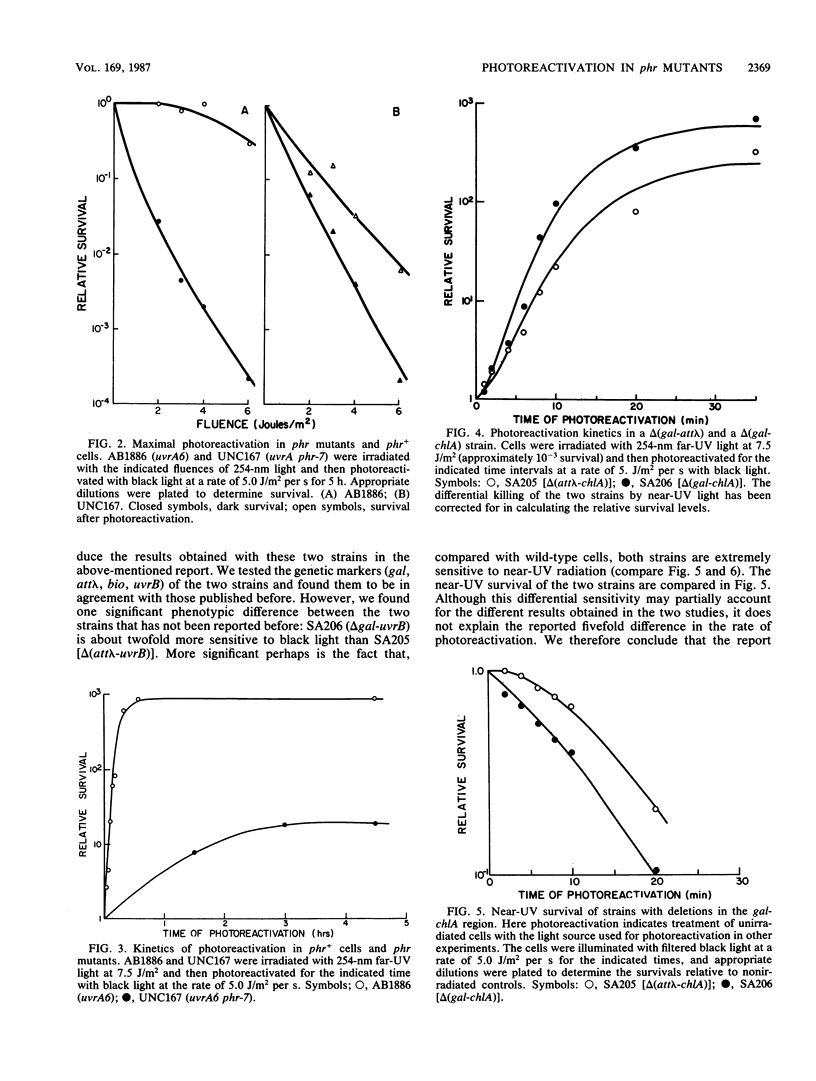
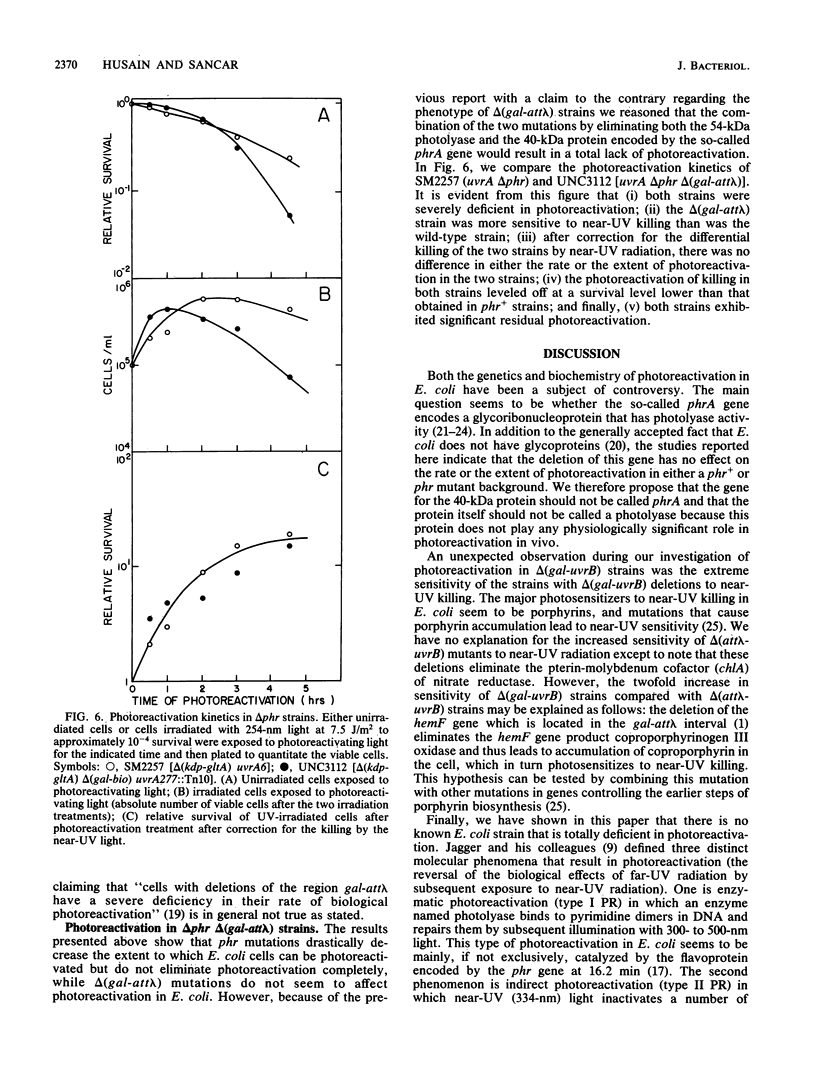
Abstract
We have investigated the genetics of photoreactivation in Escherichia coli K-12. We found that strains with point mutations or deletions in the phr gene showed a significant residual level of photoreactivation after exposure to large fluences of photoreactivating light. It had been previously proposed that a gene in the gal-att lambda interval is also involved in photoreactivation and that the residual photoreactivating activity might be due to this so-called phrA gene located at this interval. We found that deletions of the gal-att lambda region had no effect on either the rate or the final extent of photoreactivation observed in phr+ cells or phr mutants; however strains carrying the delta (gal-att lambda) deletions displayed increased sensitivity to near-UV radiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm W. Evidence for photoenzymatically repairable, lethal "non-dimer" photoproducts, formed in E. coli cells by 365-nm radiation or by sunlight greater than 360 nm. Mutat Res. 1978 Sep;51(3):301–310. doi: 10.1016/0027-5107(78)90119-7. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Martin S. J., Bhatia K. Repair of nonreplicating UV-irradiated DNA: cooperative dark repair by Escherichia coli uvr and phr functions. J Bacteriol. 1985 Feb;161(2):602–608. doi: 10.1128/jb.161.2.602-608.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenaga M., Patrick M. H., Jagger J. Photoreactivation of killing in Streptomyces. 3. Action spectra for photolysis of pyrimidine dimers and adducts in S. griseus and S. griseus PHR-1. Photochem Photobiol. 1971 Aug;14(2):175–187. doi: 10.1111/j.1751-1097.1971.tb06161.x. [DOI] [PubMed] [Google Scholar]
