Abstract
Background
Mitochondrial toxicity was described in infants exposed to long-term antiretroviral regimens (ARVs) containing nucleoside analogues for the prevention of mother-to-child transmission of HIV (PMTCT). We measured the serum lactate levels in children born to HIV-1 infected (HIV+) African women receiving short-term ARV PMTCT regimens.
Methods
A prospective study was conducted in women-child pairs from the third trimester of pregnancy to three months of life. The exposed group was formed by children exposed in utero to nucleoside analogue ARVs, zidovudine (ZDV) or ZDV + lamivudine (3TC) from 32–36 weeks of amenorrhea until delivery. All these women received nevirapine single-dose (NVPsd) at the beginning of labor. The children received ZDV during the first 7 days of life and a NVPsd at day 3. The control group was formed by infants born to HIV+ women who had received NVPsd only and not exposed to nucleoside analogue ARVs. Serum lactate levels were measured at 4, 6 and 12 weeks of life by Cobas Integra 400™.
Results
A total of 836 blood samples from 338 infants were collected (262 exposed and 76 controls). Median lactacidemia was 1.8 mmol/l, Interquartile Range [1.2–2.7 mmol/l]). Overall serum lactate levels ≥2.5 mmol/l, defining hyperlactatemia were observed in 39 of the 292 infants who had at least two serum lactate measurements, 13.4%, 95% confidence Interval [9.6–17.8%]. The three-month period prevalence of hyperlactatemia did not differ between the exposed group (13.1%) and the control group (14.3%) (p=0.84). All serum lactate levels returned to normal values in all subsequent samples No case of symptomatic hyperlactatemia was detected during the study period.
Conclusion
Increased lactate levels were identified equally in infants whose mother received a short-term of nucleoside analogues or NVPsd for PMTCT. Although not rare, hyperlactatemia was not related to short-term exposure to nucleoside analogue ARVs
Keywords: hyperlactatemia, HIV infection, children, HIV infection, mitochondrial injury, vertical transmission, Africa
Keywords: Adult; Anti-Retroviral Agents; adverse effects; pharmacokinetics; therapeutic use; Disease Transmission, Vertical; prevention & control; Female; HIV Infections; prevention & control; HIV-1; Humans; Infant; Infant, Newborn; Lactic Acid; blood; Maternal-Fetal Exchange; Mitochondria; drug effects; pathology; Pregnancy; Pregnancy Trimester, Third; Prospective Studies
Introduction
A possible mitochondrial toxicity has been hypothesized in infants exposed to long-term regimens of antiretrovirals (ARVs) used for the prevention of mother-to-child transmission of HIV (PMTCT), following a case report of symptomatic severe lactic acidosis (1). Subsequently, children with neurological symptoms and biochemical and histological signs of mitochondrial dysfunction were described within the French Perinatal Study (2, 3). In a systematic screening of neurological symptoms presented by uninfected children of this large cohort, the 18-month incidence of this phenomenon was estimated at 0.3% in children exposed perinatally to nucleoside analogues (3). Subsequently several cohorts reported that a significant number of exposed but asymptomatic children had increased lactate levels within the 6-week postnatal phase of the ARVs prophylaxis exposure (4–6) and sometimes persisting several weeks or months later. This biological abnormality was a probable consequence of an acute asymptomatic mitochondrial toxicity (3). In one of these studies, hyperlactatemia was defined by a lactate value ≥5/mmol/l and was observed in 26% of the infants (4). The serum lactate measurement was considered there as a surrogate marker of mitochondrial dysfunction and could thus be used to investigate mitochondrial toxicity in adults and infants (4, 7). However, the high potential of artefactual values of lactate level is well known for this laboratory measurement (8). Recently Noguera et al (6) confirmed these observational data with a control group and adequate biological internal controls, thus strengthening the hypothesis that zidovudine (ZDV) exposed children had a significant risk of transient asymptomatic hyperlactatemia. Long term consequences of this mitochondrial injury are not known.
In Africa, where short-term regimens of ARVs are frequently used for PMTCT since 2000 (9, 10), there has not yet been any study to explore this possible mitochondrial toxicity. We hypothesized that the screening of elevated lactate levels could help to early identify the infants presenting possible mitochondrial dysfunction. The objective of our study was to estimate the frequency of high lactate levels in infants born to HIV-1 infected women and exposed during pregnancy to short-term of ZDV or short-term of ZDV + lamivudine (3TC) used for PMTCT in comparison to infants exposed to nevirapine single-dose (NVPsd) only. We investigated also the risk factors associated to high lactate levels, including the maternal ARV regimen.
