Abstract
West Nile virus (WNV) causes a severe infection of the central nervous system in several vertebrate animals including humans. Prior studies have shown that complement plays a critical role in controlling WNV infection in complement (C) 3−/− and complement receptor 1/2−/− mice. Here, we dissect the contributions of the individual complement activation pathways to the protection from WNV disease. Genetic deficiencies in C1q, C4, factor B, or factor D all resulted in increased mortality in mice, suggesting that all activation pathways function together to limit WNV spread. In the absence of alternative pathway complement activation, WNV disseminated into the central nervous system at earlier times and was associated with reduced CD8+ T cell responses yet near normal anti-WNV antibody profiles. Animals lacking the classical and lectin pathways had deficits in both B and T cell responses to WNV. Finally, and somewhat surprisingly, C1q was required for productive infection in the spleen but not for development of adaptive immune responses after WNV infection. Our results suggest that individual pathways of complement activation control WNV infection by priming adaptive immune responses through distinct mechanisms.
The complement system is a family of more than 30 proteins and cell surface receptors that recognize pathogen-associated molecular patterns, altered-self ligands, or immune complexes. Complement activation through the classical, lectin, and alternative pathways induces several protective functions including direct pathogen opsonization and/or lysis, and enhancement of B and T responses (1). Through these innate and adaptive responses complement contributes to the development of immunity against some enveloped DNA and RNA viruses (2–5). Several of these viruses have been shown to trigger distinct pathways of complement activation in vitro. Glycoproteins of murine leukemia, HIV, and human T cell lymphotropic viruses directly interact with C1q to activate the classical pathway (6). Carbohydrates on the structural proteins of HSV, hepatitis B, and influenza viruses bind mannose binding lectins (MBLs) and activate the lectin pathway (7, 8). Multiple viruses activate the alternative pathway, including Sindbis (9), Sendai (10), measles (11, 12), and Epstein Barr viruses (13). However, the in vivo contribution of each complement activation pathway to the development of antiviral immunity has yet to be defined.
West Nile encephalitis virus (WNV) is a single-stranded positive sense RNA virus of the Flaviviridae family. WNV cycles in nature between mosquitoes and birds, but also infects human, horses, and other vertebrates. The virus is endemic in parts of Africa, Asia, Europe, and the Middle East, and has become established in North America. Infected humans generally develop a febrile illness, with a subset progressing to severe neurological disease. The elderly and patients with impaired immune systems are at greatest risk for the severe neurological manifestations of disease. Experiments in mice have begun to elucidate how an impaired host immune response results in severe WNV infection. An intact innate and adaptive immune response is required to limit central nervous system (CNS) infection as mice deficient in type I IFN, γδ T cells, B cells, soluble IgM, and CD8+ T cells are all highly susceptible to lethal infection (14–19). Additionally, complement is required to control WNV, as mice deficient in either complement (C)3 or complement receptor (CR)1/2 were vulnerable to lethal WNV infection (20).
In this study, we investigated the activation requirements for complement-mediated control of WNV dissemination and disease. We observed a marked increased in WNV susceptibility in mice deficient in any of the pathways of complement activation. However, the virologic and immunologic phenotypes of the various complement-deficient mice were distinct, suggesting that the concerted activation of the classical, lectin, and alternative pathways is required to fully prime adaptive immune responses and control WNV infection.
RESULTS
Complement activation in vivo after WNV infection
Previous studies have suggested that other pathogenic flaviviruses, such as Dengue virus, activate complement leading to consumption of complement proteins and more severe disease (21, 22). To confirm that complement activation occurs in vivo after WNV infection, we compared the levels of functional C3 and C4 in the serum of naive and WNV-infected C57BL/6 mice using an erythrocyte hemolysis assay (Fig. 1 A). On day 2 after WNV infection, a time point at which peak viremia was observed (see Fig. 3 A), a 2.5-fold decrease in C3 functional activity (P < 0.0001) was measured. Significant decreases, albeit smaller, were also noted on days 4 and 6 after infection (P ≤ 0.02). C4 activity (23) was also reduced at day 2 after WNV infection (Fig. 1 B). As the catabolism of C3 in vivo generates a C3dg fragment, Western blot analysis was performed on serum from WNV-infected mice with an anti-C3 antibody. Increased levels of the 38-kD C3dg fragment were observed in serum at day 2 after WNV infection (Fig. 1 C); the identity of this fragment was confirmed by its absence from serum of congenic C3-deficient mice. Collectively, our experiments suggest that WNV infection activates and consumes complement within days of infection.
Figure 1.
Complement is activated in vivo in response to WNV infection. Levels of functional (A) C3 and (B) C4 were determined by erythrocyte hemolysis assay of serum samples from naive and WNV-infected mice. Differences in the C3 and C4 activity between naive and WNV-infected mice were statistically significant (P < 0.05). (C) Serum complement activation was evaluated by Western blot using equal volumes of serum (20 μl of 1/50 dilution) from naive wild-type and C3−/− mice and WNV-infected (day 2) wild-type mice. Bands corresponding to the C3 α chain (100 kD), C3 β chain (75 kD), and C3dg (38 kD) are labeled.
Figure 3.
WNV infection in serum and lymphoid tissues. WNV RNA levels in the serum (A), spleen (C), and draining inguinal lymph node (D) of wild-type, C1q−/−, C4−/−, fD−/−, and fB−/− mice at the indicated days were determined by quantitative RT-PCR from at least 4–6 independent mice per time point per group. Infectious WNV burden in the spleen (B) of wild-type, C1q−/−, C4−/−, fD−/−, and fB−/− mice at the indicated days was determined by plaque assay of samples from 8–15 mice per time point per group. The dotted line indicates the limit of sensitivity of the assay. Asterisks indicate time points at which differences are statistically significant compared with wild type.
Susceptibility of complement-deficient mice to WNV infection
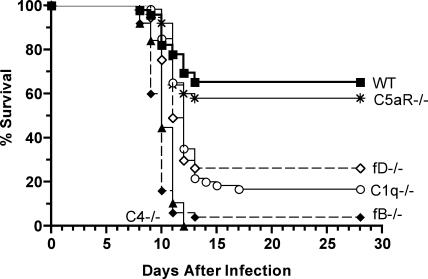
To assess the mechanism of complement-mediated protection from WNV disease and the function of the classical, classical and lectin, or alternative pathways of complement activation in control of WNV, we evaluated morbidity and mortality in wild-type and congenic C1q−/−, C4−/−, factor D (fD)−/−, and factor B (fB)−/− C57BL/6 mice. Mice in all groups exhibited clinical signs of infection including weight loss, hunching, fur ruffling, and decreased activity. However, mice deficient in any individual complement activation pathway were more vulnerable to infection and had higher mortality rates compared with wild-type mice (Fig. 2). Whereas only 35% of wild-type C57BL/6 mice succumbed to infection, 83% of C1q−/− (n = 60, P < 0.0001), 100% of C4−/− (n = 38, P < 0.0001), 74% of fD−/−, (n = 57, P = 0.0004), and 96% of fB−/− (n = 51, P < 0.0001) mice died after WNV infection (Fig. 2). Not all complement deficiencies were associated with enhanced susceptibility as infection of mice deficient in the C5a receptor (C5aR) survived at rates similar to wild-type mice (n = 50, P = 0.5). Although both fB and fD are components of the alternative pathway, a deficiency of fB caused greater mortality after WNV infection (P < 0.0001), possibly caused by residual alternative pathway activation in fD−/− mice (24). Overall, the C4−/− and fB−/− mice were more susceptible to lethal WNV infection than either C1q−/− or fD−/− mice (P < 0.0001). The increased susceptibility of fB−/− mice was also reflected by a decrease in the mean time to death from 10.9 ± 0.3 d for wild-type mice to 9.7 ± 0.1 d for the fB−/− mice (P = 0.002). No statistically significant decrease in the mean time to death was observed for C4−/− (10.3 ± 0.2 d), C1q−/− (11.7 ± 0.2 d), or fD−/− (11.0 ± 0.2 d) mice. Thus, deficiencies in all complement activation pathways resulted in increased mortality after WNV infection. The most severe phenotypes were observed in mice that had a combined deficiency in the classical and lectin pathways (C4−/−) or a complete deficiency in the alternative pathway (fB−/−).
