Abstract
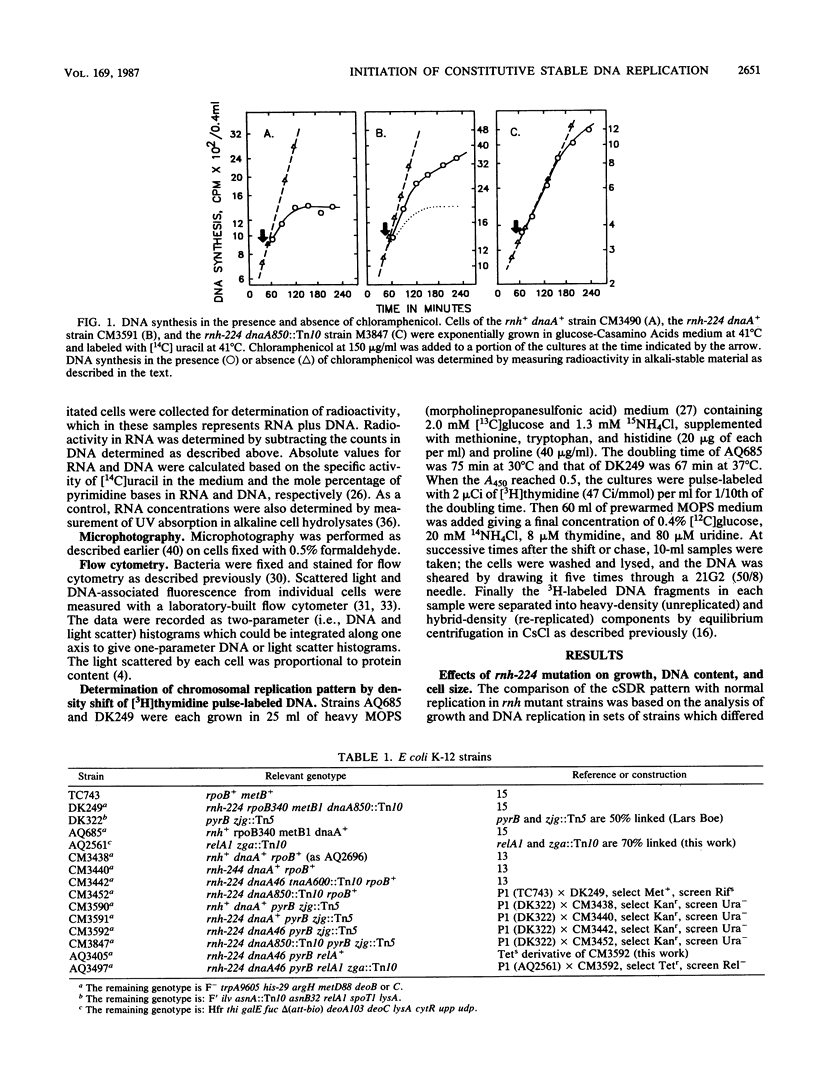