Methods
Design
The ANRS 1209 study was a prospective observational cohort set up in neonates born to HIV-1 infected women within the ANRS 1201/1202 Ditrame Plus project which evaluated the safety and field effectiveness in reducing mother-to-child transmission of HIV with short-term combinations of ZDV+NVPsd and of ZDV+3TC+NVPsd (11) followed by alternatives to prolonged breastfeeding in Abidjan, Côte d’Ivoire (12).
Ethical permissions
The ANRS 1201/1202 Ditrame Plus project was granted ethical permission in Côte d’Ivoire from the ethical committee of the National AIDS Control Programme, and in France from the institutional review board of the French Agence Nationale de Recherches sur le Sida (ANRS). As part of the Ditrame Plus project, the study presented here was included in the institutional review board approval.
Patients
Between May 2002 and February 2005, we enrolled consecutively in this sub-study three groups of infants born to HIV-1 infected pregnant women (Table 1). Cohort 1 was exposed to maternal short-term ZDV initiated at 36 weeks of amenorrhea. Cohort 2 was exposed to maternal short-term ZDV+3TC initiated at 32 weeks of amenorrhea. In these two cohorts, mothers received also NVPsd at the beginning of the labour and the newborns ZDV syrup (2mg/kg/6 hours) during their first week of life and NVPsd (2mg/kg) at Day 2. The third cohort was used as a control group formed between February 2004 and February 2005 of infants exposed to a NVPsd PMTCT regimen of known efficacy (13) and recommended by the international (14) and national guidelines implemented after the end of the Ditrame Plus cohort.
Table 1.
Antiretroviral interventions among HIV-infected mothers and infants for the prevention of mother-to-child transmission of HIV in the ANRS Ditrame Plus cohort in Abidjan, Côte d’Ivoire (2002–2005).
| Regimens
|
Mother
|
Infants
|
||
|---|---|---|---|---|
| Prepartum (gestational age at the beginning) | Intrapartum | Postpartum | Postnatal | |
| Ditrame Plus 1.0 (Cohort 1, exposed) | ZDV 300 mg (36 weeks) | ZDV 600 mg + NVP 200 mg | - | ZDV syrup 2mg/kg × 4/day for one week and NVP syrup 2mg/kg on day 2–3 |
| Ditrame Plus 1.1 (Cohort 2, exposed) | ZDV 300 mg + 3TC 150 mg (32 weeks) | ZDV 600 mg + NVP 200 mg | ZDV 300 mg + 3TC 150 mg (3 days) | ZDV syrup 2mg/kg × 4/day for one week and NVP syrup 2mg/kg on day 2–3 |
| National Program (Cohort 3, control) | - | NVP 200 mg | - | NVP syrup 2mg/kg on day 2–3 |
ZDV= zidovudine, 3TC = lamivudine, NVP= nevirapine
Biological Analyses
The lactate levels were determined according to ACTG mitochondrial dysfunction Focus Group Guidelines (15). These guidelines specify in particular how the venous lactate specimens must be collected for this purpose.
The serum lactate levels were systematically measured in children at 4, 6 and 12 weeks of life by Roche Cobas Integra 400™ in Treichville Hospital University, CeDReS laboratory in Abidjan. All measures were performed on the supernatant after deproteineization with internal quality control as suggested by the manufacturer (Roche Diagnostics, Mannheim, Germany). CeDReS also participated in an external quality control program of lactate measurements organized by a Necker University Hospital Laboratory (Paris, France). The quality control was performed in ASQUALAB “Assurance de qualité des laboratories d’analyses médicales” in Corentin Celton Hospital (Moulineaux, France).
Capillary blood was collected in EDTA microtainer tubes (Becton Dickinson) in newborns at weeks 4 and 6 for the diagnosis of pediatric HIV infection. All samples collected at week 4 were systematically processed for a plasma HIV-1 RNA viral load measurement using the bDNA assay or a real time polymerase chain reaction (PCR) with the quantitative Taqman technology (16). The same technique was applied to the 6-week sample if the first one tested was positive. Maternal CD4 count was measured using flow cytometry (FASCAN).