Figure 2.
All pathways of complement activation are required for survival of WNV infection. Wild-type (n = 95), C1q−/− (n = 60), C4−/− (n = 34), fD−/− (n = 57), fB−/− (n = 50), and C5aR−/− (n = 50) C57BL/6 mice were infected with 102 PFU by footpad injection in at least three independent experiments. Significant decreases in survival as compared with wild-type mice were noted for all complement deficient mice (P ≤ 0.0004), except C5aR−/− mice (P = 0.5).
WNV tissue burden in complement-deficient mice
To elucidate the mechanisms by which deficiencies in complement activation pathways enhanced susceptibility to WNV infection, a kinetic analysis of viral burden was performed (Fig. 3 and 4). Wild-type and complement-deficient C57BL/6 mice were infected with WNV, and viral loads in the serum, spleen, inguinal lymph node, spinal cord, and brain were determined on days 2, 4, 6, 8, and 10 after infection.
Figure 4.
WNV infection in CNS tissues. Infectious WNV burden in (A) spinal cord and (C) brain from wild-type, C1q−/−, C4−/−, fD−/−, and fB−/− mice was determined by plaque assay of samples from 8–15 mice per time point per group. Scatter plots of (B) spinal cord and (D) brain titers from individual mice at day 4 or 10 are shown to the right of each graph. The limit of sensitivity of the assay and statistical significance are as described in Fig 3.
Viremia.
Throughout the time course of infection, viremia was below the limit of detection by direct plaque assay in all samples (unpublished data). However, when viral RNA was measured by quantitative RT-PCR assay (25), additional information was obtained. In wild-type mice, viremia peaked at day 2 after infection and decreased over the next several days. No significant difference in viremia was observed at any time point after infection in C1q−/−, fB−/−, or fD−/− mice (Fig. 3 A, P ≥ 0.5). The only variation among complement-deficient mice occurred in C4−/− mice, which had slightly higher WNV RNA levels than wild-type mice (n = 8, P = 0.009), but only on day 4 after infection.
Spleen and lymph node.
Previous experiments have shown that a deficiency of C3 results in a delayed clearance of WNV from the spleen (20). To assess which complement pathway contributed to viral clearance in the spleen, we evaluated WNV burden in C1q−/−, C4−/−, fD−/−, and fB−/− mice (Fig. 3 B). Compared with wild-type mice, we observed a slight delay in clearance of WNV from the spleen in a subset of fD−/− and fB−/− mice. At day 6 after infection, 58% (7 of 12) and 73% (11 of 15) of fD−/− and fB−/− mice, respectively, had detectable infectious WNV in the spleen, whereas only 33% (5 of 15) of wild-type mice had infectious virus at this time point. On day 8 after infection 57% (8 of 14) of fB−/− mice still had measurable infectious virus in the spleen, whereas it was no longer detectable in wild-type mice. Surprisingly, infectious virus was never recovered from the spleens of C1q−/− mice at any point during the time course (n ≥ 11 for each time point). Consistent with this, at the peak of viral replication in the spleen, 120,000-fold lower levels of WNV RNA were detected by quantitative RT-PCR from the spleens of C1q−/− mice compared with wild-type mice (Fig. 3 C, P = 0.002). The peak viral burden and number of positive samples in the spleen also was significantly lower in the absence of C4 (P = 0.04). Whereas 80% (12 of 15) of wild-type spleen samples were positive for infectious virus at day 4 after infection, only 30% (4 of 12) from C4−/− mice had infectious virus. Moreover, on day 6 after infection 60,000-fold lower levels of WNV RNA were detected in spleens of C4−/− mice (P = 0.001). Thus, in the spleen, classical pathway activation appears to be necessary for productive infection, whereas the alternative pathway contributes in part to its rapid clearance of WNV.
Decreased WNV burden in spleens of C1q−/− and C4−/− mice was not predicted. Previous studies in mice indicated that F4/80+ macrophages and CD19+ B cells were the principal cellular targets for WNV infection in the spleen (18). Because infection of C1q−/− mice resulted in normal viremia, we hypothesized that virus must replicate in another peripheral tissue. Studies from our group and others (14, 26) indicate that draining lymph nodes are an early site of WNV replication. To determine one possible source of viremia in the C1q−/− and C4−/− animals, viral burden in the inguinal lymph nodes was measured by quantitative RT-PCR (Fig. 3 D). Equivalent levels of WNV RNA were detected on the first and second days after infection in wild-type, C1q−/−, and C4−/− mice (P ≥ 0.8). However, by 3 d after infection fourfold less WNV RNA was found in the draining inguinal lymph nodes of C1q (P = 0.05) and C4−/− mice (P = 0.04) compared with wild-type mice.
CNS: spinal cord.
Distinct patterns of WNV invasion and accumulation in the spinal cord were observed in C1q−/−, C4−/−, fD−/−, and fB−/− mice (Fig. 4 A). In agreement with published studies, WNV was not detected in spinal cord samples from wild-type mice until day 8 after infection (16). Similarly, infectious virus was not recovered from the spinal cords of C1q−/− or fD−/− mice until day 8 after infection. In the absence of either fB or C4, however, WNV was detected in a subset of spinal cord samples 2 d earlier. At day 6, 47% (7 of 15) fB−/− and 50% (6 of 12) of C4−/− mice had measurable infectious WNV in the spinal cord (P ≤ 0.03). Overall, all complement-deficient mice had significantly higher peak spinal cord viral burdens by day 10 after infection compared with wild-type mice (P ≤ 0.03). Additionally, whereas 100% of complement-deficient mice exhibited WNV invasion of the spinal cord by day 10 after infection, only 73% (11 of 15) of wild-type mice had infectious WNV in the spinal cord at this time point. These results suggest that the different complement activation pathways modulate the entry and accumulation of WNV in the spinal cord.
CNS: brain.
A slightly different pattern of infection was observed in the brain, as earlier viral invasion was observed in all complement-deficient mice (Fig. 4 C). Infectious virus was not recovered from the brains of wild-type adult mice until day 6, results that agree with our previous studies (16). In contrast, infectious virus was detected at day 4 in the brains of 33% of C4−/− (4 of 12), 18% of C1q−/− (2 of 11), 67% of fD−/− (8 of 12), and 60% of fB−/− (6 of 10) mice. On average, fD−/− mice had ∼30-fold higher levels of WNV in the brain than C1q−/− mice on day 4 after infection (P = 0.02). Viral burden in the brain peaked in wild-type mice on day 8 after infection. In contrast, levels of infectious WNV continued to rise in C1q−/−, C4−/−, fD−/−, and fB−/− mice such that 10–200-fold higher levels were observed by day 10 after infection (P ≤ 0.04). The analysis of viral burden in the CNS suggests that a deficiency in primarily the alternative pathway results in earlier WNV entry and accumulation, whereas a deficiency in any of the complement pathways results in increased viral load.
