Abstract
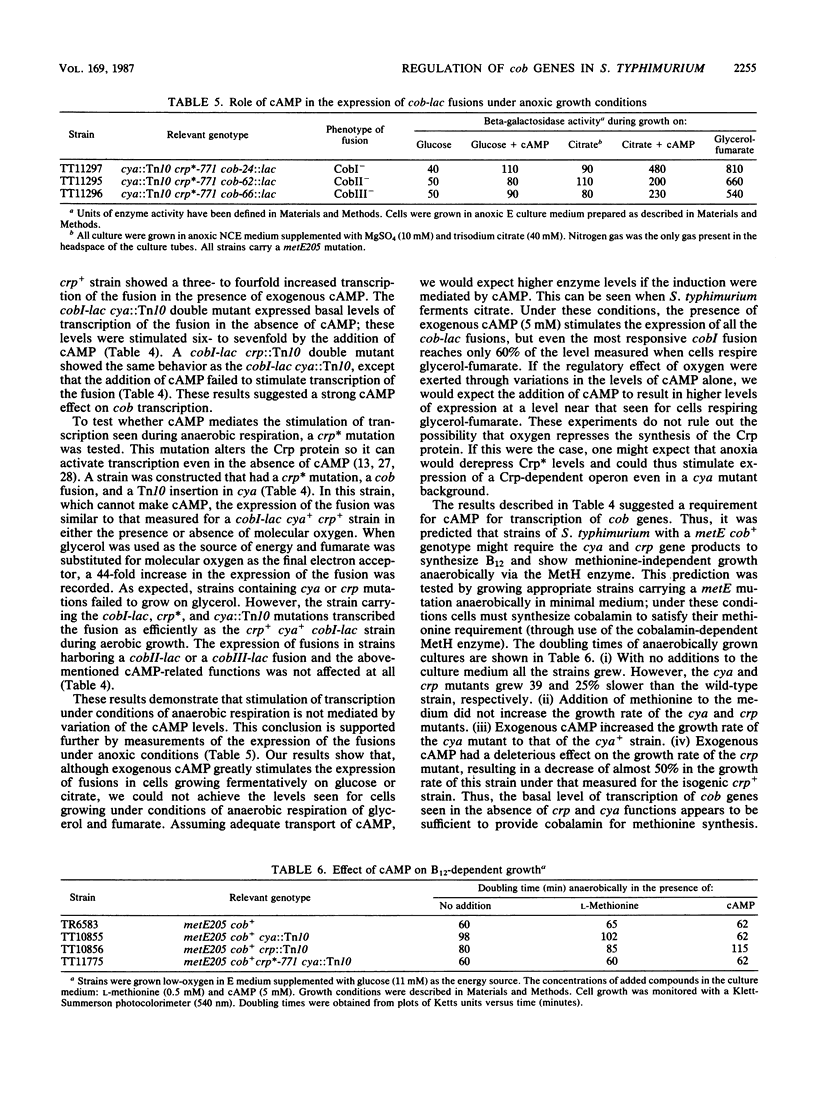
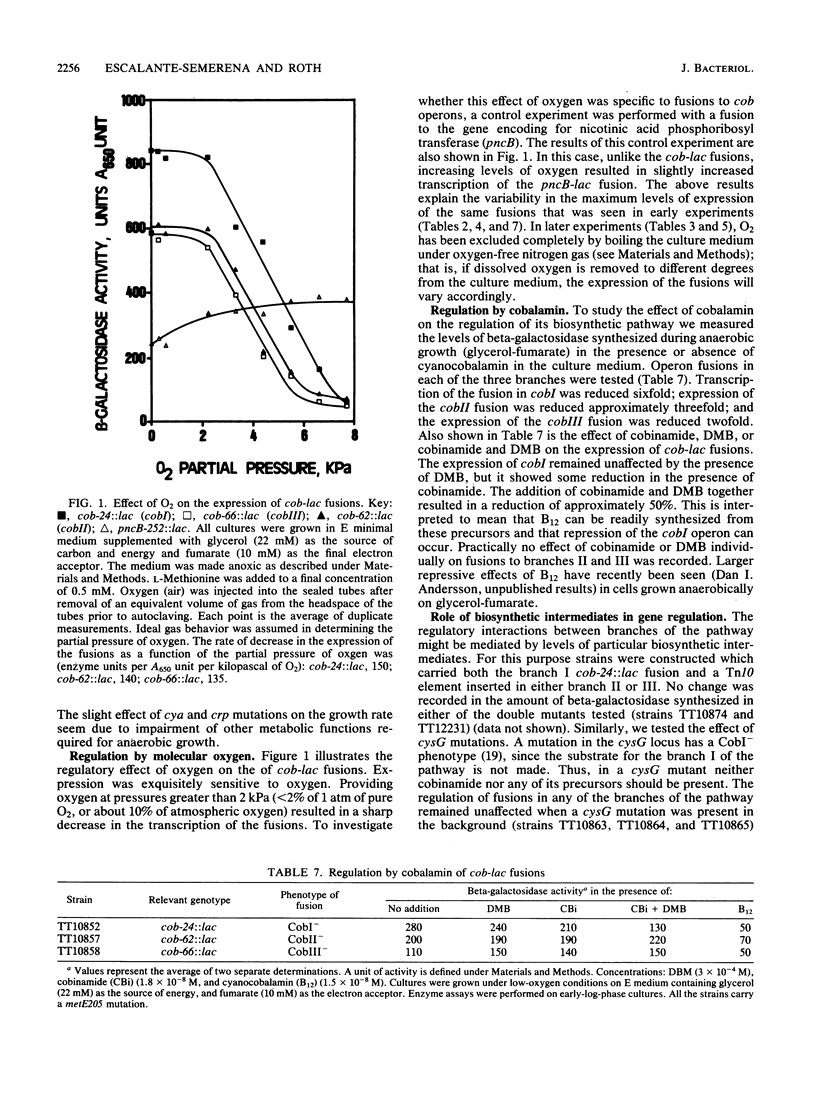
Transcription of cobalamin (cob) biosynthetic genes in Salmonella typhimurium is repressed by cobalamin and by molecular oxygen. These genes seem to be subject to catabolite repression, and they are maximally expressed under conditions of anaerobic respiration of glycerol-fumarate. A 215-fold increase in the expression of cob genes occurs when S. typhimurium shifts from aerobic growth on glucose to anaerobic respiration of glycerol-fumarate under strictly anoxic growth conditions. Exogenous cyclic AMP substantially stimulates the transcription of cob-lac fusions during aerobic growth. However, cyclic AMP is not absolutely required for the expression of the pathway, nor does it mediate the aerobic control. Cobalamin biosynthesis is not seen under aerobic growth conditions, even when transcription is stimulated by the addition of cyclic AMP. Hence, additional control mechanisms triggered by the presence of molecular oxygen must operate independently from transcription effects on the cob operons.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley G. W., Harris G., Stubbe J. The mechanism of Lactobacillus leichmannii ribonucleotide reductase. Evidence for 3' carbon-hydrogen bond cleavage and a unique role for coenzyme B12. J Biol Chem. 1986 Mar 25;261(9):3958–3964. [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby A. R., Bushell M. J., Jones C., Lewis N. G., Pfenninger A. Biosynthesis of vitamin B12: identity of fragment extruded during ring contraction to the corrin macrocycle. Proc Natl Acad Sci U S A. 1981 Jan;78(1):13–15. doi: 10.1073/pnas.78.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. II. Requirement for a cobamide coenzyme by an ethanolamine deaminase. J Biol Chem. 1965 Dec;240(12):4675–4681. [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S. H. Amidation of, and (R)-1-amino-2-propanol attachment to, the corrin ring during vitamin B-12 biosynthesis by Clostridium tetanomorphum extracts. Biochim Biophys Acta. 1985 Sep 6;841(3):306–317. doi: 10.1016/0304-4165(85)90073-x. [DOI] [PubMed] [Google Scholar]
- Fyfe J. A., Friedmann H. C. Vitamin B 12 biosynthesis. Enzyme studies on the formation of the alpha-glycosidic nucleotide precursor. J Biol Chem. 1969 Apr 10;244(7):1659–1666. [PubMed] [Google Scholar]
