Abstract
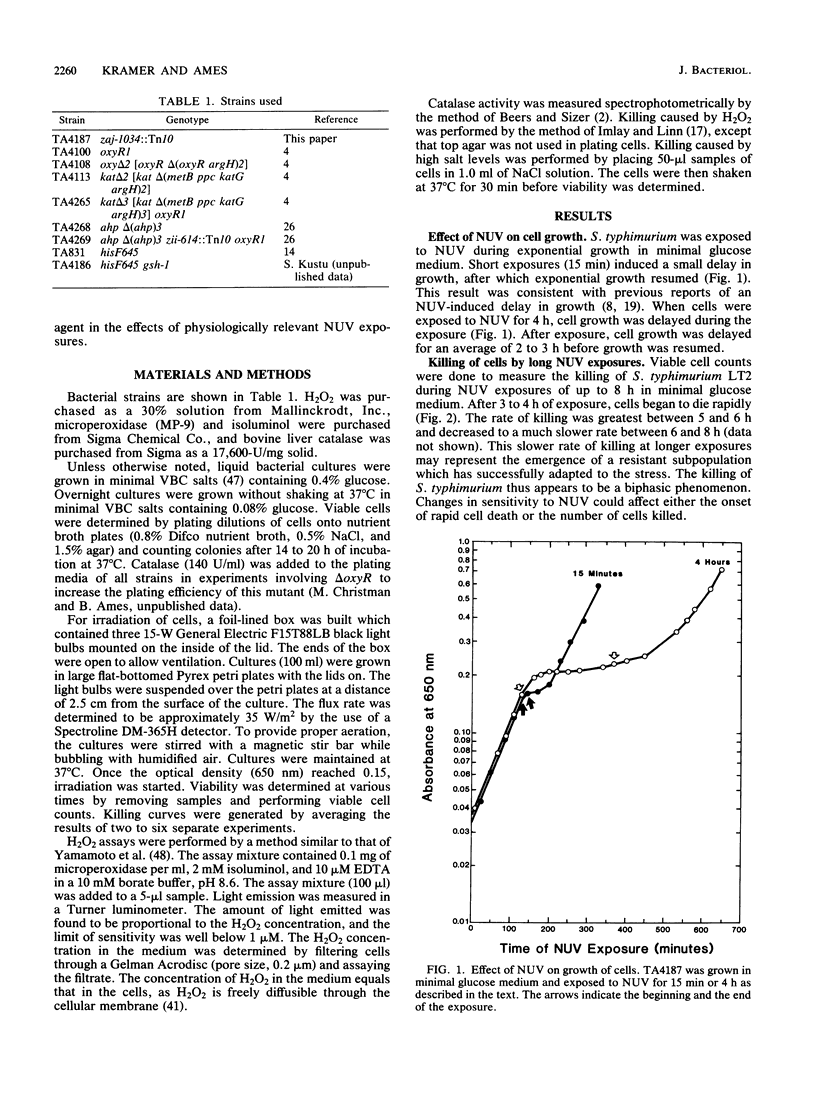
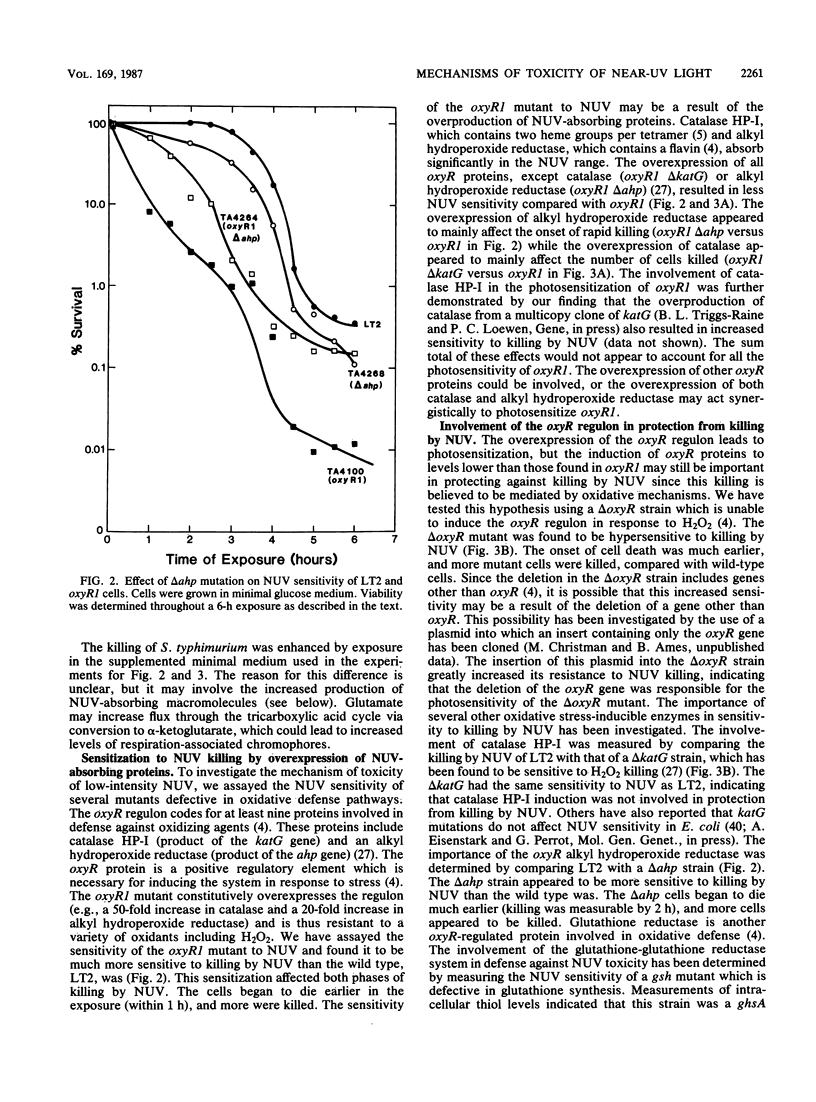
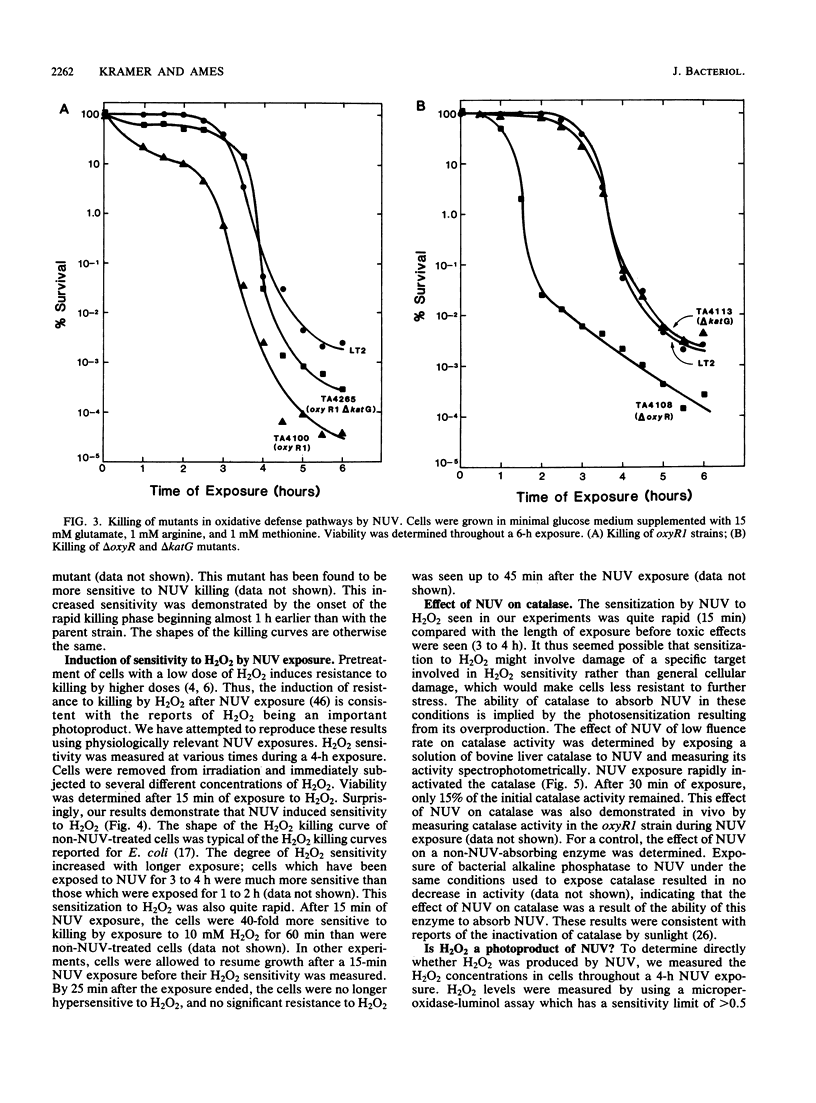
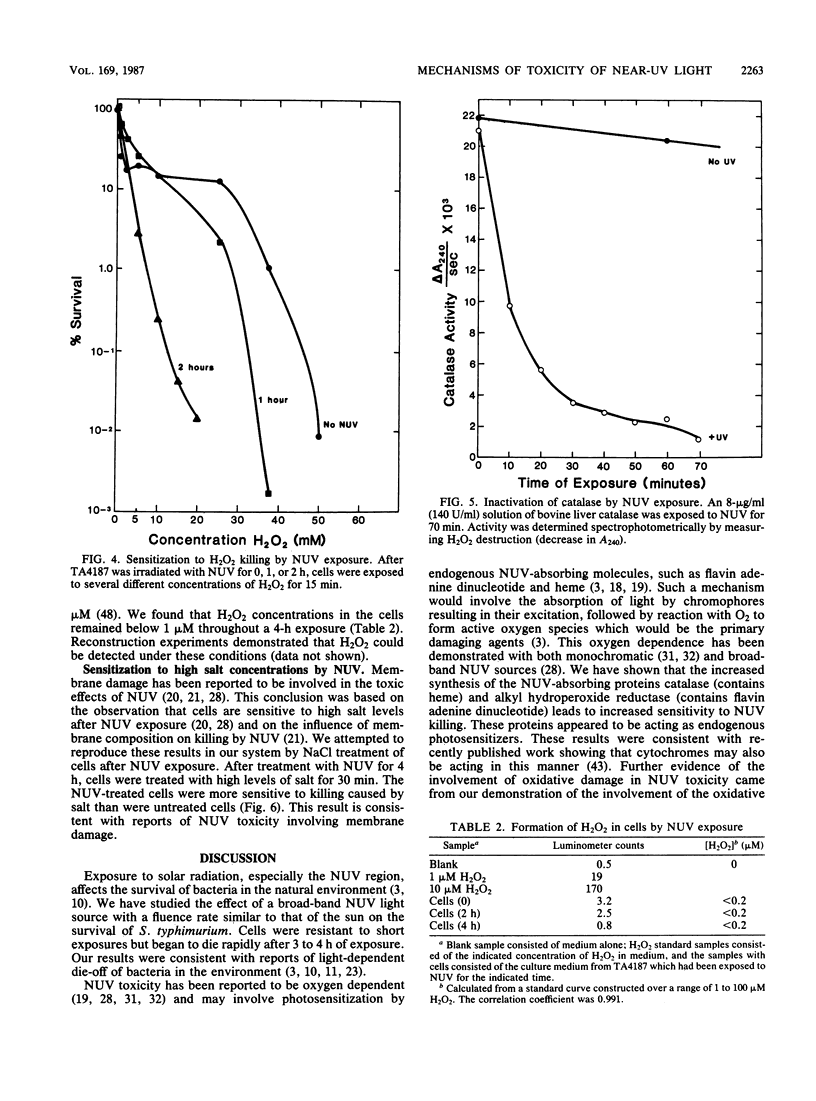
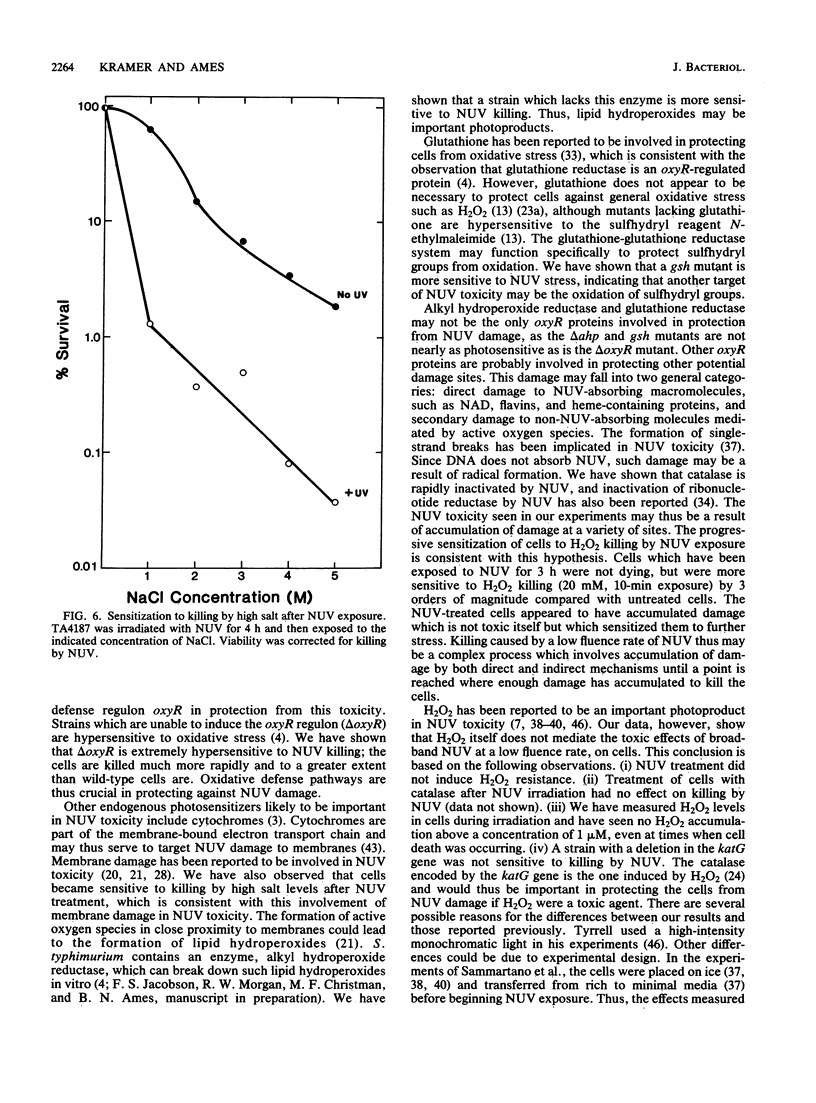
The exposure of Salmonella typhimurium to environmentally relevant near-UV light stress has been studied by the use of a low-intensity, broad-band light source. The exposure of cells to such a light source rapidly induced a growth delay; after continuous exposure for 3 to 4 h, cells began to die at a rapid rate. The oxidative defense regulon controlled by the oxyR gene was involved in protecting cells from being killed by near-UV light. This killing may be potentiated by the overexpression of near-UV-absorbing proteins. These results are consistent with near-UV toxicity involving the absorption of light by endogenous photosensitizers, leading to the production of active oxygen species. We have shown, however, that one such species, H2O2, is not a major photoproduct involved in killing by near-UV light. Strains lacking alkyl hydroperoxide reductase were more sensitive to near-UV light, indicating that such hydroperoxides may be photoproducts. Near-UV exposure induced sensitivity to high salt levels, indicating that membranes may be a target of near-UV toxicity and a possible source of alkyl hydroperoxides. The demonstration of the inactivation of the heme-containing protein catalase indicates that direct destruction of UV-absorbing macromolecules could be another factor