Abstract
We cloned the gene (c1) which encodes the repressor of vegetative function of Pseudomonas aeruginosa bacteriophage D3. The cloned gene was shown to inhibit plating of D3 and the induction of D3 lysogens by UV irradiation. The efficiency of plating and prophage induction of the heteroimmune P. aeruginosa phage F116L were not affected by the presence of the cloned c1 gene of D3. When the D3 DNA fragment containing c1 was subcloned into pBR322 and introduced into Escherichia coli, it was shown to specifically inhibit the plating of phage lambda and the induction of the lambda prophage by mitomycin C. The plating of lambda imm434 phage was not affected. Analysis in minicells indicated that these effects correspond to the presence of a plasmid-encoded protein of 36,000 molecular weight. These data suggest the possibility that coliphage lambda and the P. aeruginosa phage D3 evolved from a common ancestor. The conservation of the functional similarities of their repressors may have occurred because of the advantage to these temperate phages of capitalizing on the potential of the evolutionarily conserved RecA protein to monitor the level of damage to the host genome.
Full text
PDF
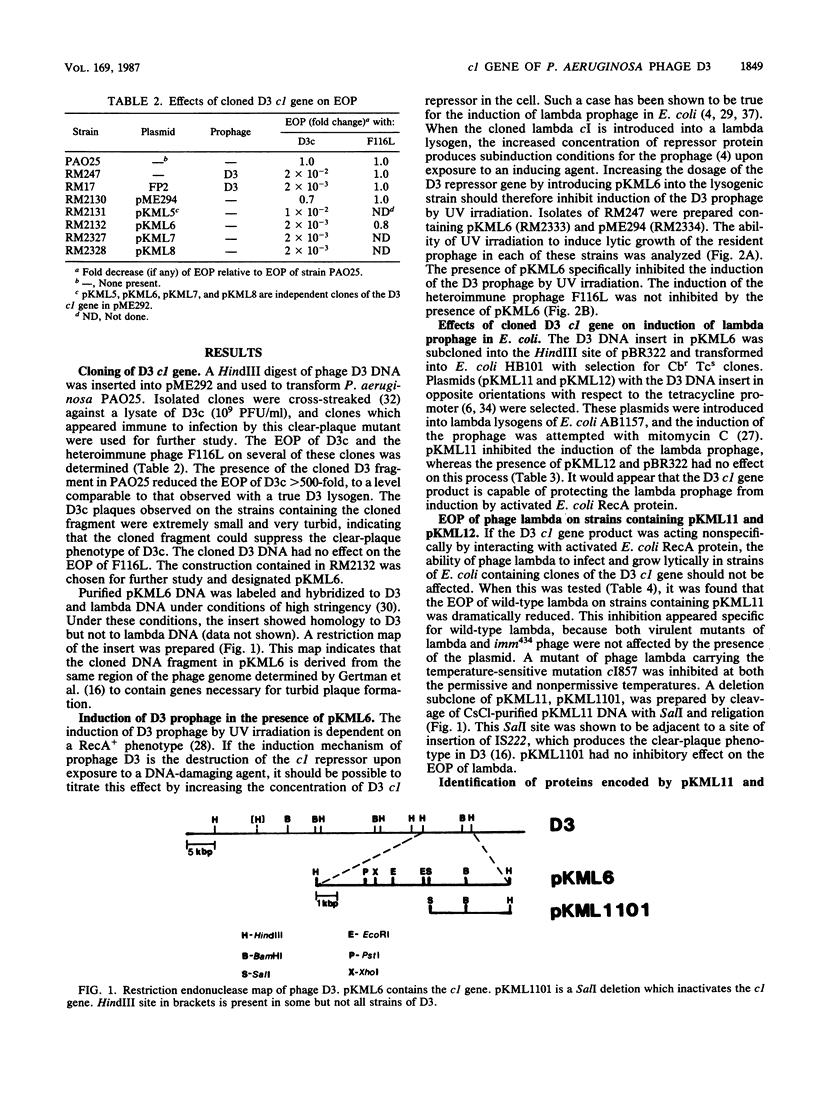
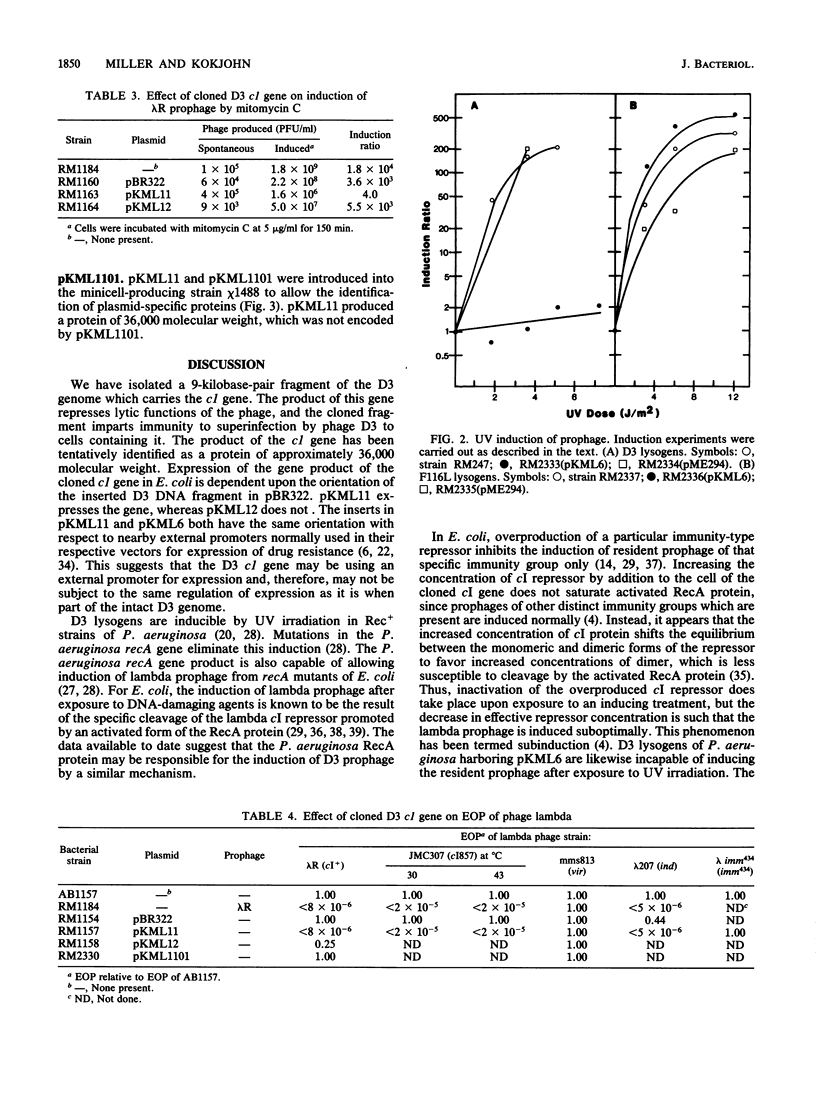
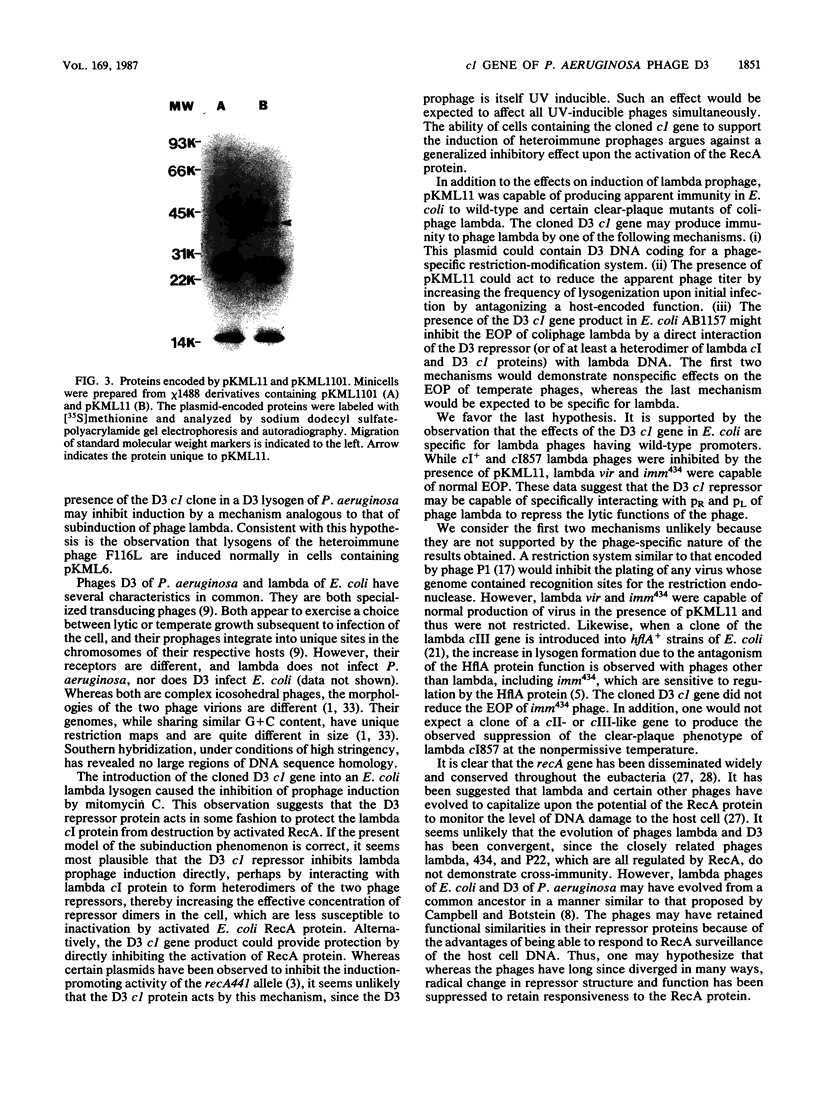

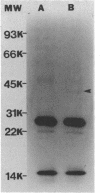
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdasarian M., Bailone A., Bagdasarian M. M., Manning P. A., Lurz R., Timmis K. N., Devoret R. An inhibitor of SOS induction, specified by a plasmid locus in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5723–5726. doi: 10.1073/pnas.83.15.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Belfort M., Wulff D. L. Genetic and biochemical investigation of the Escherichia coli mutant hfl-1 which is lysogenized at high frequency by bacteriophage lambda. J Bacteriol. 