Pattern of WNV CNS infection
To evaluate if enhanced CNS WNV infection was the result of a change in viral tropism, we examined brain and spinal cord sections for WNV antigen (Fig. 5 and unpublished data). WNV antigen localized to cells that stained positive for neuronal antigens (unpublished data) throughout the brain and spinal cord, in agreement with previous studies (16). Intense antigen staining in complement-deficient mice was observed in neurons of the cerebellum, brain stem, brain base, and cortex. Less intense antigen staining in CNS tissues was noted in wild-type mice, with sporadic infection of similar neuronal populations. Thus, more neurons throughout the brain and spinal cord were infected at higher levels in the absence of complement activation compared with wild-type animals.
Figure 5.
WNV antigen staining in the brain. The brains of wild-type, C1q−/−, C4−/−, and fD−/− mice were harvested on day 10, sectioned, and stained for WNV. Representative images of the cerebellum, cortex, and brain stem were determined after staining three to four mice per group. Bar, 200 μm.
Virus-specific antibody responses in complement-deficient mice
Because the production of anti-WNV antibody was depressed in C3−/− mice (20), we assessed the kinetics of specific antiviral IgM and IgG production in C1q−/−, C4−/−, fD−/−, and fB−/− mice. Consistent with previous studies (14–16), in wild-type mice WNV-specific IgM was detected by day 4 after infection (Fig. 6 A), and WNV-specific IgG was detected by day 6 after infection (Fig. 6 B). Near normal development of anti-WNV IgG and IgM was observed in the absence of alternative pathway activation in fD−/− and fB−/− mice (P ≥ 0.1). Additionally, in fB−/− mice, we observed no significant difference in the isotype specificity (Fig. 6 C) or neutralizing activity of the WNV antibody response (Fig. 6 D). In contrast, the development of WNV-specific IgG was delayed in C1q−/− and C4−/− mice, with three- to fourfold reduced titers on day 8 after infection, respectively (P ≤ 0.0003). However, this deficit disappeared by day 10, as normal levels of anti-WNV IgG were subsequently observed (P ≥ 0.5). Throughout the time course the anti-WNV IgM response was reduced only in the absence of C4 (P ≤ 0.03). Collectively, our serologic data establish that the IgM response to WNV is C4 dependent, yet C1q, fD, and fB independent, suggesting a possible role for the lectin pathway. Consistent with this, C1q−/− × fD−/− mice had normal anti-WNV IgM and IgG responses on day 10 after infection (unpublished data; P = 0.7).
Figure 6.
Humoral responses against WNV. Serum samples from wild-type, C1q−/−, C4−/−, fD−/−, and fB−/− mice were collected at the indicated time points. Titers of specific IgM (A) and IgG (B) against WNV were calculated after incubating serum samples with absorbed control or WNV E proteins. The isotype of WNV-specific IgG responses (C) was analyzed on day 8 after infection. Neutralizing activity (D) of serum samples from wild-type and fB−/− mice on day 8 after infection was determined by a flow cytometry–based neutralization assay. Data are an average of at least three independent experiments performed in duplicate and reflect 5–10 mice per group. Asterisks indicate time points at which differences are statistically significant compared with wild type (P < 0.05).
T cell response in C4−/−, C1q−/−, fD−/−, and fB−/− mice
CD8+ T cells are required for clearance of WNV from the CNS (16, 17). Studies with influenza and lymphocytic choriomeningits virus (LCMV) have found that virus-specific T cell priming is inhibited in the absence of C3 (4, 27). Because enhanced WNV accumulation in the CNS is observed in all complement-deficient mice, we examined how the individual complement pathway deficiencies affected the response of CD8+ T cells to WNV infection (Fig. 7 A). We evaluated intracellular IFNγ expression in splenocytes from mock- and WNV-infected C1q−/−, C4−/−, fD−/−, and fB−/− mice. By day 8 after infection, there was a ∼2.5-fold increase in the total number of splenic CD4+ and CD8+ cells that expressed IFNγ in both wild-type (P = 0.0007) and C1q−/− mice (P = 0.03). This increase in IFNγ expression was not observed in CD4+ or CD8+ splenocytes from WNV-infected C4−/−, fD−/−, or fB−/− mice. Thus, our data suggests that C4 and the alternative pathway of complement activation both are required for full T cell responsiveness after WNV infection.
Figure 7.
T cell activation and trafficking after WNV infection. (A) Mock or WNV-infected splenocytes from wild-type, C1q−/−, C4−/−, fD−/−, and fB−/− mice on day 8 were harvested and stimulated ex vivo. Cells were stained for CD4 or CD8 and intracellular IFNγ and analyzed by flow cytometry. Data are an average of at least three independent experiments and reflect 6–10 mice per group. Asterisks indicate differences from mock infected that are statistically significant (*P < 0.05; **P < 0.005). (B) Representative flow cytometry profiles showing intracellular IFNγ staining of splenic CD8+ T cells after WNV infection in wild-type or complement-deficient mice. The percentage of double-positive cells is indicated in the top right corner. (C) Brains were harvested from WNV-infected wild-type, C1q−/−, C4−/−, and fB−/− mice on day 9. Leukocytes were isolated by percoll gradient centrifugation and double-stained for CD3 and CD4 or CD8. The total number of brain infiltrating CD4+ or CD8+ T cells was determined by multiplying the total number of leukocytes from three pooled brains by the percentage of double-positive cells as measured by flow cytometry. Data are an average of at least three independent experiments and reflect at least five groups of three mice. Asterisks indicate differences from wild type that are statistically significant (*P < 0.05; **P < 0.005). (D) Representative flow cytometry profiles showing CD3+CD8+ T cells in the brain after WNV infection in wild-type or complement-deficient mice.
T cell trafficking to WNV-infected CNS
Trafficking of effector T cells into WNV-infected CNS tissues requires cellular activation and up-regulation of select chemokine receptors (28). As an independent measure of the T cell activity, we tested which pathway of complement activation was required for T cell migration into the CNS of WNV-infected animals. We isolated brain-infiltrating leukocytes on day 9 after infection in wild-type, C4−/−, C1q−/−, and fB−/− mice and quantified T cells by flow cytometry (Fig. 7 C). We isolated an average of 2.3 ± 0.5 × 104 CD3+CD4+ T cells and 4.2 ± 0.6 × 104 CD3+CD8+ T cells from the brains (n = 18) of wild-type mice. Consistent with our IFNγ expression data, the numbers of infiltrating CD4+ and CD8+ T cells were reduced significantly in the absence of either fB or C4 (n ≥ 12, P ≤ 0.04). No difference in CNS T cell migration was observed in the absence of C1q in either the CD4+ or CD8+ compartment (n = 15, P ≥ 0.3). Collectively, our data suggests that the lectin and alternative pathways of complement activation are required for normal T cell responsiveness and CNS trafficking after WNV infection.