in near-UV toxicity. Cells which have been exposed to near-UV light for long, but sublethal, periods of time (up to 4 h) can recover and resume growth if the UV exposure is stopped but become progressively more sensitive to further stresses, such as H2O2. This result indicates that cells gradually accumulated damage during near-UV exposure until toxic levels were reached.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Claiborne A., Fridovich I. Purification of the o-dianisidine peroxidase from Escherichia coli B. Physicochemical characterization and analysis of its dual catalatic and peroxidatic activities. J Biol Chem. 1979 May 25;254(10):4245–4252. [PubMed] [Google Scholar]
- Demple B., Halbrook J. Inducible repair of oxidative DNA damage in Escherichia coli. Nature. 1983 Aug 4;304(5925):466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory L. P. Polonium-210 in leaf tobacco from four countries. Science. 1965 Oct 1;150(3692):74–76. doi: 10.1126/science.150.3692.74-a. [DOI] [PubMed] [Google Scholar]
- Hartman P. E., Hartman Z., Stahl R. C. Classification and mapping of spontaneous and induced mutations in the histidine operon of Salmonella. Adv Genet. 1971;16:1–34. doi: 10.1016/s0065-2660(08)60352-1. [DOI] [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Killing of Escherichia coli K-12 by near-ultraviolet radiation in the presence of hydrogen peroxide: role of double-strand DNA breaks in absence of recombinational repair. Mutat Res. 1980 Aug;72(1):31–42. doi: 10.1016/0027-5107(80)90217-1. [DOI] [PubMed] [Google Scholar]