- JAGGER J. Photoreactivation. Bacteriol Rev. 1958 Jun;22(2):99–142. doi: 10.1128/br.22.2.99-142.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger J., Stafford R. S., Snow J. M. Thymine-dimer and action-spectrum evidence for indirect photoreactivation in Escherichia coli. Photochem Photobiol. 1969 Dec;10(6):383–395. doi: 10.1111/j.1751-1097.1969.tb05703.x. [DOI] [PubMed] [Google Scholar]
- Jagger J., Takebe H., Snow J. M. Photoreactivation of killing in Streptomyces: action spectra and kinetic studies. Photochem Photobiol. 1970 Sep;12(3):185–196. doi: 10.1111/j.1751-1097.1970.tb06050.x. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Moehring M. P., Webb R. B. Ultraviolet action spectra for DNA dimer induction, lethality, and mutagenesis in Escherichia coli with emphasis on the UVB region. Photochem Photobiol. 1984 Nov;40(5):613–620. doi: 10.1111/j.1751-1097.1984.tb05349.x. [DOI] [PubMed] [Google Scholar]
- RUPERT C. S., GOODGAL S. H., HERRIOTT R. M. Photoreactivation in vitro of ultraviolet-inactivated Hemophilus influenzae transforming factor. J Gen Physiol. 1958 Jan 20;41(3):451–471. doi: 10.1085/jgp.41.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. B., Laimins L., Epstein W. Functional organization of the kdp genes of Escherichia coli K-12. J Bacteriol. 1978 Aug;135(2):445–452. doi: 10.1128/jb.135.2.445-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert C. S. Relation of photoreactivation to photoenzymatic repair of DNA in Escherichia coli. Photochem Photobiol. 1966 Mar;4(2):271–275. doi: 10.1111/j.1751-1097.1965.tb05746.x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupert C. S. Penicillin selection of Escherichia coli deoxyribonucleic acid repair mutants. J Bacteriol. 1979 Jun;138(3):779–782. doi: 10.1128/jb.138.3.779-782.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. Escherichia coli DNA photolyase is a flavoprotein. J Mol Biol. 1984 Jan 15;172(2):223–227. doi: 10.1016/s0022-2836(84)80040-6. [DOI] [PubMed] [Google Scholar]
