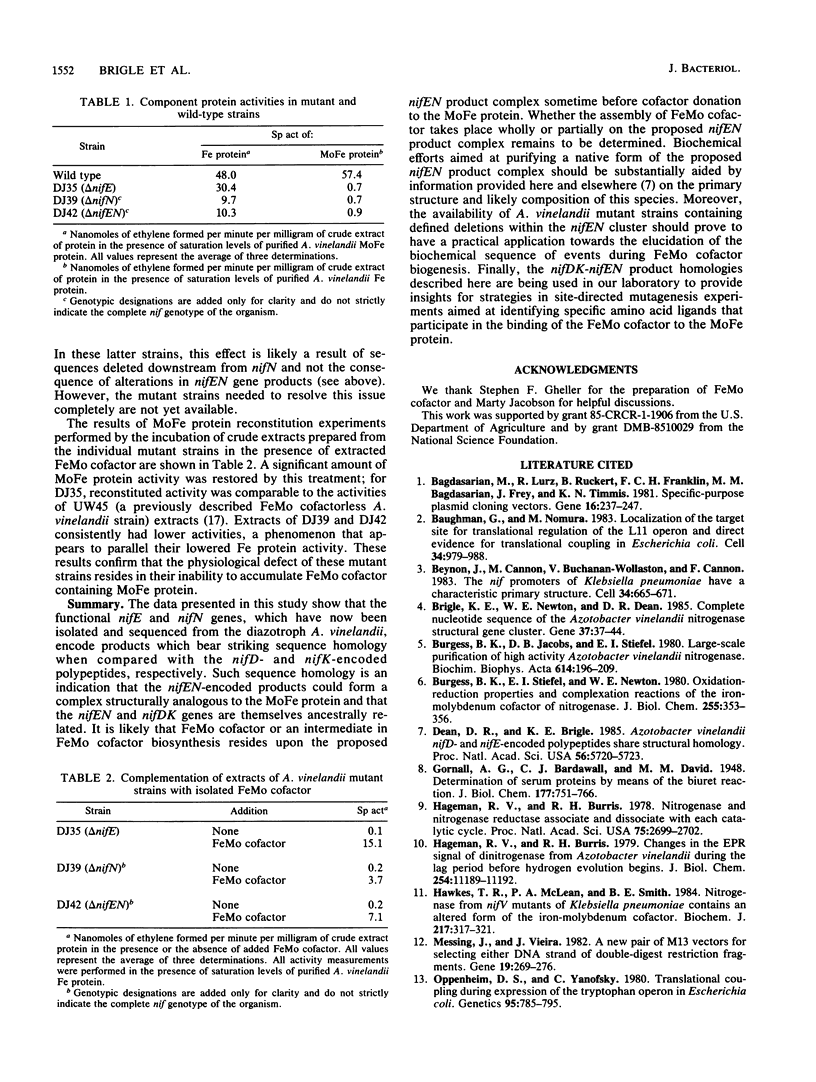
The genes from Azotobacter vinelandii, which are homologous to the iron-molybdenum cofactor biosynthetic genes, nifE and nifN, from Klebsiella pneumoniae, have been cloned and sequenced. These genes comprise a single transcription unit and are located immediately downstream from the nitrogenase structural gene cluster (nifHDK). DNA sequence analysis has revealed that the products of the nifE and nifN genes contain considerable homology when compared with the nifD (MoFe protein alpha subunit) and the nifK (MoFe protein beta subunit) gene products, respectively. These striking sequence homologies indicate a structural and functional relationship between a proposed nifEN product complex and the nitrogenase MoFe protein as well as imply an ancestral relationship between these gene clusters. The isolation and characterization of strains which contain deletions within the nifEN gene cluster demonstrate a role for these products in iron-molybdenum cofactor biosynthesis in A. vinelandii.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Baughman G., Nomura M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell. 1983 Oct;34(3):979–988. doi: 10.1016/0092-8674(83)90555-x. [DOI] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Brigle K. E., Newton W. E., Dean D. R. Complete nucleotide sequence of the Azotobacter vinelandii nitrogenase structural gene cluster. Gene. 1985;37(1-3):37–44. doi: 10.1016/0378-1119(85)90255-0. [DOI] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Burgess B. K., Stiefel E. I., Newton W. E. Oxidation-reduction properties and complexation reactions of the iron-molybdenum cofactor of nitrogenase. J Biol Chem. 1980 Jan 25;255(2):353–356. [PubMed] [Google Scholar]
- Dean D. R., Brigle K. E. Azotobacter vinelandii nifD- and nifE-encoded polypeptides share structural homology. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5720–5723. doi: 10.1073/pnas.82.17.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Changes in the EPR signal of dinitrogenase from Azotobacter vinelandii during the lag period before hydrogen evolution begins. J Biol Chem. 1979 Nov 25;254(22):11189–11192. [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Molecular basis of biological nitrogen fixation. Annu Rev Biophys Biophys Chem. 1985;14:419–459. doi: 10.1146/annurev.bb.14.060185.002223. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. C., Burgess B. K., Dean D. R. Activity, reconstitution, and accumulation of nitrogenase components in Azotobacter vinelandii mutant strains containing defined deletions within the nitrogenase structural gene cluster. J Bacteriol. 1986 Apr;166(1):180–186. doi: 10.1128/jb.166.1.180-186.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg G. W., Wilson P. W. Formation of the nitrogen-fixing enzyme system in Azotobacter vinelandii. Can J Microbiol. 1968 Jan;14(1):25–31. doi: 10.1139/m68-005. [DOI] [PubMed] [Google Scholar]
- Ugalde R. A., Imperial J., Shah V. K., Brill W. J. Biosynthesis of iron-molybdenum cofactor in the absence of nitrogenase. J Bacteriol. 1984 Sep;159(3):888–893. doi: 10.1128/jb.159.3.888-893.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