DISCUSSION
In this study, we report that different pathways of complement activation can exert independent protective effects in response to viral infection (Table I). WNV infection activates the complement system in vivo, and all three complement activation pathways are required for protection against WNV. However, differences in priming of adaptive immune responses to WNV were observed in mice lacking individual components of all three pathways of complement activation. Deficiencies in the alternative pathway were associated with slightly delayed splenic clearance, blunted T cell responses, yet near normal anti-WNV antibody profiles. Deficiencies in classical and lectin pathway activation were associated with decreased splenic infection and significant deficits in both B and T cell responses against WNV. A deficiency in the classical pathway alone was associated with markedly decreased splenic infection, yet normal T cell responses and near normal antibody responses. Importantly, not all defects in the complement system predispose mice to enhanced WNV susceptibility, as mice lacking the C5aR exhibited no increase in mortality after infection. This latter result was not unexpected as C5aR activity primarily modulates granulocyte responses, a cell type that has not been implicated in the pathogenesis or immune response to WNV infection (29).
Table I.
Summary of phenotypes observed during WNV infection of complement-deficient mice
| Viremia | Spleen | CNS | IgM | IgG | T cell activity | Survival | |
|---|---|---|---|---|---|---|---|
| WT | + | + | + | + | + | + | 65% |
| C4−/− | D4 ++ | − | ++ | − | D8 − | − | 0% |
| C1q−/− | + | None | ++ | + | D8 − | + | 17% |
| fB−/− | + | D6 ++ | ++ | + | + | − | 4% |
| fD−/− | + | D6 ++ | ++ | + | + | ND | 26% |
| C1q × fD−/− | ND | ND | ND | D10 + | D10 + | ND | 10% |
Viremia, infectious burden in the spleen and CNS, virus-specific IgM and IgG responses, and T cell activity after WNV infection of wild-type, C4−/−, C1q−/−, fB−/−, fD−/−, and C1q × fD−/− mice are summarized above. Differences from WNV infection in wild-type mice (+) at specific days after infection are indicated. Comparative levels are indicated by – (low), + (normal), or ++ (high). ND indicates experiments were not performed.
All pathways of complement activation are required to control WNV infection
Although clinical studies have suggested that increased complement activation may be associated with more severe forms of infection with two related viruses of the Flaviviridae family, dengue virus (21) and hepatitis C virus (30), C3 was essential for controlling WNV infection (20). Although the studies in C3−/− mice were consistent with models of complement-dependent control of other viral infections including HSV, influenza, and LCMV (2–4, 31, 32), no investigation had systematically addressed how the different activation pathways coordinate protection. Our experiments demonstrate that all complement activation pathways are required for control of severe WNV disease. The virologic experiments suggest that complement limits viral spread and facilitates clearance of WNV within the CNS. As many complement proteins are constitutively expressed within the CNS (33) and up-regulated after infection with other neurotropic viruses (34–36), complement activation in the CNS could be critical for destruction of viral particles or virus-infected neuronal cells. Alternatively, the defects observed in priming adaptive immune responses to WNV in complement-deficient mice (see below) may result in earlier spread and/or decreased clearance.
Most complement-deficient mice had near wild-type levels of viral RNA in serum throughout the time course, suggesting that the dominant antiviral role of complement activation may not be direct virolysis. Because IgM fixes complement by the classical pathway and WNV particles can be neutralized efficiently in vitro by exogenous rabbit complement (20), we anticipated increased viremia in C1q−/− and C4−/− mice. However, we only observed a transient increase in viremia in C4−/− mice, which may be secondary to depressed levels of antiviral IgM in these mice. Viral clearance from serum is known to correlate with the induction of WNV-specific IgM at days 4 and 6 after infection (15). The relatively low viral neutralizing activity of serum complement in mice may be caused by the reduced function of the mouse classical pathway C5 convertase (37, 38). Our prior in vivo experiments support this, as antibody-mediated protection from WNV disease was not significantly attenuated in C1q−/− or C4−/− mice (39).
Although the magnitude and kinetics of viremia were roughly equivalent, earlier CNS viral invasion was observed in all complement-deficient mice. This early CNS seeding was most consistently observed in mice lacking fB or fD. Although the mechanism remains unclear, alternative pathway activation during the initial phases of infection could induce inflammatory mediators that facilitate viral blood-brain barrier crossing. TNF-α generation in peripheral lymphoid tissues has been suggested to modulate blood-brain barrier permeability and WNV neuroinvasion (40). Notably, genetic disruption or antibody blockade of alternative pathway activation or CR3 function significantly inhibited TNF-α secretion from human or murine leukocytes after exposure to group B Streptococcus (41). Experiments that examine the relationship between CNS viral entry and cytokine profiles after WNV infection in complement-sufficient and -deficient mice may elucidate the molecular basis for this stage in pathogenesis.
Classical pathway activation facilitates secondary lymphoid organ WNV infection
A surprising result was the requirement of C1q and C4 for normal levels of WNV infection in the spleen and draining lymph node. In the absence of C1q, infectious virus was not detected in the spleen, and 100,000-fold less WNV RNA was measured on day 6 after infection. Additionally, WNV infection of the draining inguinal lymph nodes was reduced in the absence of C1q beginning on day 3 after infection. In the absence of C4, fewer spleen samples contained less infectious virus than those of wild-type animals, and less WNV RNA was detected in the spleen and draining inguinal lymph node. At first glance, this observation appears consistent with earlier in vitro studies that showed complement and antiviral IgM enhanced WNV infection in macrophages (42, 43). Although these studies suggested that IgM-dependent fixation of C3 split products (e.g., C3b, C3dg, or iC3b) on WNV enhanced infection, our prior studies with secreted IgM−/− or C3−/− mice showed normal WNV splenic infection (15, 20). Based on this, we speculate that C1q directly or indirectly interacts with WNV to facilitate splenic infection. Direct C1q interaction with viral glycoproteins has been observed for HIV-1, human T cell lymphotropic virus-1, and Moloney murine leukemia virus (6), and C1q-dependent enhancement of infection has been demonstrated in vitro for both HIV-1 and Ebola viruses (44, 45). Our data clearly establishes that WNV infection in the spleen does not significantly contribute to viremia or CNS seeding. Somewhat paradoxically, decreased productive splenic infection did not dramatically alter the induction of primary antiviral B and T cell immune responses to WNV. This apparently contrasts with studies which show that follicular localization in the spleen is complement dependent and required for the development of antibody responses to soluble antigen (46) and vesicular stomatitis virus (2). For WNV, we hypothesize that T and B cell priming may occur in other secondary lymphoid tissues. Alternatively, despite an absence of productive viral infection in the spleen, sufficient complement-dependent antigen trapping occurs for antigen presentation and priming of antiviral adaptive immune responses.
