Abstract
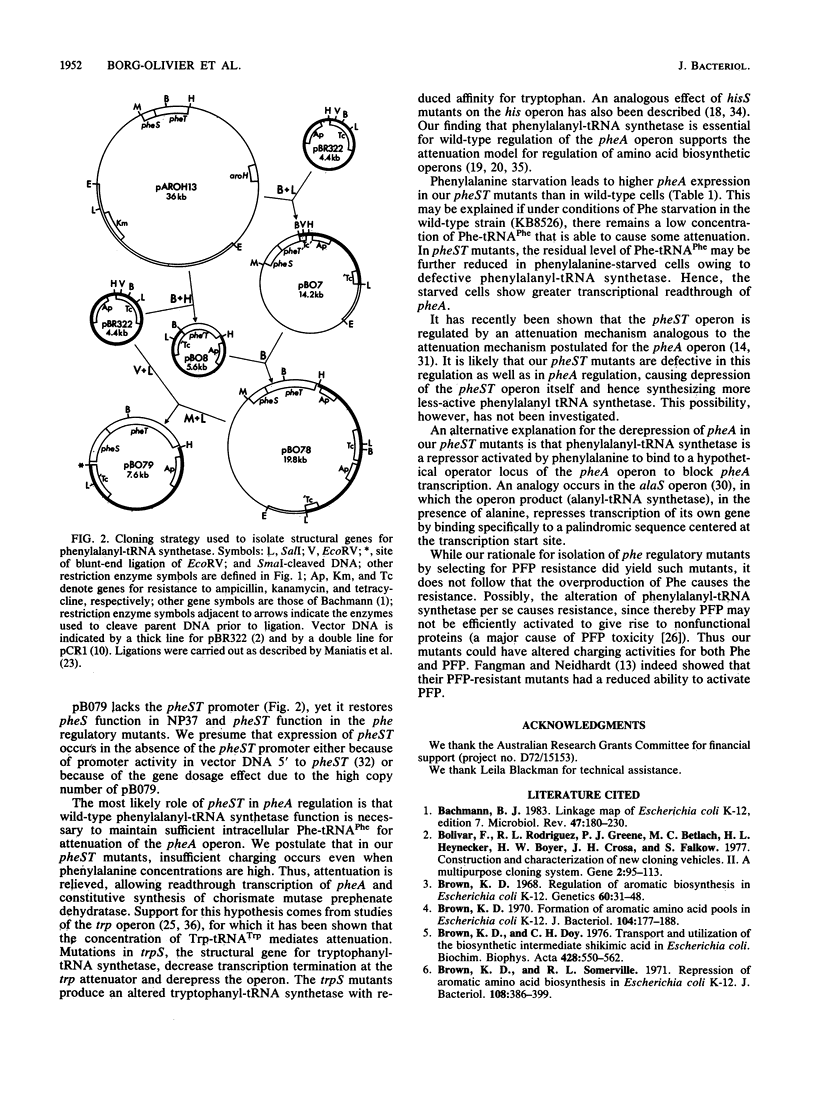
Among mutants of Escherichia coli resistant to p-fluorophenylalanine (PFP) were some with constitutive expression of the phenylalanine biosynthetic operon (the pheA operon). This operon is repressed in the wild type by phenylalanine. The mutation in three of these mutants mapped in the aroH-aroD region of the E. coli chromosome at 37 min. A plasmid bearing wild-type DNA from this region restored p-fluorophenylalanine sensitivity and wild-type repression of the pheA operon. Analysis of subclones of this plasmid and comparison of its restriction map with published maps indicated that the mutations affecting regulation of the pheA operon lie in the structural genes for phenylalanyl-tRNA synthetase, pheST, probably in pheS. Thus, the pheST operon has a role in the regulation of phenylalanine biosynthesis, the most likely being that wild-type phenylalanyl-tRNA synthetase maintains a sufficient intracellular concentration of Phe-tRNA(Phe) for attenuation of the pheA operon in the presence of phenylalanine. A revised gene order for the 37-min region of the chromosome is reported. Read clockwise, the order is aroD, aroH, pheT, and pheS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brown K. D., Doy C. H. Transport and utilization of the biosynthetic intermediate shikimic acid in Escherichia coli. Biochim Biophys Acta. 1976 May 28;428(3):550–562. doi: 10.1016/0304-4165(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Brown K. D. Formation of aromatic amino acid pools in Escherichia coli K-12. J Bacteriol. 1970 Oct;104(1):177–188. doi: 10.1128/jb.104.1.177-188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D. Regulation of aromatic amino acid biosynthesis Escherichia coli K12. Genetics. 1968 Sep;60(1):31–48. doi: 10.1093/genetics/60.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Somerville R. L. Repression of aromatic amino acid biosynthesis in Escherichia coli K-12. J Bacteriol. 1971 Oct;108(1):386–399. doi: 10.1128/jb.108.1.386-399.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer M. M., Böck A. Genes for the alpha and beta subunits of the phenylalanyl-transfer ribonucleic acid synthetase of Escherichia coli. J Bacteriol. 1976 Aug;127(2):923–933. doi: 10.1128/jb.127.2.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey C., Richardson D., Carbon J. A method for the deletion of restriction sites in bacterial plasmid deoxyribonucleic acid. Mol Gen Genet. 1976 May 7;145(2):155–158. doi: 10.1007/BF00269587. [DOI] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Gallagher P., Hoffman A., Weinberg B., Schwartz I. Molecular cloning and regulation of expression of the genes for initiation factor 3 and two aminoacyl-tRNA synthetases. J Bacteriol. 1982 Oct;152(1):357–362. doi: 10.1128/jb.152.1.357-362.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANGMAN W. L., NEIDHARDT F. C. DEMONSTRATION OF AN ALTERED AMINOACYL RIBONUCLEIC ACID SYNTHETASE IN A MUTANT OF ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1839–1843. [PubMed] [Google Scholar]
- Fayat G., Mayaux J. F., Sacerdot C., Fromant M., Springer M., Grunberg-Manago M., Blanquet S. Escherichia coli phenylalanyl-tRNA synthetase operon region. Evidence for an attenuation mechanism. Identification of the gene for the ribosomal protein L20. J Mol Biol. 1983 Dec 15;171(3):239–261. doi: 10.1016/0022-2836(83)90092-x. [DOI] [PubMed] [Google Scholar]
