Abstract
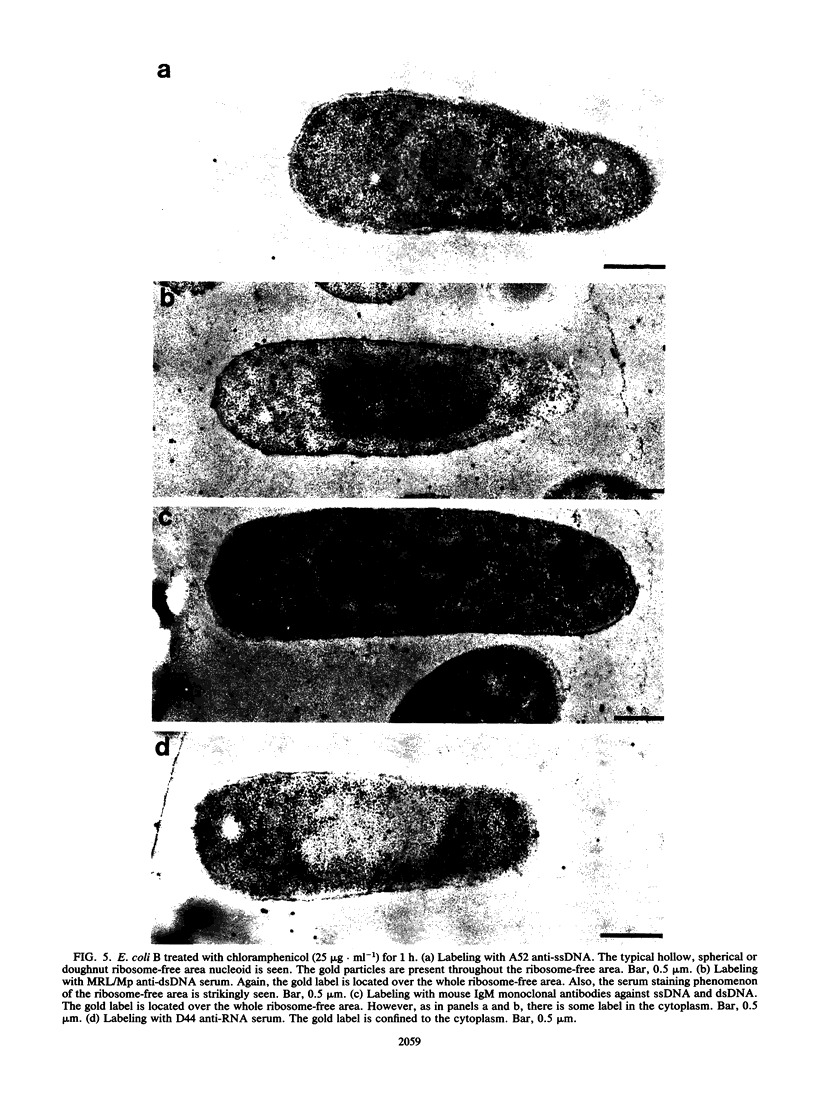
Escherichia coli cells were very rapidly frozen and substituted at a low temperature with 3% glutaraldehyde in acetone. Infiltration and embedding with Lowicryl K4M were carried out at -35 degrees C. This procedure resulted in good structural preservation of both the nucleoid morphology and its DNA plasm, such that immunolabeling with the protein-A gold technique could be carried out. With antibodies specific for either double-stranded DNA (dsDNA) or single-stranded DNA (ssDNA), it was shown that dsDNA was present throughout the nucleoid but that ssDNA was located on the nucleoid periphery. Chloramphenicol-treated cells, in which protein synthesis but not DNA replication is stopped, produced a characteristic ringlike nucleoid shape and had both dsDNA and ssDNA present throughout the annular section of the DNA plasm. The relationship between metabolically active DNA and overall bacterial genome organization is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlemalm E., Villiger W., Hobot J. A., Acetarin J. D., Kellenberger E. Low temperature embedding with Lowicryl resins: two new formulations and some applications. J Microsc. 1985 Oct;140(Pt 1):55–63. doi: 10.1111/j.1365-2818.1985.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Fischel R., Laskov R. Antibodies to RNA from autoimmune NZB/NZW mice recognize a similar antigenic determinant and show a large idiotypic diversity. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3818–3822. doi: 10.1073/pnas.79.12.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe K., Ovrebö S., Lossius I. The bacterial nucleoid. J Gen Microbiol. 1979 May;112(1):1–13. doi: 10.1099/00221287-112-1-1. [DOI] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A. Visualization of RNA synthesis on chromosomes. Int Rev Cytol. 1972;33:1–25. doi: 10.1016/s0074-7696(08)61446-1. [DOI] [PubMed] [Google Scholar]
- Munns T. W., Liszewski M. K., Hahn B. H. Antibody-nucleic acid complexes. Antigenic domains within nucleosides as defined by solid-phase immunoassay. Biochemistry. 1984 Jun 19;23(13):2958–2964. doi: 10.1021/bi00308a017. [DOI] [PubMed] [Google Scholar]
- Newman G. R., Jasani B., Williams E. D. A simple post-embedding system for the rapid demonstration of tissue antigens under the electron microscope. Histochem J. 1983 Jun;15(6):543–555. doi: 10.1007/BF01954145. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]