- Garges S., Adhya S. Sites of allosteric shift in the structure of the cyclic AMP receptor protein. Cell. 1985 Jul;41(3):745–751. doi: 10.1016/s0092-8674(85)80055-6. [DOI] [PubMed] [Google Scholar]
- Halpern J. Mechanisms of coenzyme B12-dependent rearrangements. Science. 1985 Feb 22;227(4689):869–875. doi: 10.1126/science.2857503. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Conditionally transposition-defective derivative of Mu d1(Amp Lac). J Bacteriol. 1984 Jul;159(1):130–137. doi: 10.1128/jb.159.1.130-137.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllriegl V., Lamm L., Rowold J., Hörig J., Renz P. Biosynthesis of vitamin B12. Different pathways in some aerobic and anaerobic microorganisms. Arch Microbiol. 1982 Aug;132(2):155–158. doi: 10.1007/BF00508722. [DOI] [PubMed] [Google Scholar]
- Hörig J. A., Renz P. Biosynthesis of vitamin B12. Some properties of the 5,6-dimethylbenzimidazole-forming system of Propionibacterium freudenreichii and Propionibacterium shermanii. Eur J Biochem. 1980 Apr;105(3):587–592. doi: 10.1111/j.1432-1033.1980.tb04536.x. [DOI] [PubMed] [Google Scholar]
- Hörig J. A., Renz P., Heckmann G. [5-15N]Riboflavin as precursor in the biosynthesis of the 5,6-dimethylbenzimidazole moiety of vitamin B12. A study by 1H and 15N magnetic resonance spectroscopy. J Biol Chem. 1978 Oct 25;253(20):7410–7414. [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H. F., Spears C., Greene R. C., Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B 12 and methionine. Arch Biochem Biophys. 1972 May;150(1):23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lütgens M., Gottschalk G. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J Gen Microbiol. 1980 Jul;119(1):63–70. doi: 10.1099/00221287-119-1-63. [DOI] [PubMed] [Google Scholar]
- Mombelli L., Nussbaumer C., Weber H., Müller G., Arigoni D. Biosynthesis of vitamin B12: nature of the volatile fragment generated during formation of the corrin ring system. Proc Natl Acad Sci U S A. 1981 Jan;78(1):11–12. doi: 10.1073/pnas.78.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Margolin W., Krueger J. H., Walker G. C. Mutations affecting regulation of methionine biosynthetic genes isolated by use of met-lac fusions. J Bacteriol. 1982 Aug;151(2):609–619. doi: 10.1128/jb.151.2.609-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer C., Imfeld M., Wörner G., Müller G., Arigoni D. Biosynthesis of vitamin B12: mode of incorporation of factor III into cobyrinic acid. Proc Natl Acad Sci U S A. 1981 Jan;78(1):9–10. doi: 10.1073/pnas.78.