- Gollub E. G., Liu K. P., Sprinson D. B. Regulatory gene of phenylalanine biosynthesis (pheR) in Salmonella typhimurium. J Bacteriol. 1973 Jul;115(1):121–128. doi: 10.1128/jb.115.1.121-128.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J., Pittard J. Regulation of phenylalanine biosynthesis in Escherichia coli K-12: control of transcription of the pheA operon. J Bacteriol. 1982 Jun;150(3):1130–1137. doi: 10.1128/jb.150.3.1130-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S. W., Pittard J. Phenylalanine biosynthesis in Escherichia coli K-12: mutants derepressed for chorismate mutase P-prephenate dehydratase. J Bacteriol. 1971 Jun;106(3):784–790. doi: 10.1128/jb.106.3.784-790.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNIER R., COHEN G. N. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes au cours de leur synthèse in vivo. Biochim Biophys Acta. 1959 Feb;31(2):378–391. doi: 10.1016/0006-3002(59)90011-3. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fayat G., Blanquet S. Sequence of the Escherichia coli pheST operon and identification of the himA gene. J Bacteriol. 1985 Aug;163(2):787–791. doi: 10.1128/jb.163.2.787-791.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Morse A. N. Dual-control of the tryptophan operon is mediated by both tryptophanyl-tRNA synthetase and the repressor. J Mol Biol. 1976 May 15;103(2):209–226. doi: 10.1016/0022-2836(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Escherichia coli phenylalanyl-tRNA synthetase operon: characterization of mutations isolated on multicopy plasmids. J Bacteriol. 1982 Nov;152(2):650–660. doi: 10.1128/jb.152.2.650-660.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M. Genes for the two subunits of phenylalanyl-tRNA synthesis of Escherichia coli are transcribed from the same promoter. J Mol Biol. 1980 Dec 25;144(4):595–600. doi: 10.1016/0022-2836(80)90341-1. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Springer M., Graffe M., Goursot R., Grunberg-Manago M. Physical localisation and cloning of the structural gene for E. coli initiation factor IF3 from a group of genes concerned with translation. Gene. 1980 Oct;11(1-2):33–42. doi: 10.1016/0378-1119(80)90084-0. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981 Jun 25;291(5817):632–635. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- Springer M., Trudel M., Graffe M., Plumbridge J., Fayat G., Mayaux J. F., Sacerdot C., Blanquet S., Grunberg-Manago M. Escherichia coli phenylalanyl-tRNA synthetase operon is controlled by attenuation in vivo. J Mol Biol. 1983 Dec 15;171(3):263–279. doi: 10.1016/0022-2836(83)90093-1. [DOI] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wyche J. H., Ely B., Cebula T. A., Snead M. C., Hartman P. E. Histidyl-transfer ribonucleic acid synthetase in positive control of the histidine operon in Salmonella typhimurium. J Bacteriol. 1974 Feb;117(2):708–716. doi: 10.1128/jb.117.2.708-716.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yanofsky C. Mutations affecting tRNATrp and its charging and their effect on regulation of transcription termination at the attenuator of the tryptophan operon. J Mol Biol. 1977 Jul 15;113(4):663–677. doi: 10.1016/0022-2836(77)90229-7. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]