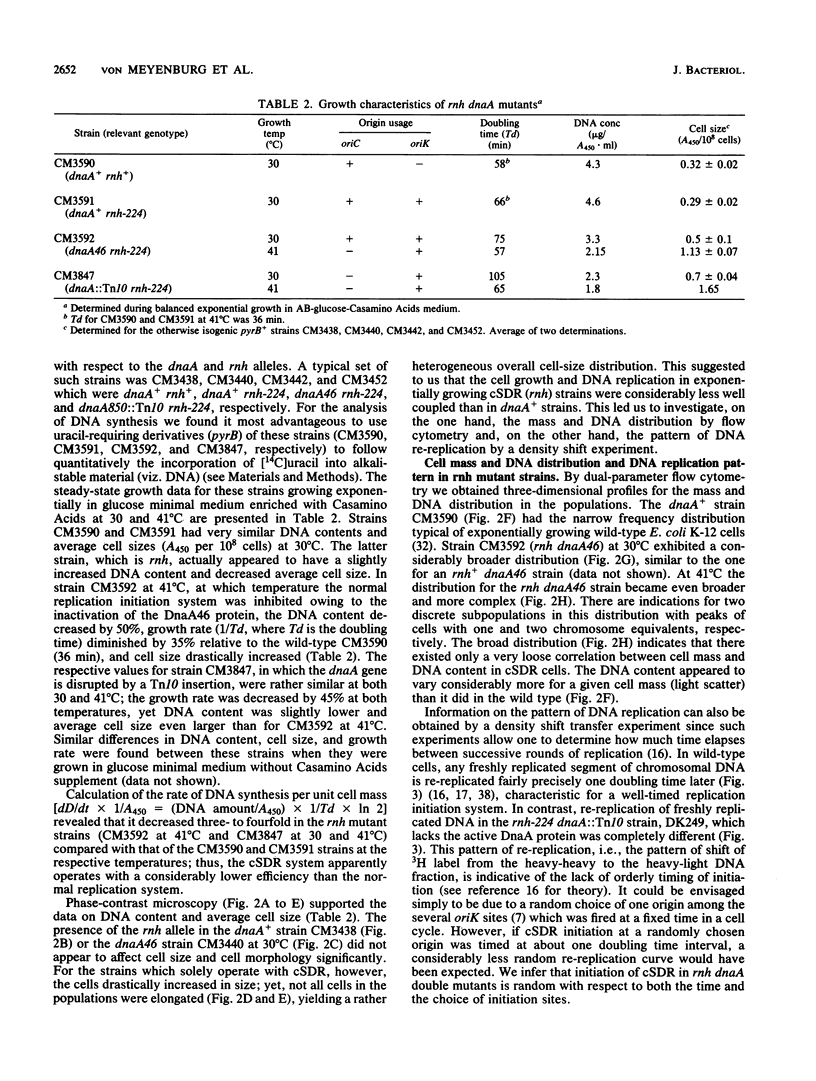
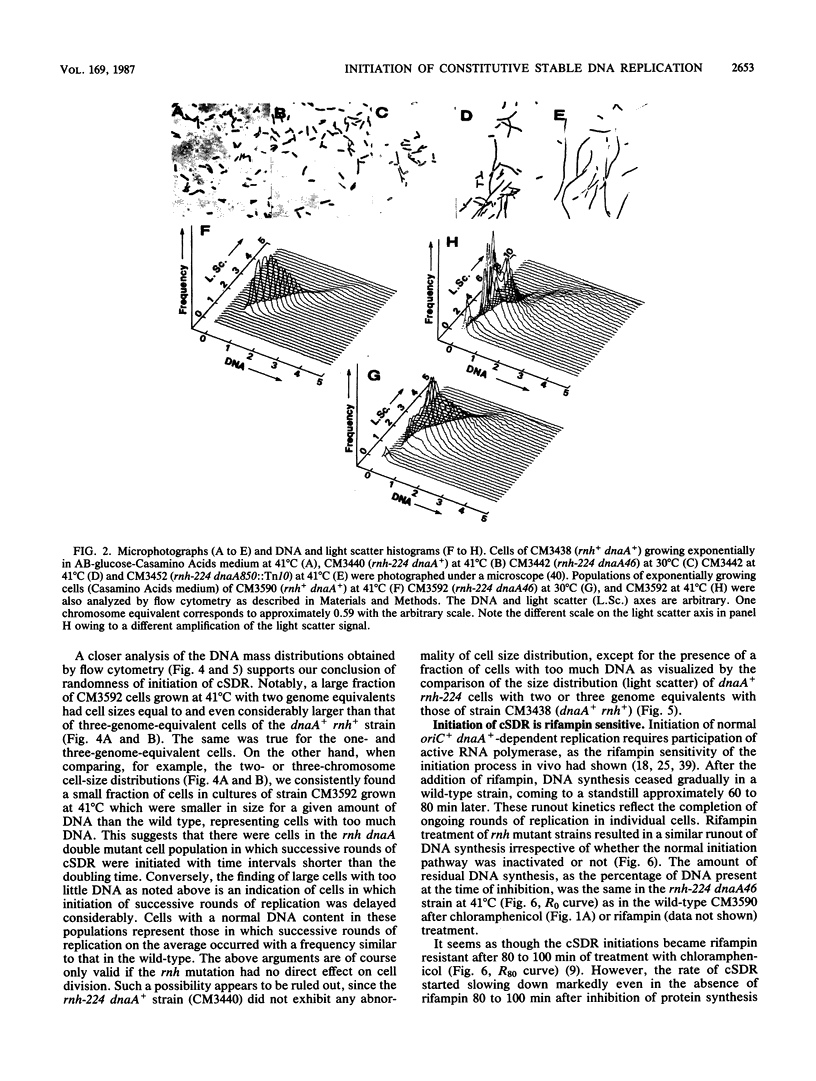