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetti V., Pfaltz A., Kratky C., Eschenmoser A. Ring contraction of hydroporphinoid to corrinoid complexes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):16–19. doi: 10.1073/pnas.78.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlett F. A., Turner J. M. Microbial metabolism of amino alcohols. Ethanolamine catabolism mediated by coenzyme B12-dependent ethanolamine ammonia-lyase in Escherichia coli and Klebsiella aerogenes. J Gen Microbiol. 1976 Jul;95(1):173–176. doi: 10.1099/00221287-95-1-173. [DOI] [PubMed] [Google Scholar]
- Schmieger H. A method for detection of phage mutants with altered transducing ability. Mol Gen Genet. 1971;110(4):378–381. doi: 10.1007/BF00438281. [DOI] [PubMed] [Google Scholar]
- Schmieger H., Backhaus H. The origin of DNA in transducing particles in P22-mutants with increased transduction-frequencies (HT-mutants). Mol Gen Genet. 1973 Jan 24;120(2):181–190. doi: 10.1007/BF00267246. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Roth J. R., Artz S. W. Regulation of histidine operon does not require hisG enzyme. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5021–5025. doi: 10.1073/pnas.72.12.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoeman R., Coleman T., Redfield B., Greene R. C., Smith A. A., Saint-Girons I., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: effect of metJ gene product and S-adenosylmethionine on the in vitro expression of the metB, metL and metJ genes. Biochem Biophys Res Commun. 1985 Dec 17;133(2):731–739. doi: 10.1016/0006-291x(85)90965-9. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Brot N., Weissbach H., Greene R. C., Smith A. A., Saint-Girons I., Zakin M. M., Cohen G. N. Regulation of the methionine regulon in Escherichia coli. Bioessays. 1985 Nov;3(5):210–213. doi: 10.1002/bies.950030506. [DOI] [PubMed] [Google Scholar]
- Shoeman R., Redfield B., Coleman T., Greene R. C., Smith A. A., Brot N., Weissbach H. Regulation of methionine synthesis in Escherichia coli: Effect of metJ gene product and S-adenosylmethionine on the expression of the metF gene. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3601–3605. doi: 10.1073/pnas.82.11.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]