Complement activation pathways and the humoral response
Combined with previous work, our current experiments indicate that the development of a protective anti-WNV antibody response, in part, requires C3, C4, and CR1/2 (20). Because near normal anti-WNV antibody responses were observed in fB−/− or fD−/− mice, activation of the alternative pathway is not required for priming the antibody response against WNV. Our results are consistent with studies which demonstrate that fB−/− mice exhibit normal B cell priming to T cell–independent and T cell–dependent antigens (47). We observed a transient delay in the development of antiviral IgG but not IgM in the absence of C1q, consistent with previous reports of defects in primary T cell–dependent IgG but not IgM responses in C1q−/− mice (46). Our data suggests the contribution of the classical pathway to anti-WNV antibody priming is relatively minor and could be secondary to the defect observed in splenic infection. The most severe defect in anti-WNV antibody development was observed in the absence of C4, where IgM responses were blunted and IgG responses were delayed. Since the C1q−/− mice exhibited normal IgM responses, the C4-dependent lectin pathway could play an important role in priming the antiviral IgM against WNV. Although no study has directly evaluated the contribution of the lectin pathway to antiviral antibody responses, this idea is consistent with work on HSV in which both the primary and memory antiviral humoral responses were blunted in C4−/− mice (3, 48). Defects in MBL function and lectin pathway activation have been suggested to predispose individuals to HIV-1 (49), HSV-2 (7), severe acute respiratory syndrome (50), and chronic hepatitis B infection (51). We speculate that the lectin pathway may have an essential role in the priming of humoral responses against some pathogens.
Complement activation pathways and T cell activation
An emerging literature has suggested an essential role for complement in priming antiviral T cell immunity. T cell responses to influenza and LCMV require C3 activation (4, 27), possibly by enhancing antigen uptake and presentation or T cell trafficking to infected organs. Efficient CD8+ CTL responses are critical for development of WNV immunity (16, 17), and complement-deficient mice with the greatest mortality (i.e., C4−/− and fB−/−) had associated T cell activation and trafficking deficits. Because previous studies with soluble antigens suggested that C1q-mediated immune complex trapping contributed to T cell priming (46, 52), we expected C1q−/− mice to have T cell deficits; however, this was not observed. We speculate that WNV antigen uptake and presentation may be reduced in the absence of lectin pathway activity and/or C4b deposition. Lectin pathway recognition induces opsonization of influenza virus, HSV-2, and HIV-1 (53), and C4b deposition and CR1 binding can activate and enhance antigen presentation in human cells (54). Nonetheless, the defects in antiviral T cell responses in C4−/− mice could be indirect and attributed to the depressed anti-WNV IgM responses and decreased immune complex clearance. Although more experiments are necessary to identify the exact mechanism, our study is novel as it describes a role for C4 in T cell function after viral infection.
We also observed a requirement for the alternative pathway in the development of T cell responses after WNV infection. Because others have demonstrated an 80% reduction in classical pathway-dependent membrane attack complex formation in the absence of alternative pathway activity (55, 56), WNV antigen presentation may be reduced in the absence of fB because of a failure to amplify classical and/or lectin pathway-initiated responses. This may attenuate C3b-directed targeting, uptake, and presentation of WNV in lymphoid tissues (2, 4, 27). Alternatively, C3b engagement of CR3 may promote an appropriate cytokine environment for antiviral T cell priming: production of IFNγ and IL-12 were reduced by treatment of human monocytes with CR3 blocking antibodies or natural CR3 ligands (57).
Complement activation plays a critical role in innate recognition and neutralization of pathogens in vivo (3, 4, 58). Our study expands the current knowledge of the in vivo mechanisms of complement-mediated antiviral immunity, and in particular suggests that activation of complement by distinct pathways may control pathogens by triggering different effector arms of antiviral immunity. A limitation of our study is that it does not address the precise mechanism by which the three complement activation pathways stimulate different adaptive immune responses; the differences could be because of quantitative or qualitative variation in the generation of C3 or C5 activation fragments. Regardless, a fully functional complement cascade is clearly important for controlling and clearing WNV given that antibody and CD8+ T cells both are essential for protection (14, 16, 17). It is intriguing to consider that severe WNV infection in humans, which occurs infrequently (∼1/150), could be more common in patients with dysfunctional complement responses. Although complete deficiencies of complement proteins are rare, heterozygous deficiencies of C4 and MBL are common (59) and have been associated with an increased risk of viral diseases (30, 49, 51, 60). As therapies against WNV become available, it will be important to target high-risk populations. Depressed levels of complement proteins or reduced complement activity could explain why some seemingly healthy patients develop severe disease after WNV infection. Genetic studies that evaluate the risk factors of neuroinvasive WNV infection in humans should include an analysis of common complement gene allelic polymorphisms.
MATERIALS AND METHODS
Viruses and cells.
The WNV strain 3000.0259 was isolated in New York in 2000 and used for all studies (61). All mouse experiments used a low passage stock (P = 2) that was propagated once in C6/36 Aedes albopictus cells.
Mice.
C4−/− (62), C1q−/− (63), fD−/− (24), C1q × fD−/−, fB−/− (47), and C5aR−/− (64) mice on a pure C57BL/6 (H-2b) background and were obtained from colleagues (C. Gerard, Harvard Medical School, Boston, MA; M. Botto, Imperial College, London, UK; M. Carroll, Harvard Medical School, Boston, MA; G. Stahl, Harvard Medical School, Boston, MA; and H. Molina, Washington University School of Medicine). The congenic wild-type C57BL/6 control mice were purchased commercially (Jackson Laboratories). All mice were housed in the pathogen-free BSL3 mouse facility at Washington University School of Medicine. Studies were performed in compliance with the guidelines of the Washington University School of Medicine Animal Safety Committee. 8–12-wk-old mice were inoculated subcutaneously via footpad injection with 102 PFUs diluted in Hanks balanced salt solution and 1% heat inactivated FBS.
Complement hemolysis assays.
Levels of functional mouse C4 were determined by an erythrocyte hemolysis assay as previously described (23). In brief, sheep erythrocytes (Colorado Serum Co.) were coated with rabbit anti–sheep erythrocyte IgG (Rockland Immunochemical) at 37°C for 30 min. After extensive washing, antibody-coated sheep erythrocytes (5 × 106) were mixed with C4-depleted guinea pig serum (Complement Technologies, Inc.) and serial dilutions of mouse serum from naive or WNV-infected C57BL/6 mice. Reactions were incubated at 37°C for 1 h and stopped by the addition of 1 vol of PBS at 0°C. Erythrocytes were pelleted, supernatants were removed, and lysis was measured by using a 96-well plate reader at an OD405. Levels of C3 were determined as above using C3-depleted normal human serum (Complement Technologies). The 50% value of hemolytic complement activity (CH50) was determined as described previously (65).
C3 Western blotting.
Complement activation was assessed by Western blot as previously described (24). Serum samples were collected from naive or WNV-infected C57BL/6 mice on day 2 after infection, diluted in PBS, boiled in SDS reducing sample buffer, and subjected to SDS PAGE using a 4–20% gradient gel. After transfer to a PVDF membrane, C3 and its cleavage products were detected with goat anti–mouse C3 IgG (MP Biomedicals) followed by horseradish peroxidase–conjugated donkey anti–goat IgG F(ab)′2 (Jackson ImmunoResearch). C3 bands were visualized using the ECL Plus chemiluminescent substrate system (BD Biosciences).
Quantification of viral burden.
For analysis of viral burden, organs were recovered at days 2, 4, 6, 8, and 10 postinfection after cardiac perfusion with 10 ml of PBS. Tissues were weighed, homogenized using a Bead-beater apparatus, and titrated for WNV by plaque assay on BHK21-15 cells as described previously (14). Serum was obtained from whole blood by phlebotomy of the axillary vein immediately before sacrifice. Viral RNA was prepared from serum by using a Qia-Amp viral RNA recovery kit (Qiagen) and quantified by real-time fluorogenic RT-PCR on an ABI 7000 sequence detection system (Applied Biosystems) using primers and probes corresponding to a segment of the E gene as described previously (25). Viral RNA was extracted from lymph node and spleen tissue homogenates using the RNeasy isolation kit (Qiagen) and quantified by fluorogenic RT-PCR as described above. Values were normalized to the level of 18S RNA as determined by fluorogenic RT-PCR with a commercially available kit (Applied Biosystems).