- Hartman P. S., Eisenstark A. Synergistic killing of Escherichia coli by near-UV radiation and hydrogen peroxide: distinction between recA-repairable and recA-nonrepairable damage. J Bacteriol. 1978 Feb;133(2):769–774. doi: 10.1128/jb.133.2.769-774.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol. 1986 May;166(2):519–527. doi: 10.1128/jb.166.2.519-527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. R., Moss S. H., Davies D. J. Recovery of Escherichia coli K-12 from near-ultraviolet radiation-induced membrane damage. Photochem Photobiol. 1983 Jun;37(6):617–622. doi: 10.1111/j.1751-1097.1983.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Klamen D. L., Tuveson R. W. The effect of membrane fatty acid composition on the near-UV (300-400 nm) sensitivity of Escherichia coli K1060. Photochem Photobiol. 1982 Feb;35(2):167–173. doi: 10.1111/j.1751-1097.1982.tb03827.x. [DOI] [PubMed] [Google Scholar]
- Lang H., Riesenberg D., Zimmer C., Bergter F. Fluence-rate dependence of monophotonic reactions of nucleic acids in vitro and in vivo. Photochem Photobiol. 1986 Nov;44(5):565–570. doi: 10.1111/j.1751-1097.1986.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Loewen P. C., Switala J., Triggs-Raine B. L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985 Nov 15;243(1):144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- Miguel A. G., Tyrrell R. M. Repair of near-ultraviolet (365 nm)-induced strand breaks in Escherichia coli DNA. The role of the polA and recA gene products. Biophys J. 1986 Feb;49(2):485–491. doi: 10.1016/S0006-3495(86)83658-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. H., Smith K. C. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Feb;33(2):203–210. doi: 10.1111/j.1751-1097.1981.tb05325.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978 Jul;14(3):545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- Peak J. G., Peak M. J. Lethality in repair-proficient Escherichia coli after 365 nm ultraviolet light irradiation is dependent on fluence rate. Photochem Photobiol. 1982 Jul;36(1):103–105. doi: 10.1111/j.1751-1097.1982.tb04348.x. [DOI] [PubMed] [Google Scholar]
- Peak J. G., Peak M. J., Tuveson R. W. Ultraviolet action spectra for aerobic and anaerobic inactivation of Escherichia coli strains specifically sensitive and resistant to near ultraviolet radiations. Photochem Photobiol. 1983 Nov;38(5):541–543. doi: 10.1111/j.1751-1097.1983.tb03380.x. [DOI] [PubMed] [Google Scholar]
- Peak M. J., Peak J. G., Nerad L. The role of 4-thiouridine in lethal effects and in DNA backbone breakage caused by 334 nm ultraviolet light in Escherichia coli. Photochem Photobiol. 1983 Feb;37(2):169–172. doi: 10.1111/j.1751-1097.1983.tb04453.x. [DOI] [PubMed] [Google Scholar]
- Peters J. In vivo photoinactivation of Escherichia coli ribonucleotide reductase by near-ultraviolet light. Nature. 1977 Jun 9;267(5611):546–548. doi: 10.1038/267546a0. [DOI] [PubMed] [Google Scholar]