1973 Jul;115(1):299–306. doi: 10.1128/jb.115.1.299-306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenagh M. M., Miller R. V. Specialized transduction of Pseudomonas aeruginosa PAO by bacteriophage D3. J Bacteriol. 1986 Feb;165(2):448–452. doi: 10.1128/jb.165.2.448-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Curtiss R., 3rd Analysis of recombinant DNA using Escherichia coli minicells. Methods Enzymol. 1983;101:347–362. doi: 10.1016/0076-6879(83)01026-5. [DOI] [PubMed] [Google Scholar]
- De Anda J., Poteete A. R., Sauer R. T. P22 c2 repressor. Domain structure and function. J Biol Chem. 1983 Sep 10;258(17):10536–10542. [PubMed] [Google Scholar]
- EGAN J. B., HOLLOWAY B. W. Genetic studies on lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1961 Feb;39:9–17. doi: 10.1038/icb.1961.2. [DOI] [PubMed] [Google Scholar]
- Früh R., Watson J. M., Haas D. Construction of recombination-deficient strains of Pseudomonas aeruginosa. Mol Gen Genet. 1983;191(2):334–337. doi: 10.1007/BF00334835. [DOI] [PubMed] [Google Scholar]
- Gertman E., White B. N., Berry D., Kropinski A. M. IS222, a new insertion element associated with the genome of Pseudomonas aeruginosa. J Bacteriol. 1986 Jun;166(3):1134–1136. doi: 10.1128/jb.166.3.1134-1136.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOWAY B. W., EGAN J. B., MONK M. Lysogeny in Pseudomonas aeruginosa. Aust J Exp Biol Med Sci. 1960 Aug;38:321–329. doi: 10.1038/icb.1960.34. [DOI] [PubMed] [Google Scholar]
- Hinkle N. F., Miller R. V. pMG7-mediated restriction of Pseudomonas aeruginosa phage DNAs is determined by a class II restriction endonuclease. Plasmid. 1979 Jul;2(3):387–393. doi: 10.1016/0147-619x(79)90022-2. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Haas D. Cloning vectors derived from the Pseudomonas plasmid pVS1. Gene. 1985;36(1-2):27–36. doi: 10.1016/0378-1119(85)90066-6. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Watson J. M., Haas D., Leisinger T. Genetic and molecular characterization of the Pseudomonas plasmid pVS1. Plasmid. 1984 May;11(3):206–220. doi: 10.1016/0147-619x(84)90027-1. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Characterization of the Pseudomonas aeruginosa recA analog and its protein product: rec-102 is a mutant allele of the P. aeruginosa PAO recA gene. J Bacteriol. 1987 Apr;169(4):1499–1508. doi: 10.1128/jb.169.4.1499-1508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer A. A., Loutit J. S. Transformation and transfection of Pseudomonas aeruginosa: effects of metal ions. J Bacteriol. 1979 Oct;140(1):37–42. doi: 10.1128/jb.140.1.37-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Ku C. M. Characterization of Pseudomonas aeruginosa mutants deficient in the establishment of lysogeny. J Bacteriol. 1978 Jun;134(3):875–883. doi: 10.1128/jb.134.3.875-883.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Pemberton J. M., Richards K. E. F116, D3 and G101: temperate bacteriophages of Pseudomonas aeruginosa. Virology. 1974 Jun;59(2):566–569. doi: 10.1016/0042-6822(74)90466-8. [DOI] [PubMed] [Google Scholar]
- Peden K. W. Revised sequence of the tetracycline-resistance gene of pBR322. Gene. 1983 May-Jun;22(2-3):277–280. doi: 10.1016/0378-1119(83)90112-9. [DOI] [PubMed] [Google Scholar]
- Phizicky E. M., Roberts J. W. Kinetics of RecA protein-directed inactivation of repressors of phage lambda and phage P22. J Mol Biol. 1980 May 25;139(3):319–328. doi: 10.1016/0022-2836(80)90133-3. [DOI] [PubMed] [Google Scholar]
- Quillardet P., Moreau P. L., Ginsburg H., Mount D. W., Devoret R. Cell survival, UV-reactivation and induction of prophage lambda in Escherichia coli K12 overproducing RecA protein. Mol Gen Genet. 1982;188(1):37–43. doi: 10.1007/BF00332993. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]