Abstract
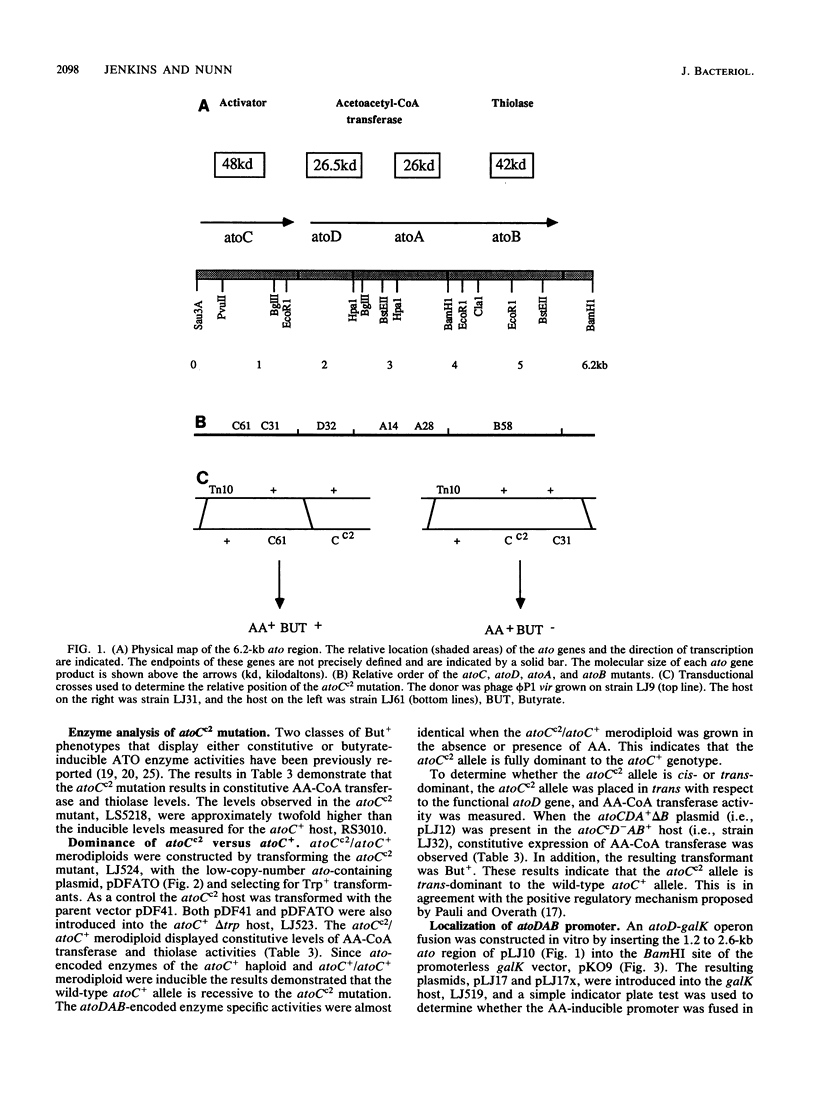
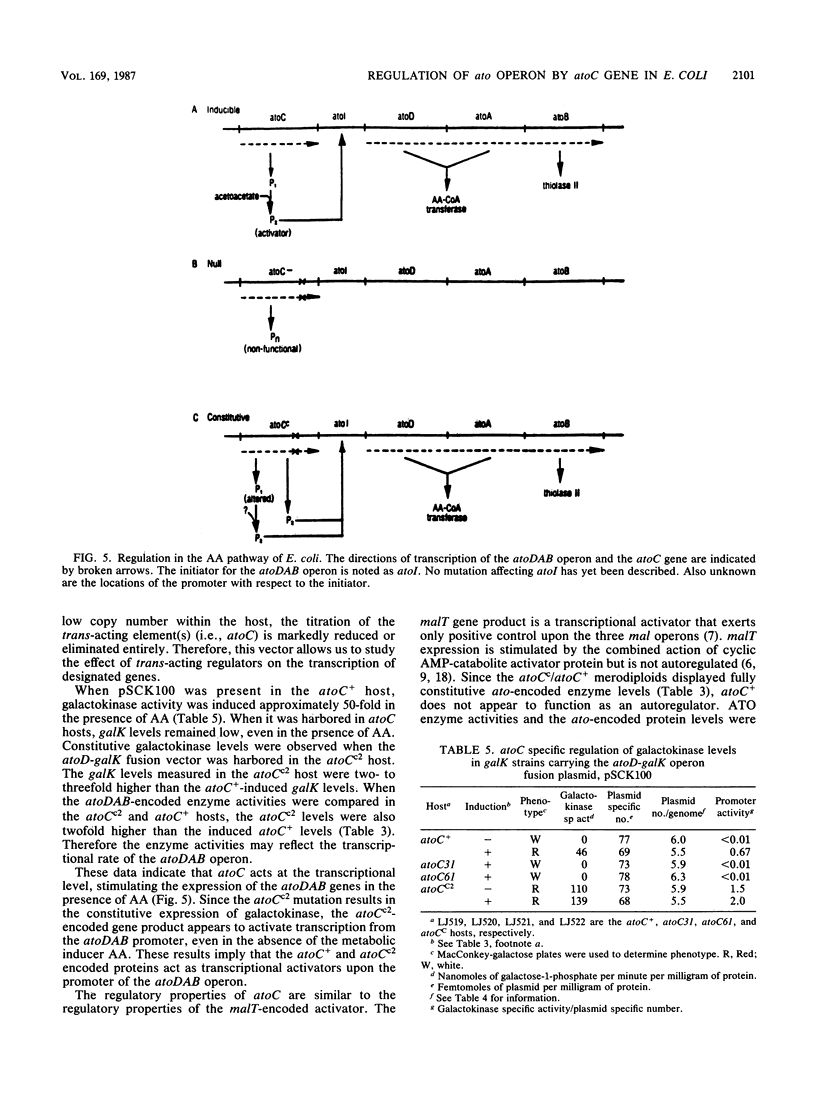
The expression of the Ato enzymes, acetyl coenzyme A:acetoacetyl coenzyme A transferase and thiolase II, is required for growth of Escherichia coli on short-chain fatty acids. The structural genes for these enzymes, atoD, atoA, and atoB, respectively, make up the ato operon. A 48-kilodalton protein encoded by atoC was required for the synthesis or activation of the Ato enzymes. The expression of Ato enzyme activities was inducible in atoC+ strains, constitutive in atoCc strains, and noninducible in atoC mutants. Merodiploid studies demonstrated that the atoCc allele is trans-dominant to the atoC+ allele. To study the action of the trans-acting atoC-encoded activator, the promoter of the ato operon was fused to the promoterless galK gene and introduced into a low-copy-number vector. The resulting low-copy-number fusion plasmid was introduced into atoC+, atoC, and atoCc hosts. The expression of the fused galK gene was inducible in the atoC+ host, noninducible in atoC host strains, and constitutive when harbored in the atoCc host. This indicated that the atoC+ and atoCc gene products act at the level of transcription, stimulating the expression of the ato operon. A working model consistent with these results is presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Hatfield G. W. Effects of promoter strengths and growth conditions on copy number of transcription-fusion vectors. J Biol Chem. 1984 Jun 25;259(12):7399–7403. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J. Regulation of the regulatory gene for the arabinose pathway, araC. J Mol Biol. 1976 Jul 5;104(3):557–566. doi: 10.1016/0022-2836(76)90120-0. [DOI] [PubMed] [Google Scholar]
- Chapon C., Kolb A. Action of CAP on the malT promoter in vitro. J Bacteriol. 1983 Dec;156(3):1135–1143. doi: 10.1128/jb.156.3.1135-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe G. R., Frerman F. E. Molecular and catalytic properties of the acetoacetyl-coenzyme A thiolase of Escherichia coli. Arch Biochem Biophys. 1976 Sep;176(1):159–170. doi: 10.1016/0003-9861(76)90152-1. [DOI] [PubMed] [Google Scholar]
- Débarbouillé M., Shuman H. A., Silhavy T. J., Schwartz M. Dominant constitutive mutations in malT, the positive regulator gene of the maltose regulon in Escherichia coli. J Mol Biol. 1978 Sep 15;124(2):359–371. doi: 10.1016/0022-2836(78)90304-2. [DOI] [PubMed] [Google Scholar]
- Gutierrez C., Raibaud O. Point mutations that reduce the expression of malPQ, a positively controlled operon of Escherichia coli. J Mol Biol. 1984 Jul 25;177(1):69–86. doi: 10.1016/0022-2836(84)90058-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto-Gotoh T., Franklin F. C., Nordheim A., Timmis K. N. Specific-purpose plasmid cloning vectors. I. Low copy number, temperature-sensitive, mobilization-defective pSC101-derived containment vectors. Gene. 1981 Dec;16(1-3):227–235. doi: 10.1016/0378-1119(81)90079-2. [DOI] [PubMed] [Google Scholar]