Outcomes
We defined hyperlactatemia as at least two consecutives measures of lactate levels higher than 2.5 mmol/L at either 4 weeks (S4) and 6 weeks (S6) or S6 and month 3 (M3). Repeated measurements were also realized at 4–6 months in case of diagnosis of hyperlactatemia at an earlier age, for studying further the kinetics of the lactate levels in this subgroup.
The available infant data included in the analysis of the determinants of hyperlactatemia were gender, HIV infection status, anthropometric data at birth (weight, length) and compliance to ZDV syrup intake for the post-exposure prophylaxis. Maternal data included the ARV regimen (type and term) during pregnancy, intrapartum dose intake, age, clinical stage and CD4 count.
Statistical analysis
The prevalence of hyperlactatemia was estimated with its 95 percent confidence interval (CI). The group comparisons used Student’s t-test or the non parametric Mann-Whitney U test or one-way analysis of the variance (ANOVA) for quantitative variables and the Chi-2 test or Fisher’s exact test for qualitative variables.
All factors potentially associated with hyperlactatemia were studied in univariate then multivariate logistic regression. All tests were two-sided and a p-value <0.05 was considered significant. All the analyses were performed with STATA™ 8.0 (Stata Corporation, College Station, TX, USA).
Results
Description of the study sample
Between May 2002 and February 2005, 836 blood samples were collected from 338 infants (140 in cohort 1, 122 in cohort 2 and 76 in the control group). Altogether, 23 infants were diagnosed as HIV-infected at 4 weeks: 6 (4.4%) in the ZDV group, 6 (4.9%) in the ZDV+3TC group and 11 (14.9%) in the sdNVP group. Among these infants, 159 (47%) initiated breastfeeding and 179 (53%) received formula feeding. Overall, three lactate measurements were performed in median per infant (range: 1–5) and the mean lactate level was 2.2 mmol/L, (standard deviation [SD] 1.4 mmol/L). No statistical difference of mean lactate level was observed between the three groups (p=0.242) and between the exposed and the controls groups (p=0.530) when adjusting on the timing of the blood samples collected (Table 2).
Table 2.
Lactate values (mmol/L) according to the exposure to antiretroviral prophylaxis and the timing of the blood collection. ANRS 1209 study, Abidjan, Côte d’Ivoire (2002–2005).
| Overall | Control group
|
Exposedgroup
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cohort 3 sdNVP | All | Cohort 1 ZDV+ sdNVP | Cohort 2 ZDV+3TC+ sdNVP | p* | p** | p*** | ||
| Lactate values (total) | ||||||||
| Number of samples | 836 | 194 | 642 | 323 | 319 | |||
| Min-Max | 0.2–11.7 | 0.6–6.3 | 0.3–11.7 | 0.3–10.5 | 0.2–11.7 | |||
| Mean (SD) mmol/L | 2.2 (1.4) | 2.3 (1.1) | 2.2 (1.5) | 2.0 (1.1) | 2.3 (1.6) | 0.009 | 0.530 | 0.242 |
| Lactate values (week-4) | ||||||||
| Age of infants (days) | 29 [28–31] | 31 [29–34] | 28 [28–30] | 29 [28–30] | 28 [28–30] | 0.570 | ||
| Number of samples | 299 | 73 | 226 | 117 | 109 | |||
| Min-Max | 0.2–11.7 | 0.9–5.7 | 0.2–11.7 | 0.6–7.5 | 0.2–11.7 | |||
| Mean (SD) mmol/L | 2.2 (1.4) | 2.2 (0.9) | 2.2 (1.5) | 2.1 (1.1) | 2.3 (1.8) | 0.283 | 0.976 | 0.499 |
| Lactate values (week-6) | ||||||||