- Peters J., Jagger J. Inducible repair of near-UV radiation lethal damage in E. coli. Nature. 1981 Jan 15;289(5794):194–195. doi: 10.1038/289194a0. [DOI] [PubMed] [Google Scholar]
- Ramabhadran T. V., Jagger J. Mechanism of growth delay induced in Escherichia coli by near ultraviolet radiation. Proc Natl Acad Sci U S A. 1976 Jan;73(1):59–63. doi: 10.1073/pnas.73.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W., Davenport R. Control of sensitivity to inactivation by H2O2 and broad-spectrum near-UV radiation by the Escherichia coli katF locus. J Bacteriol. 1986 Oct;168(1):13–21. doi: 10.1128/jb.168.1.13-21.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. Escherichia coli xthA mutants are sensitive to inactivation by broad-spectrum near-UV (300- to 400-nm) radiation. J Bacteriol. 1983 Nov;156(2):904–906. doi: 10.1128/jb.156.2.904-906.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. Hydrogen peroxide induced resistance to broad-spectrum near-ultraviolet light (300-400 nm) inactivation in Escherichia coli. Photochem Photobiol. 1985 Mar;41(3):367–370. doi: 10.1111/j.1751-1097.1985.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Sammartano L. J., Tuveson R. W. The effects of exogenous catalase on broad-spectrum near-UV (300-400 nm) treated Escherichia coli cells. Photochem Photobiol. 1984 Nov;40(5):607–612. doi: 10.1111/j.1751-1097.1984.tb05348.x. [DOI] [PubMed] [Google Scholar]