Serologic analysis.
WNV-specific IgM and IgG levels were determined by an envelope (E) protein–specific ELISA, as described previously (14). In brief, a soluble WNV E-protein with a COOH-terminal hexa-histidine (His6) tag was purified from baculovirus-infected Hi5 insect cells by nickel-affinity chromatography (39) and used to coat Maxi-Sorp microtiter plates (Nalgene Nunc) overnight at 4°C. After blocking with PBS 1% BSA, 3% horse serum, 0.05% Tween-20, and 0.025% NaN3, plates were incubated with fourfold serial dilutions of heat-inactivated serum samples overnight at 4°C. Plates were washed, incubated with biotin-conjugated goat anti–mouse IgM, IgG, IgG1, IgG2a, IgG2c, or IgG3 (Southern Biotech), followed by strepatavidin-HRP (Invitrogen), and developed with tetramethylbenzidine substrate (Dako). The OD450 was determined, and the adjusted OD was determined by subtracting the OD450 value for each sample on blocked control wells. Titers represent the serum dilution yielding an adjusted OD450 value equivalent to three standard deviations above the background of the assay.
The neutralizing activity of the WNV-specific antibody response was determined using a previously described high-throughput flow cytometry–based neutralization assay with WNV virus-like particles that express a reporter gene (green fluorescent protein [GFP]) (66). WNV virus-like particles were incubated with serial dilutions of heat-inactivated serum samples before incubation with permissive Raji cells expressing DC-SIGN-R (67). 2 d later, cells were fixed in 1% paraformaldehyde, analyzed by flow cytometry for GFP expression, and the titer of 50% inhibition (EC50) was determined using GraphPad Prism software.
Immunohistochemistry.
Brain and spinal cord were harvested from infected mice on day 10 postinfection after perfusion with 20 ml of PBS. Tissues were fixed in 4% paraformaldehyde in PBS for 24 h at 4°C, then embedded in paraffin, sectioned, and stained for WNV infection using mAbs (E18, E22, and E33) against the WNV E protein (18), after an antigen retrieval step (Dako Cytomation). Staining was detected after incubation with streptavidin-conjugated horseradish peroxidase and development with diaminobenzidine. Slides were counterstained with hematoxylin to facilitate visualization of tissue histology.
Intracellular IFN-γ staining.
Intracellular IFN-γ staining of wild-type and complement-deficient splenocytes was performed as described previously (19). In brief, at day 8 after infection, splenocytes were harvested from wild-type and C1q−/−, fD−/−, fB−/−, or C4−/− mice. Erythrocytes were lysed with ACK lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) and splenocytes were counted. In a 96-well plate, 106 splenocytes were stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 1 μM Ionomycin (Sigma-Aldrich) in the presence of Golgi plug (BD PharMingen) for 4 h at 37°C. Cells were harvested and stained with either FITC-conjugated anti-CD4 (L3T4) or anti-CD8α (Ly-2) antibody (BD PharMingen). Cells were then washed and fixed in 4% paraformaldehyde, permeabilized with 0.5% saponin, and stained with AlexaFluor 647–conjugated rat anti–mouse IFN-γ antibody or rat IgG1 isotype control (BD PharMingen), and analyzed by flow cytometry. The total numbers of IFNγ-expressing CD8+ cells was determined by multiplying the percentage of IFNγ+ CD8+ cells by the total numbers of splenocytes harvested.
Brain T cell isolation.
Brain infiltrating CD4+ and CD8+ T cells were isolated and quantified as previously described (68). Complement-deficient and wild-type mice were anaesthetized and perfused with 20 ml of PBS on day 9 after infection with 102 PFU of WNV. Individual brains were harvested into RPMI supplemented with 5% FBS and homogenized by pressing through a 70-μm mesh tissue strainer (BD Biosciences). The cell homogenates were centrifuged, and the cell pellets were resuspended in RPMI with 5% FBS and overlaid on a 70–30% isotonic percoll (Pharmacia) step gradient. The gradients were centrifuged (800 g for 25 min at 25°C), and the leukocytes were collected from between the 70 and 30% interface. Leukocytes from three brains were pooled, washed twice, and stained for CD4+ or CD8+ T cells with anti-CD3 (17A2) and anti-CD8α (Ly-2) or anti-CD4 (L3T4) antibodies (BD PharMingen) in the presence of 5% goat serum after Fc block (2.4G2; BD PharMingen). After washing, cells were fixed with 1% paraformaldehyde in PBS and analyzed by FACscan (Becton Dickinson) with CellQuest software. The total numbers of CD4+ or CD8+ T cells from each brain were determined by multiplying the percentage of CD3+CD4+ or CD3+CD8+ cells by the total numbers of leukocytes harvested.
Data analysis.
For survival analysis, Kaplan-Meier survival curves were plotted using Prism software (GraphPad) and analyzed by the log rank test. Statistical significance of complement levels, survival time, viral burdens, antiviral antibody titers, and total number of activated T cells were analyzed by an unpaired t test or Mann-Whitney test.
Acknowledgments
The authors thank J. Atkinson, X. Wu, M. Engle, K.A. Brett, R. Klein, and the Ophthalmology Core Facilities at Washington University for technical assistance, and J. Atkinson, H. Virgin, and M. Carroll for critical reading of the manuscript and experimental advice. We also gratefully acknowledge the generous gifts of complement-deficient mice by G. Stahl, M. Carroll, H. Molina, and M. Botto.
The work was supported by grants from the National Institutes of Health grant U54 AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research.
The authors have no conflicting financial interests.
Abbreviations used: C, complement; CNS, central nervous system; CR, complement receptor; E, envelope; fB, factor B; fD, factor D; LCMV, lymphocytic choriomeningits virus; MBL, mannose binding lectin; WNV, West Nile virus.