| Age of infants (days) | 44 [42–46] | 46 [44–50] | 44 [42–46] | 44 [42–46] | 44 [42–46] | |||
| Number of samples | 264 | 65 | 199 | 101 | 98 | |||
| Min-Max | 0.3–10.5 | 0.9–5.7 | 0.3–9.9 | 0.6–10.5 | 0.3–9.9 | |||
| Mean (SD) mmol/L | 2.4 (1.5) | 2.3 (1.2) | 2.2 (1.5) | 2.2 (1.5) | 2.6 (1.9) | 0.149 | 0.638 | 0.273 |
| Lactate values (month-3) | ||||||||
| Age of infants (days) | 92 [90–93] | 93 [92–100] | 91 [90–92] | 93 [92–94] | 91 [90–92] | |||
| Number of samples | 264 | 65 | 170 | 79 | 91 | |||
| Min-Max | 0.3–8.1 | 0.9–6.3 | 0.3–8.1 | 0.3–6.3 | 0.4–8.1 | |||
| Mean (SD) mmol/L | 2.1 (1.1) | 2.3 (1.1) | 2.0 (1.2) | 1.7 (1.1) | 2.2 (1.1) | 0.006 | 0.104 | 0.006 |
sdNVP = single-dose of nevirapine, ZDV = zidovudine, 3TC = lamivudine
Comparison between the two exposed groups,
Comparison between exposed (all) and control groups,
Comparison between the three groups
SD: Standard deviation
Frequency of hyperlactatemia
A total of 292 infants (86%) who had at least two consecutive lactate measurements were available for this estimation, and 39 of them had hyperlactatemia. Thus, the prevalence of hyperlactatemia in this population was 13.4%, (CI: 9.6–17.8%). It was 11.6% (CI: 6.3–19.0%) among 112 infants from cohort 1, 14.5%, (CI: 8.5–22.5%) in 110 infants of cohort 2 and 14.3%, (CI: 7.1%–24.7%) in 70 infants of control group (p=0.79) (Figure 1). The prevalence of hyperlactatemia was 13.1% in the overall exposed group and 14.3% in the control group (p=0.84). Serious lactate levels (≥ 5 mmol/L) were identified in 34/836 (4.0%) blood samples collected and five children presented confirmed (≥ 2 samples) severe hyperlactatemia among 292 infants who had two consecutive measurements. No difference was observed between the three groups (p=0.574).
Figure 1. Frequency of hyperlactatemia* in infants born to HIV-infected mothers and exposed to antiretovirals during pregnancy. ANRS 1269 study, Abidjan, Côte d’Ivoire (2002–2005).
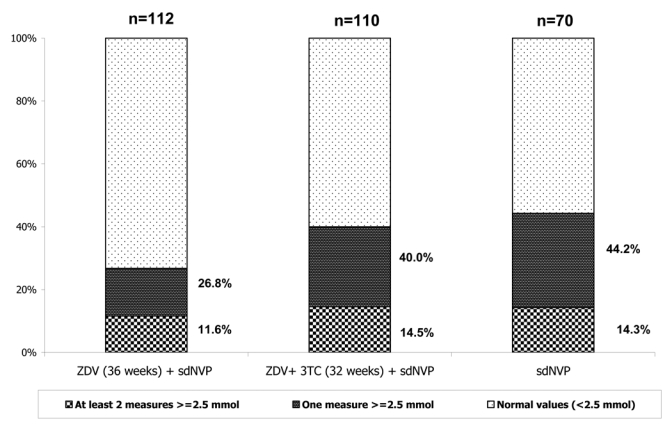
sdNVP: single-dose of nevirapine
ZDV+sdNVP: zidovudine (ZDV) from 36 weeks of gestation and single-dose of nevirapine
ZDV+3TC+sdNVP: zidovudine (ZDV) + lamivudine (3TC) from 32 weeks of gestation and single-dose of nevirapine
*>=2.5 mmol/L on two consecutives measurements in a series of three
Factors associated with hyperlactatemia in infants exposed to nucleoside antiretrovirals
In univariate then multivariate analyses, none of the following variables was found to be significantly associated with hyperlactatemia: child characteristics (gender, HIV status at week 4, twin birth, infant ZDV prophylaxis, birth weight) and maternal characteristics (duration of prepartum prophylaxis with ZDV, CD4, WHO clinical stage and age). No difference of frequency of hyperlactatemia was found according to the infant HIV status at week-4 (4.8% in HIV-infected infant versus 14.0% among the non HIV-infected, p=0.229).
Evolution of neonatal hyperlactatemia
The kinetics of hyperlactatemia is documented in 28 of 31 infants exposed to ZDV (n=12) or ZDV+3TC (n=19) and shows a return to normal values for 25 children (Figure 2). Three infants had persistent hyperlactatemia at month 6 and their lactate levels were respectively 3.3 mmol/L (ZDV group), 3.6 mmol/L (ZDV group) and 5.7 mmol/L (ZDV+3TC group) at that time.