- Schwartz C. E., Krall J., Norton L., McKay K., Kay D., Lynch R. E. Catalase and superoxide dismutase in Escherichia coli. J Biol Chem. 1983 May 25;258(10):6277–6281. [PubMed] [Google Scholar]
- Tsai S. C., Jagger J. The roles of the rel+ gene and of 4-thiouridine in killing and photoprotection of Escherichia coli by near-ultraviolet radiation. Photochem Photobiol. 1981 Jun;33(6):825–834. doi: 10.1111/j.1751-1097.1981.tb05499.x. [DOI] [PubMed] [Google Scholar]
- Tuveson R. W., Sammartano L. J. Sensitivity of hemA mutant Escherichia coli cells to inactivation by near-UV light depends on the level of supplementation with delta-aminolevulinic acid. Photochem Photobiol. 1986 Jun;43(6):621–626. doi: 10.1111/j.1751-1097.1986.tb05637.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. A common pathway for protection of bacteria against damage by solar UVA (334 nm, 365 nm) and an oxidising agent (H2O2). Mutat Res. 1985 May;145(3):129–136. doi: 10.1016/0167-8817(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Rec A+-dependent synergism between 365 NM and ionizing radiation in log-phase Escherichia coli: a model for oxygen-dependent near-UV inactivation by disruption of DNA repair. Photochem Photobiol. 1976 Jan;23(1):13–20. doi: 10.1111/j.1751-1097.1976.tb06764.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R. M. Synergistic lethal action of ultraviolet violet radiations and mild heat in Escherichia coli. Photochem Photobiol. 1976 Oct;24(4):345–351. doi: 10.1111/j.1751-1097.1976.tb06835.x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]