- Jenkins L. S., Nunn W. D. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J Bacteriol. 1987 Jan;169(1):42–52. doi: 10.1128/jb.169.1.42-52.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Roth J., Botstein D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol. 1977 Oct 15;116(1):125–159. doi: 10.1016/0022-2836(77)90123-1. [DOI] [PubMed] [Google Scholar]
- Lee N. L., Gielow W. O., Wallace R. G. Mechanism of araC autoregulation and the domains of two overlapping promoters, Pc and PBAD, in the L-arabinose regulatory region of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):752–756. doi: 10.1073/pnas.78.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli G., Overath P. ato Operon: a highly inducible system for acetoacetate and butyrate degradation in Escherichia coli. Eur J Biochem. 1972 Sep 25;29(3):553–562. doi: 10.1111/j.1432-1033.1972.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Wegener W. S. Growth of Escherichia coli on short-chain fatty acids: growth characteristics of mutants. J Bacteriol. 1971 Nov;108(2):885–892. doi: 10.1128/jb.108.2.885-892.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanitro J. P., Wegener W. S. Growth of Escherichia coli on short-chain fatty acids: nature of the uptake system. J Bacteriol. 1971 Nov;108(2):893–901. doi: 10.1128/jb.108.2.893-901.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Egan P. A., Chute H. T., Nunn W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. J Bacteriol. 1980 May;142(2):621–632. doi: 10.1128/jb.142.2.621-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt S. K., Black P. N., Ragozzino M. M., Nunn W. D. Cloning, mapping, and expression of genes involved in the fatty acid-degradative multienzyme complex of Escherichia coli. J Bacteriol. 1984 May;158(2):535–542. doi: 10.1128/jb.158.2.535-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt S. K., Ginsburgh C. L., Nunn W. D. Isolation and genetic characterization of Escherichia coli mutants defective in propionate metabolism. J Bacteriol. 1981 Jun;146(3):1166–1169. doi: 10.1128/jb.146.3.1166-1169.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sramek S. J., Frerman F. E. Purification and properties of Escherichia coli coenzyme A-transferase. Arch Biochem Biophys. 1975 Nov;171(1):14–26. doi: 10.1016/0003-9861(75)90002-8. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Reeves H. C., Rabin R., Ajl S. J. Alternate pathways of metabolism of short-chain fatty acids. Bacteriol Rev. 1968 Mar;32(1):1–26. doi: 10.1128/br.32.1.1-26.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