Figure 2. Kinetics of evolution of lactate measurements & in 28 infants born from HIV-infected mothers presenting hyperlactatemia in first three months of life. ANRS 1269 study in the Ditrame Plus cohort in Abidjan, Côte d’Ivoire (2002–2005).
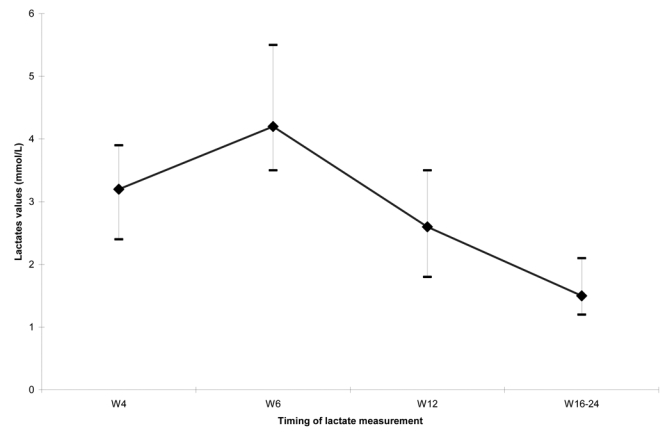
W4 = 4 weeks of life
W8 = 6 weeks of life
W12=12 weeks of life
W16–W24=between 16 and 24 weeks of life
& Median range and interquartile values of lactate measurements
Clinical manifestations
None of the children who presented a biologically confirmed hyperlactatemia in the first three months of life developed any of the following symptomatic clinical manifestations: abdominal pain, muscular or neurological symptoms, either before or after the biological diagnosis was made. There was not record of special clinical manifestation in the three children with persistent hyperlactatemia. All these infants with abnormal biological values were subsequently clinically followed at least until their second birthday and one male infant death was reported at week 38, whose presumptive cause was severe anemia. His serum lactate values were 2.6 mmol/L at week-4, 3.3 mmol/L at week-6, 1.8 mmol/L at month 3 and 2.7 mmol at month 6.
Discussion
In adults and children, the rise of lactate levels may be observed in physiological circumstances such as during and immediately after exercise, in hypermetabolic states and in the context of disease conditions (8). Thus, the diagnosis of nucleoside reverse transcriptase inhibitors related hyperlactatemia requires exclusion of others causes, such as dehydratation, vigorous exercise, alcohol intoxication, renal failure, hyperthyroidism and exposure to others drugs (17). However, hyperlactatemia is commonly artefactual due to sampling methods: in vivo by the use of tourniquets or as a consequence of fist clenching or hand pumping when venous specimens are drawn and in vitro if adequate plasma collection tubes are not used (18, 19).
In order to understand the significance of elevated lactate levels in infants exposed to ARVs, our study included also a control group formed by infants born to HIV-infected mothers and exposed to sdNVP, a non nucleoside reverse transcriptase inhibitor.
We have also undertaken internal and external quality controls with an independent laboratory to validate the lactate measurements. Moreover, we defined high lactate levels as two consecutives levels >2.5 mmol/L, thus taking into account only high and sustained lactatemia. We believe therefore our estimates are minimally biased regarding an artefactual measure. In our study, the overall frequency of high lactate levels was estimated at 13% and did not differ according to the type of ARV exposure. No difference was also found between groups when comparing the mean value of serum lactates of the exposed and control groups.
These results are in relative contradiction with those from a controlled study in Spain, where half of exposed children presented hyperlactatemia (6). This discrepancy could be explained by a longer exposure to NRTIs in the Spanish study, both for prenatal and postnatal periods. In industrialized countries, prophylactic treatment are generally initiated at the beginning of the second trimester of pregnancy and continued in the newborn for 6 weeks (20, 21). In addition a high dose of ZDV is given intravenously during labor, while children in our study were exposed in utero to oral ZDV alone in median for four weeks or to ZDV+3TC in median for eight weeks, and the labor dose was only given orally. As expected, we observed that the infants from cohort 2 had higher mean lactates than infants from cohort 1 or 3. This could be related to either the longer period of exposure or an exposure to two analogues nucleosides such as ZDV and 3TC.