- Sancar G. B., Smith F. W., Sancar A. Identification and amplification of the E. coli phr gene product. Nucleic Acids Res. 1983 Oct 11;11(19):6667–6678. doi: 10.1093/nar/11.19.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T. J., Benson S. A., Emr S. D. Mechanisms of protein localization. Microbiol Rev. 1983 Sep;47(3):313–344. doi: 10.1128/mr.47.3.313-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M., Sutherland B. M. Escherichia coli photoreactivating enzyme: purification and properties. Biochemistry. 1980 Sep 2;19(18):4201–4208. doi: 10.1021/bi00559a010. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Court D., Chamberlin M. J. Studies on the DNA photoreactivating enzyme from Escherichia coli. I. Transduction of the phr gene by bacteriophage lambda. Virology. 1972 Apr;48(1):87–93. doi: 10.1016/0042-6822(72)90116-x. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Hausrath S. G. Multiple loci affecting photoreactivation in Escherichia coli. J Bacteriol. 1979 May;138(2):333–338. doi: 10.1128/jb.138.2.333-338.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B. M., Oliveira O. M., Ciarrocchi G., Brash D. E., Haseltine W. A., Lewis R. J., Hanawalt P. C. Substrate range of the 40,000-dalton DNA-photoreactivating enzyme from Escherichia coli. Biochemistry. 1986 Feb 11;25(3):681–687. doi: 10.1021/bi00351a026. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Sammartano L. J. Sensitivity of hemA mutant Escherichia coli cells to inactivation by near-UV light depends on the level of supplementation with delta-aminolevulinic acid. Photochem Photobiol. 1986 Jun;43(6):621–626. doi: 10.1111/j.1751-1097.1986.tb05637.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Induction of pyrimidine dimers in bacterial DNA by 365 nm radiation. Photochem Photobiol. 1973 Jan;17(1):69–73. doi: 10.1111/j.1751-1097.1973.tb06334.x. [DOI] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]



