Abstract
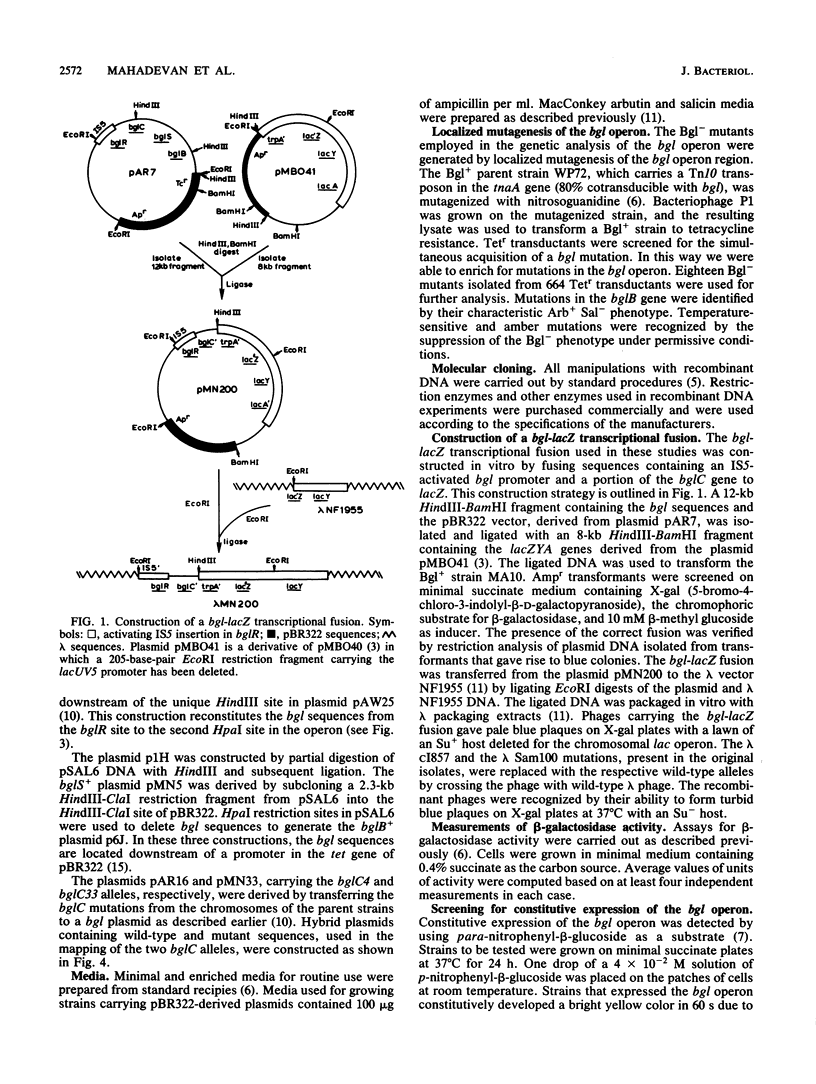
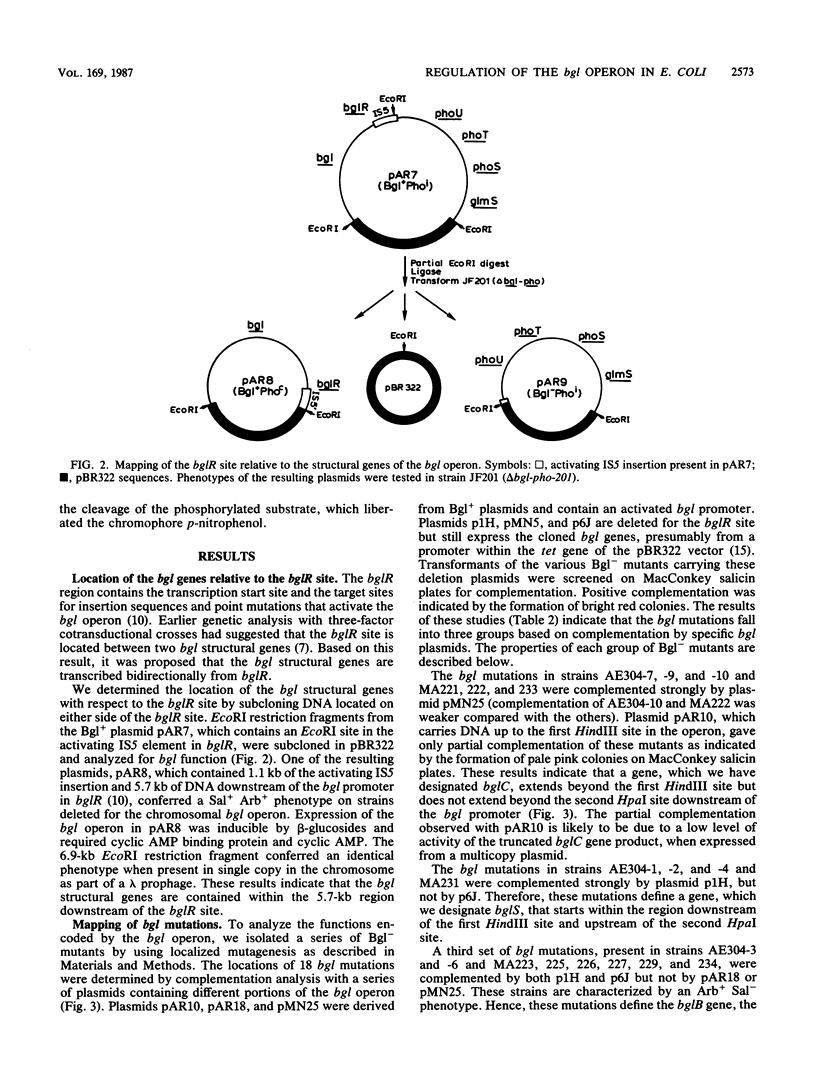
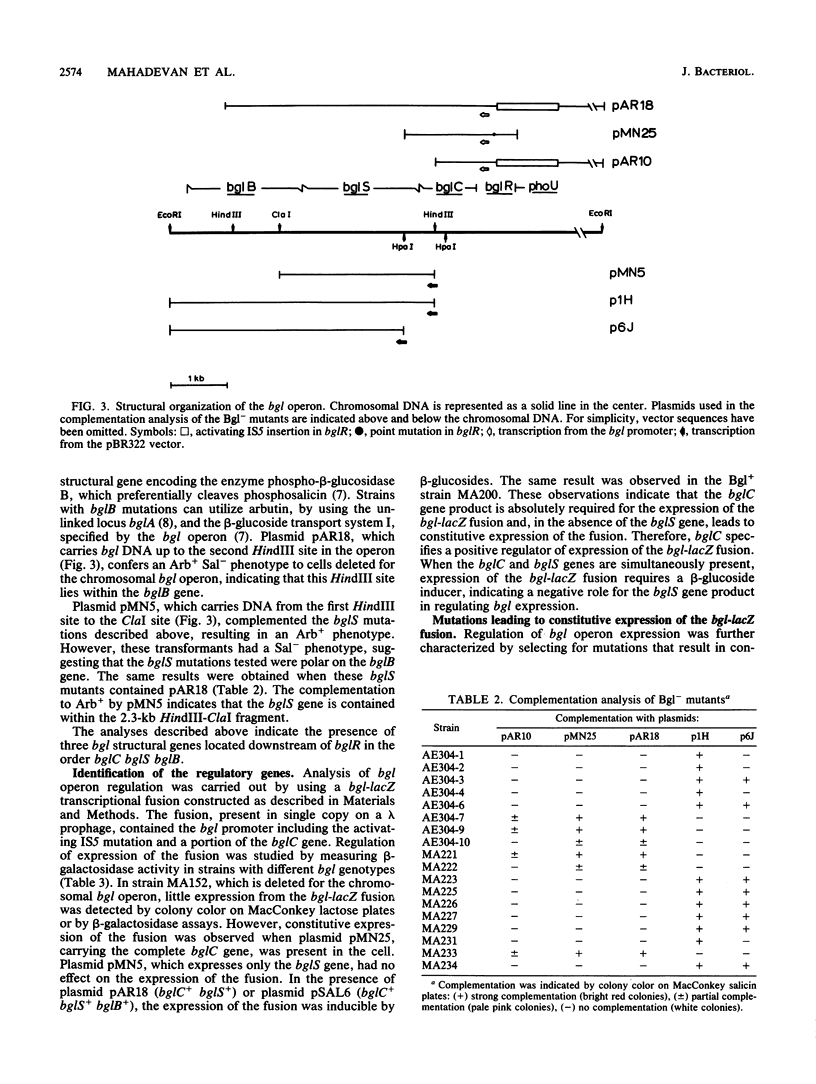
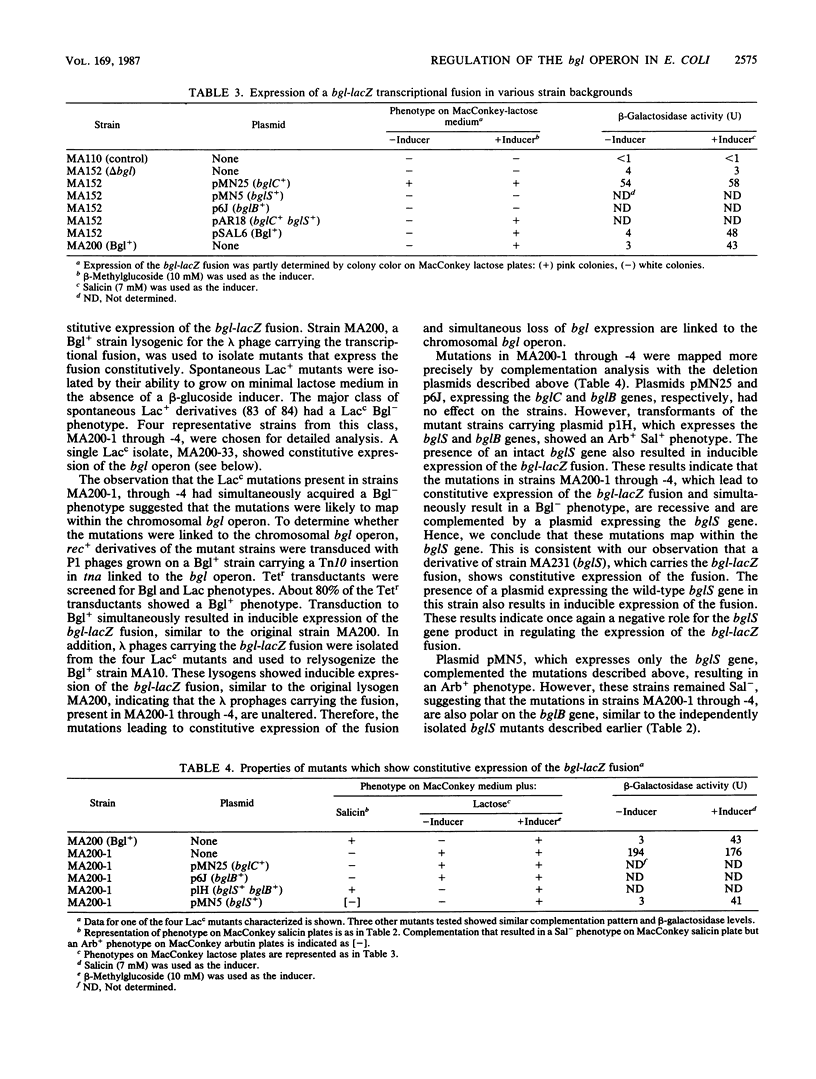
We have analyzed the functions encoded by the bgl operon in Escherichia coli K-12. Based on the ability of cloned regions of the operon to complement a series of Bgl- point mutations, we show that the three bgl structural genes, bglC, bglS, and bglB, are located downstream of the regulatory locus bglR in the order indicated. Using a bgl-lacZ transcriptional fusion, we show that bglC and bglS are involved in regulating operon expression. The presence of the bglC gene in trans is absolutely required for the expression of the fusion, which is constitutive when only the bglC gene is present. When the bglC and the bglS genes are both present in the cell, expression of the fusion requires a beta-glucoside inducer. From these observations, we conclude that (i) the bglC gene encodes a positive regulatory of bgl operon expression and (ii) the bglS gene encodes a negative regulator of operon expression, causing the requirement for a beta-glucoside inducer. These conclusions are supported by our observations that (i) a majority of bglC mutants exhibits a Bgl- phenotype, whereas rare trans-dominant mutations in bglC result in constitutive expression of the bgl operon and the fusion, and (ii) mutations in the bglS