References
- 1.Carroll, M.C. 2004. The complement system in regulation of adaptive immunity. Nat. Immunol. 5:981–986. [DOI] [PubMed] [Google Scholar]
- 2.Ochsenbein, A.F., D.D. Pinschewer, B. Odermatt, M.C. Carroll, H. Hengartner, and R.M. Zinkernagel. 1999. Protective T cell–independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Da Costa, X.J., M.A. Brockman, E. Alicot, M. Ma, M.B. Fischer, X. Zhou, D.M. Knipe, and M.C. Carroll. 1999. Humoral response to herpes simplex virus is complement-dependent. Proc. Natl. Acad. Sci. USA. 96:12708–12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopf, M., B. Abel, A. Gallimore, M. Carroll, and M.F. Bachmann. 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat. Med. 8:373–378. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch, R.L., D.E. Griffin, and J.A. Winkelstein. 1980. Role of complement in viral infections: participation of terminal complement components (C5 to C9) in recovery of mice from Sindbis virus infection. Infect. Immun. 30:899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishore, U., C. Gaboriaud, P. Waters, A.K. Shrive, T.J. Greenhough, K.B. Reid, R.B. Sim, and G.J. Arlaud. 2004. C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol. 25:551–561. [DOI] [PubMed] [Google Scholar]
- 7.Gadjeva, M., S.R. Paludan, S. Thiel, V. Slavov, M. Ruseva, K. Eriksson, G.B. Lowhagen, L. Shi, K. Takahashi, A. Ezekowitz, and J.C. Jensenius. 2004. Mannan-binding lectin modulates the response to HSV-2 infection. Clin. Exp. Immunol. 138:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thielens, N.M., P. Tacnet-Delorme, and G.J. Arlaud. 2002. Interaction of C1q and mannan-binding lectin with viruses. Immunobiology. 205:563–574. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch, R.L., J.A. Winkelstein, and D.E. Griffin. 1980. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J. Immunol. 124:2507–2510. [PubMed] [Google Scholar]
- 10.Okada, N., H. Shibuta, and H. Okada. 1979. Activation of the alternative pathway of guinea pig complement by Sendai virus-treated cells. Microbiol. Immunol. 23:689–692. [DOI] [PubMed] [Google Scholar]
- 11.Devaux, P., D. Christiansen, S. Plumet, and D. Gerlier. 2004. Cell surface activation of the alternative complement pathway by the fusion protein of measles virus. J. Gen. Virol. 85:1665–1673. [DOI] [PubMed] [Google Scholar]
- 12.Sissons, J.G., M.B. Oldstone, and R.D. Schreiber. 1980. Antibody-independent activation of the alternative complement pathway by measles virus-infected cells. Proc. Natl. Acad. Sci. USA. 77:559–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mold, C., B.M. Bradt, G.R. Nemerow, and N.R. Cooper. 1988. Activation of the alternative complement pathway by EBV and the viral envelope glycoprotein, gp350. J. Immunol. 140:3867–3874. [PubMed] [Google Scholar]
- 14.Diamond, M.S., B. Shrestha, A. Marri, D. Mahan, and M. Engle. 2003. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 77:2578–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond, M.S., E.M. Sitati, L.D. Friend, S. Higgs, B. Shrestha, and M. Engle. 2003. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 198:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha, B., and M.S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, Y., M. Lobigs, E. Lee, and A. Mullbacher. 2003. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 77:13323–13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel, M.A., and M.S. Diamond. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, T., E. Scully, Z. Yin, J.H. Kim, S. Wang, J. Yan, M. Mamula, J.F. Anderson, J. Craft, and E. Fikrig. 2003. IFN-gamma-producing gammadelta T cells help control murine West Nile virus infection. J. Immunol. 171:2524–2531. [DOI] [PubMed] [Google Scholar]
- 20.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M.S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 79:7466–7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokisch, V.A., F.H. Top Jr., P.K. Russell, F.J. Dixon, and H.J. Muller-Eberhard. 1973. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N. Engl. J. Med. 289:996–1000. [DOI] [PubMed] [Google Scholar]
- 22.Avirutnan, P., N. Punydee, S. Noisakran, C. Komoltri, S. Thiemmeca, K. Auethavornanan, A. Jairungsri, R. Kanlaya, N. Tangthawornchaikul, C. Puttikhunt, et al. 2006. Vascular leekage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 193:1078–1088. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson, J.P., K. McGinnis, and D. Shreffler. 1980. Development and characterization of a hemolytic assay for mouse C4. J. Immunol. Methods. 33:351–368. [DOI] [PubMed] [Google Scholar]
- 24.Xu, Y., M. Ma, G.C. Ippolito, H.W. Schroeder Jr., M.C. Carroll, and J.E. Volanakis. 2001. Complement activation in factor D-deficient mice. Proc. Natl. Acad. Sci. USA. 98:14577–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanciotti, R.S., A.J. Kerst, R.S. Nasci, M.S. Godsey, C.J. Mitchell, H.M. Savage, N. Komar, N.A. Panella, B.C. Allen, K.E. Volpe, et al. 2000. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 38:4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston, L.J., G.M. Halliday, and N.J. King. 2000. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J. Invest. Dermatol. 114:560–568. [DOI] [PubMed] [Google Scholar]
- 27.Suresh, M., H. Molina, M.S. Salvato, D. Mastellos, J.D. Lambris, and M. Sandor. 2003. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 170:788–794. [DOI] [PubMed] [Google Scholar]
- 28.Glass, W.G., J.K. Lim, R. Cholera, A.G. Pletnev, J.L. Gao, and P.M. Murphy. 2005. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 202:1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond, M.S., B. Shrestha, E. Mehlhop, E. Sitati, and M. Engle. 2003. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 16:259–278. [DOI] [PubMed] [Google Scholar]
- 30.Pasta, L., G. Pietrosi, C. Marrone, G. D'Amico, M. D'Amico, A. Licata, G. Misiano, S. Madonia, F. Mercadante, and L. Pagliaro. 2004. C4BQ0: a genetic marker of familial HCV-related liver cirrhosis. Dig. Liver Dis. 36:471–477. [DOI] [PubMed] [Google Scholar]
- 31.Verschoor, A., M.A. Brockman, D.M. Knipe, and M.C. Carroll. 2001. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J. Immunol. 167:2446–2451. [DOI] [PubMed] [Google Scholar]
- 32.Verschoor, A., M.A. Brockman, M. Gadjeva, D.M. Knipe, and M.C. Carroll. 2003. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J. Immunol. 171:5363–5371. [DOI] [PubMed] [Google Scholar]
- 33.Yu, J.X., B.M. Bradt, and N.R. Cooper. 2002. Constitutive expression of proinflammatory complement components by subsets of neurons in the central nervous system. J. Neuroimmunol. 123:91–101. [DOI] [PubMed] [Google Scholar]
- 34.Bruder, C., M. Hagleitner, G. Darlington, I. Mohsenipour, R. Wurzner, I. Hollmuller, H. Stoiber, C. Lass-Florl, M.P. Dierich, and C. Speth. 2004. HIV-1 induces complement factor C3 synthesis in astrocytes and neurons by modulation of promoter activity. Mol. Immunol. 40:949–961. [DOI] [PubMed] [Google Scholar]
- 35.Speth, C., G. Stockl, I. Mohsenipour, R. Wurzner, H. Stoiber, C. Lass-Florl, and M.P. Dierich. 2001. Human immunodeficiency virus type 1 induces expression of complement factors in human astrocytes. J. Virol. 75:2604–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speth, C., K. Williams, M. Hagleitner, S. Westmoreland, G. Rambach, I. Mohsenipour, J. Schmitz, R. Wurzner, C. Lass-Florl, H. Stoiber, et al. 2004. Complement synthesis and activation in the brain of SIV-infected monkeys. J. Neuroimmunol. 151:45–54. [DOI] [PubMed] [Google Scholar]