It is also important to underline that our control group took into account the pregnancy effect as well as the maternal HIV infection status, that was not performed in the Spanish study (6). We can conclude therefore that, in our study the high lactate levels although not rare, were not related to short-term exposure to the nucleoside analogues.
There are potential limitations to our investigation. An enrolment bias could be discussed because we have enrolled the control group in a more limited time period than the other groups. However the impact of this selection procedure on the lactate measurements should be limited as all the laboratory assessments were performed by the same laboratory. We were not able to measure arterial pH to identify lactic acidosis, as well as the lactate-pyruvate ratio to explore mitochondrial function, for logistical reasons. However, the strengths of this observational study remain the large sample size and the use of a control group which has taken into account both the HIV infection status of the mother and the exposure to ARV drugs. Indeed, with 53 infants in each group we had a 80% statistical power to detect a mean difference between the infants exposed to NRTIs with mean lactate levels estimated at 2.88 mmol/L and those not exposed to NRTIs with mean lactate levels estimated at 1.61/mmol/L according to the results by Nogurera et al (6).
Our study allows drawing some public health conclusions. It appears clearly that lactate measurement is neither a specific nor a sensitive marker of mitochondrial injury in this context, although one cannot rule out the occurrence of mitochondrial injury in these cohorts (2–3). Based on our findings as well as the literature from industrialized countries (3), there is no argument to justify any routine screening of hyperlactatemia in infants exposed perinatally to ARVs. Long-term follow-up including clinical investigations as well as more advanced biological investigations of HIV-infected infants exposed perinatally to ARVs for PMTCT could help to detect all abnormalities which could be drug-related. The creation of an international registry to collect short and long-term ARV toxicity in such infants could help in the future to document the overall impact of all ARV drugs used for PMTCT.
Acknowledgments
The authors wish to acknowledge the support of the Developing Country Unit of the ANRS, particularly Drs Brigitte Bazin, Séverine Blesson and Pr Michel Kazatchkine, Director of the ANRS. Zidovudine and lamivudine were provided by Glaxo Smith Kline International. Special thanks to Anne Vassaux for the laboratory update and her help to conduct external control and to Laurence Becquet for the administrative management of the study. Finally, we would like to thank the women and children who accepted to participate to the DITRAME PLUS project.
Abbreviations
- ANRS
Agence Nationale de Recherches sur le Sida et les hépatites virales
- ARV
antiretroviral therapy
- CI
Confidence Interval
- HIV
Human Immunodeficiency Virus
- NRTI
nucleoside analogue reverse transcriptase inhibitor
- NNRTI
non-nucleoside analogue reverse transcriptase inhibitor
- SD
Standard Deviation
- sdNVP
single-dose of nevirapine
- 3TC
lamivudine
- ZDV (AZT)
zidovudine
APPENDIX
The ANRS 1209 Ditrame Plus study was coordinated by Didier K. Ekouevi and François Dabis. Ramata Toure and François Rouet were responsible for all laboratory aspects of this study. This study was part of the ANRS 1201/1202 Ditrame Plus project, of which members are listed below.
Composition of the ANRS 1201/1202 and 1209 DITRAME PLUS Study Group
Principal Investigators: François Dabis, Valériane Leroy, Marguerite Timite-Konan, Christiane Welffens-Ekra.
Coordination in Abidjan: Laurence Bequet, Didier K. Ekouévi, Besigin Tonwe-Gold, Ida Viho. Methodology, biostatistics and data management: Gérard Allou, Renaud Becquet, Katia Castetbon, Laurence Dequae-Merchadou, Charlotte Sakarovitch, Dominique Touchard.
Clinical team: Clarisse Amani-Bosse, Ignace Ayekoe, Gédéon Bédikou, Nacoumba Coulibaly, Christine Danel, Patricia Fassinou, Apollinaire Horo, Ruffin Likikouët, Hassan Toure.
Laboratory team: André Inwoley, Hervé Menan, François Rouet, Ramata Touré.
Psycho-social team: Hortense Aka-Dago, Alphonse Sihé.
Social sciences team: Hélène Agbo, Hermann Brou, Annabel Desgrées-du-Loû, Annick Tijou-Traoré, Benjamin Zanou.
Scientific Committee: Stéphane Blanche, Jean-François Delfraissy, Philippe Lepage, Laurent Mandelbrot, Christine Rouzioux, Roger Salamon.