gene lead to constitutive expression of the fusion. Based on several lines of evidence presented, we propose that the bglS gene product has an additional role as a component of the beta-glucoside transport system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Wilson G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Mar;59(3):988–995. doi: 10.1073/pnas.59.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H., Rahaim P. T., Hoffman C. S., Baghdoyan D., O'Connor M. B., Miller J. F. A frameshift mutation at the junction of an IS1 insertion within lacZ restores beta-galactosidase activity via formation of an active lacZ-IS1 fusion protein. J Mol Biol. 1985 Feb 20;181(4):551–555. doi: 10.1016/0022-2836(85)90427-9. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Roth J. R. Regulation of proline utilization in Salmonella typhimurium: characterization of put::Mu d(Ap, lac) operon fusions. J Bacteriol. 1983 May;154(2):561–568. doi: 10.1128/jb.154.2.561-568.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Schaefler S. Regulation of the beta-glucoside system in Escherchia coli K-12. J Bacteriol. 1974 Nov;120(2):638–650. doi: 10.1128/jb.120.2.638-650.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad I., Young B., Schaefler S. Genetic determination of the constitutive biosynthesis of phospho- -glucosidase A in Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):909–915. doi: 10.1128/jb.114.3.909-915.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. E., Felton J., Wright A. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature. 1981 Oct 22;293(5834):625–629. doi: 10.1038/293625a0. [DOI] [PubMed] [Google Scholar]
- Reynolds A. E., Mahadevan S., LeGrice S. F., Wright A. Enhancement of bacterial gene expression by insertion elements or by mutation in a CAP-cAMP binding site. J Mol Biol. 1986 Sep 5;191(1):85–95. doi: 10.1016/0022-2836(86)90424-9. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetz K., Toloczyki C., Rak B. Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol. 1987 Jun;169(6):2579–2590. doi: 10.1128/jb.169.6.2579-2590.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Fisher S. H., Sonenshein A. L. Regulation of expression from the glnA promoter of Bacillus subtilis requires the glnA gene product. Proc Natl Acad Sci U S A. 1985 May;82(10):3375–3379. doi: 10.1073/pnas.82.10.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stüber D., Bujard H. Organization of transcriptional signals in plasmids pBR322 and pACYC184. Proc Natl Acad Sci U S A. 1981 Jan;78(1):167–171. doi: 10.1073/pnas.78.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin B. P., Rosenberg H., Cox G. B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985 Jan;161(1):189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