- 37.Ebanks, R.O., and D.E. Isenman. 1996. Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol. Immunol. 33:297–309. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson, J.P., K. McGinnis, L. Brown, J. Peterein, and D. Shreffler. 1980. A murine C4 molecule with reduced hemolytic efficiency. J. Exp. Med. 151:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliphant, T., M. Engle, G.E. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K.M. Chung, et al. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, T., T. Town, L. Alexopoulou, J.F. Anderson, E. Fikrig, and R.A. Flavell. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10:1366–1373. [DOI] [PubMed] [Google Scholar]
- 41.Levy, O., R.M. Jean-Jacques, C. Cywes, R.B. Sisson, K.A. Zarember, P.J. Godowski, J.L. Christianson, H.K. Guttormsen, M.C. Carroll, A. Nicholson-Weller, et al. 2003. Critical role of the complement system in group B streptococcus-induced tumor necrosis factor alpha release. Infect. Immun. 71:6344–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardosa, M.J., S. Gordon, S. Hirsch, T.A. Springer, and J.S. Porterfield. 1986. Interaction of West Nile virus with primary murine macrophages: role of cell activation and receptors for antibody and complement. J. Virol. 57:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cardosa, M.J., J.S. Porterfield, and S. Gordon. 1983. Complement receptor mediates enhanced flavivirus replication in macrophages. J. Exp. Med. 158:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prohaszka, Z., J. Nemes, T. Hidvegi, F.D. Toth, K. Kerekes, A. Erdei, J. Szabo, E. Ujhelyi, N. Thielens, M.P. Dierich, et al. 1997. Two parallel routes of the complement-mediated antibody-dependent enhancement of HIV-1 infection. AIDS. 11:949–958. [DOI] [PubMed] [Google Scholar]
- 45.Takada, A., H. Feldmann, T.G. Ksiazek, and Y. Kawaoka. 2003. Antibody-dependent enhancement of Ebola virus infection. J. Virol. 77:7539–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cutler, A.J., M. Botto, D. van Essen, R. Rivi, K.A. Davies, D. Gray, and M.J. Walport. 1998. T cell–dependent immune response in C1q-deficient mice: defective interferon gamma production by antigen-specific T cells. J. Exp. Med. 187:1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto, M., W. Fukuda, A. Circolo, J. Goellner, J. Strauss-Schoenberger, X. Wang, S. Fujita, T. Hidvegi, D.D. Chaplin, and H.R. Colten. 1997. Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc. Natl. Acad. Sci. USA. 94:8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gadjeva, M., A. Verschoor, M.A. Brockman, H. Jezak, L.M. Shen, D.M. Knipe, and M.C. Carroll. 2002. Macrophage-derived complement component C4 can restore humoral immunity in C4-deficient mice. J. Immunol. 169:5489–5495. [DOI] [PubMed] [Google Scholar]
- 49.Mombo, L.E., C.Y. Lu, S. Ossari, I. Bedjabaga, L. Sica, R. Krishnamoorthy, and C. Lapoumeroulie. 2003. Mannose-binding lectin alleles in sub-Saharan Africans and relation with susceptibility to infections. Genes Immun. 4:362–367. [DOI] [PubMed] [Google Scholar]
- 50.Ip, W.K., K.H. Chan, H.K. Law, G.H. Tso, E.K. Kong, W.H. Wong, Y.F. To, R.W. Yung, E.Y. Chow, K.L. Au, et al. 2005. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 191:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thio, C.L., T. Mosbruger, J. Astemborski, S. Greer, G.D. Kirk, S.J. O'Brien, and D.L. Thomas. 2005. Mannose binding lectin genotypes influence recovery from hepatitis B virus infection. J. Virol. 79:9192–9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stager, S., J. Alexander, A.C. Kirby, M. Botto, N.V. Rooijen, D.F. Smith, F. Brombacher, and P.M. Kaye. 2003. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat. Med. 9:1287–1292. [DOI] [PubMed] [Google Scholar]
- 53.Holmskov, U., S. Thiel, and J.C. Jensenius. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547–578. [DOI] [PubMed] [Google Scholar]
- 54.Thieblemont, N., N. Haeffner-Cavaillon, A. Haeffner, B. Cholley, L. Weiss, and M.D. Kazatchkine. 1995. Triggering of complement receptors CR1 (CD35) and CR3 (CD11b/CD18) induces nuclear translocation of NF-kappa B (p50/p65) in human monocytes and enhances viral replication in HIV-infected monocytic cells. J. Immunol. 155:4861–4867. [PubMed] [Google Scholar]
- 55.Harboe, M., G. Ulvund, L. Vien, M. Fung, and T.E. Mollnes. 2004. The quantitative role of alternative pathway amplification in classical pathway induced terminal complement activation. Clin. Exp. Immunol. 138:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta-Bansal, R., J.B. Parent, and K.R. Brunden. 2000. Inhibition of complement alternative pathway function with anti-properdin monoclonal antibodies. Mol. Immunol. 37:191–201. [DOI] [PubMed] [Google Scholar]
- 57.Marth, T., and B.L. Kelsall. 1997. Regulation of interleukin-12 by complement receptor 3 signaling. J. Exp. Med. 185:1987–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ren, B., M.A. McCrory, C. Pass, D.C. Bullard, C.M. Ballantyne, Y. Xu, D.E. Briles, and A.J. Szalai. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506–7512. [DOI] [PubMed] [Google Scholar]
- 59.Yang, Y., K. Lhotta, E.K. Chung, P. Eder, F. Neumair, and C.Y. Yu. 2004. Complete complement components C4A and C4B deficiencies in human kidney diseases and systemic lupus erythematosus. J. Immunol. 173:2803–2814. [DOI] [PubMed] [Google Scholar]
- 60.Hentges, F., A. Hoffmann, F. Oliveira de Araujo, and R. Hemmer. 1992. Prolonged clinically asymptomatic evolution after HIV-1 infection is marked by the absence of complement C4 null alleles at the MHC. Clin. Exp. Immunol. 88:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ebel, G.D., A.P. Dupuis, II, K. Ngo, D. Nicholas, E. Kauffman, S.A. Jones, D. Young, J. Maffei, P.Y. Shi, K. Bernard, and L.D. KrAm. 2001. Partial genetic characterization of West Nile virus strains, New York State, 2000. Emerg. Infect. Dis. 7:650–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer, M.B., M. Ma, S. Goerg, X. Zhou, J. Xia, O. Finco, S. Han, G. Kelsoe, R.G. Howard, T.L. Rothstein, et al. 1996. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J. Immunol. 157:549–556. [PubMed] [Google Scholar]
- 63.Botto, M., C. Dell'Agnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. [DOI] [PubMed] [Google Scholar]
- 64.Gerard, C., L. Bao, O. Orozco, M. Pearson, D. Kunz, and N.P. Gerard. 1992. Structural diversity in the extracellular faces of peptidergic G-protein-coupled receptors. Molecular cloning of the mouse C5a anaphylatoxin receptor. J. Immunol. 149:2600–2606. [PubMed] [Google Scholar]
- 65.Morgan, B.P. 2000. Measurement of complement hemolytic activity, generation of complement-depleted sera, and production of hemolytic intermediates. Methods Mol. Biol. 150:61–71. [DOI] [PubMed] [Google Scholar]
- 66.Pierson, T.C., M.D. Sanchez, B.A. Puffer, A.A. Ahmed, B.J. Geiss, L.E. Valentine, L.A. Altamura, M.S. Diamond, and R.W. Doms. 2005. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology. 346:53–65. [DOI] [PubMed] [Google Scholar]
- 67.Davis, C.W., H.Y. Nguyen, S.L. Hanna, M.D. Sanchez, R.W. Doms, and T.C. Pierson. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80:1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein, R.S., E. Lin, B. Zhang, A.D. Luster, J. Tollett, M.A. Samuel, M. Engle, and M.S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457–11466. [DOI] [PMC free article] [PubMed] [Google Scholar]