Footnotes
See appendix
This study was presented, in part, at the second International AIDS Society Conference on HIV Pathogenesis and Treatment, (Paris, France), July 2003, abstract N° 664 and at the XV International AIDS conference (Bangkok, Thailand), July 2004 (ThPeC7292).
Sponsorship
The primary sponsor was the French Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS), France. Didier K. Ekouevi was a fellow of the French Charity Sidaction and is now a fellow of the European Clinical Trial Partnership (EDCTP). François Rouet was supported by the French Ministry of Foreign Affairs. Renaud Becquet was a fellow of the French Ministry of Education, Research and Technology and is now a fellow of the French Charity Sidaction. Zidovudine was provided by Glaxo-Smith Kline International.
References
- 1.Scalfaro P, Chesaux JJ, Buchwalder PA, Biollaz J, Micheli JL. Severe transient neonatal lactic acidosis during prophylactic zidovudine treatment. Intensive Care Med. 1998;24(3):247–250. doi: 10.1007/s001340050558. [DOI] [PubMed] [Google Scholar]
- 2.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354(9184):1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 3.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17(12):1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 4.Alimenti A, Burdge DR, Ogilvie GS, Money DM, Forbes JC. Lactic acidemia in human immunodeficiency virus-uninfected infants exposed to perinatal antiretroviral therapy. Pediatr Infect Dis J. 2003;22(9):782–789. doi: 10.1097/01.inf.0000086400.93257.74. [DOI] [PubMed] [Google Scholar]
- 5.Giaquinto C, De Romeo A, Giacomet V, et al. Lactic acid levels in children perinatally treated with antiretroviral agents to prevent HIV transmission. AIDS. 2001;15(8):1074–1075. doi: 10.1097/00002030-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 6.Noguera A, Fortuny C, Munoz-Almagro C, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114(5):e598–603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman K. Management of hyperlactatemia: no need for routine lactate measurements. AIDS. 2001;15(6):795–7. doi: 10.1097/00002030-200104130-00016. [DOI] [PubMed] [Google Scholar]
- 8.Carr A. Lactic acidemia in infection with human immunodeficiency virus. Clin Infect Dis. 2003;36(Suppl 2):S96–S100. doi: 10.1086/367565. [DOI] [PubMed] [Google Scholar]
- 9.Dabis F, Msellati P, Meda N, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Cote d’Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mere-Enfant. Lancet. 1999;353(9155):786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 10.Petra study team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359(9313):1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 11.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19(3):309–318. [PMC free article] [PubMed] [Google Scholar]
- 12.Becquet R, Ekouevi DK, Viho I, et al. Acceptability of exclusive breastfeeding with early cessation to prevent HIV transmission through breastmilk, ANRS 1201/1202 Ditrame Plus, Abidjan, Côte d’Ivoire. J Acquir Immune Defic Syndr. 2005;40(5):600–608. doi: 10.1097/01.qai.0000171726.17436.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Care, treatment, and support for women living with HIV/AIDS and their children in resource-constrained settings. Antiretroviral drugs for treatment in pregnant women and for prevention of HIV infection in infants and young children. [Accessed 13 January 2006];2004 Revision. Available from http://www.who.int/hiv/pub/mtct/guidelines/en/
- 15. [Accessed 13 January 2006];Adult AIDS Clinical Trial Group Mitochondrial Dysfunction Focus Group Lactis Acidosis Guideline. Available from http://aactg.s-3.com/members/psmet.htm.
- 16.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a west African resource-limited setting. Journal of Clinical Microbiology. 2005;43(6):2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizock BA, Falk JL. Lactic acidosis in critical illness. Crit Care Med. 1992;20(1):80–93. doi: 10.1097/00003246-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Andersen O, Haugaard SB, Jorgensen LT, et al. Preanalytical handling of samples for measurement of plasma lactate in HIV patients. Scand J Clin Lab Invest. 2003;63(6):449–454. doi: 10.1080/00365510310005128. [DOI] [PubMed] [Google Scholar]
- 19.Wohl DA, Pilcher CD, Evans S, et al. Absence of sustained hyperlactatemia in HIV-infected patients with risk factors for mitochondrial toxicity. J Acquir Immune Defic Syndr. 2004;35(3):274–278. doi: 10.1097/00126334-200403010-00008. [DOI] [PubMed] [Google Scholar]
- 20.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288(2):189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 21.Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA. 2001;285(16):2083–93. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]