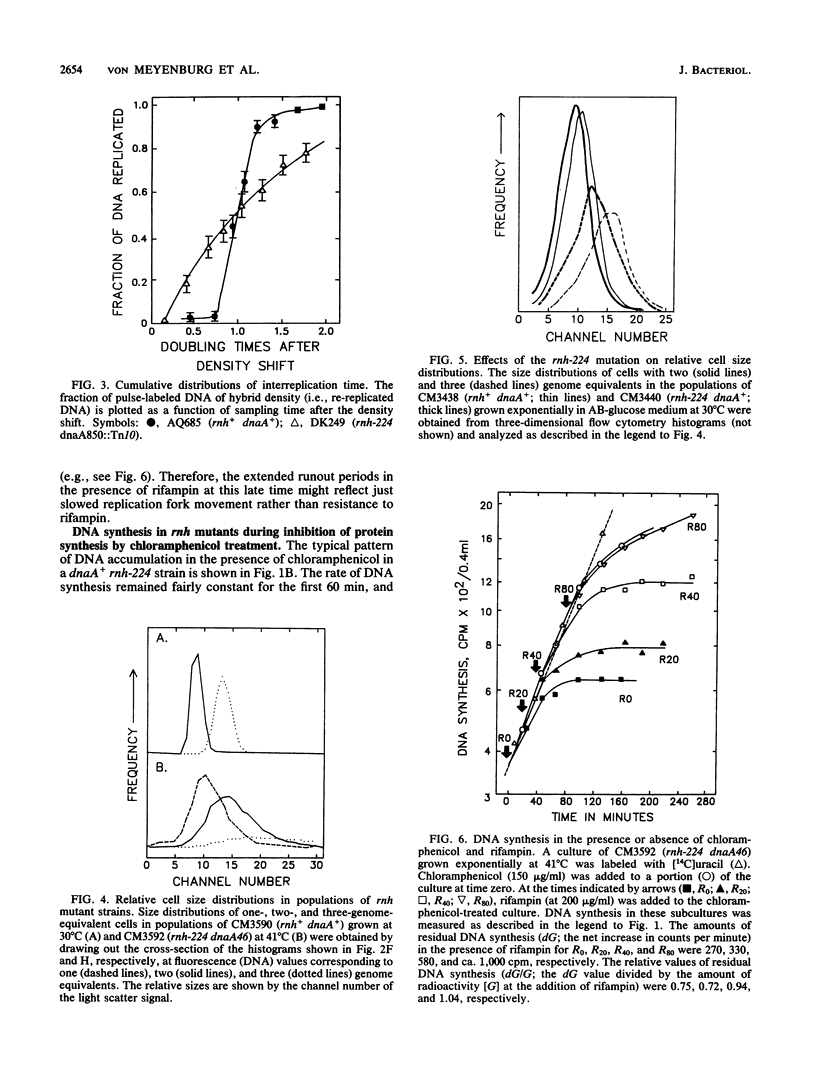
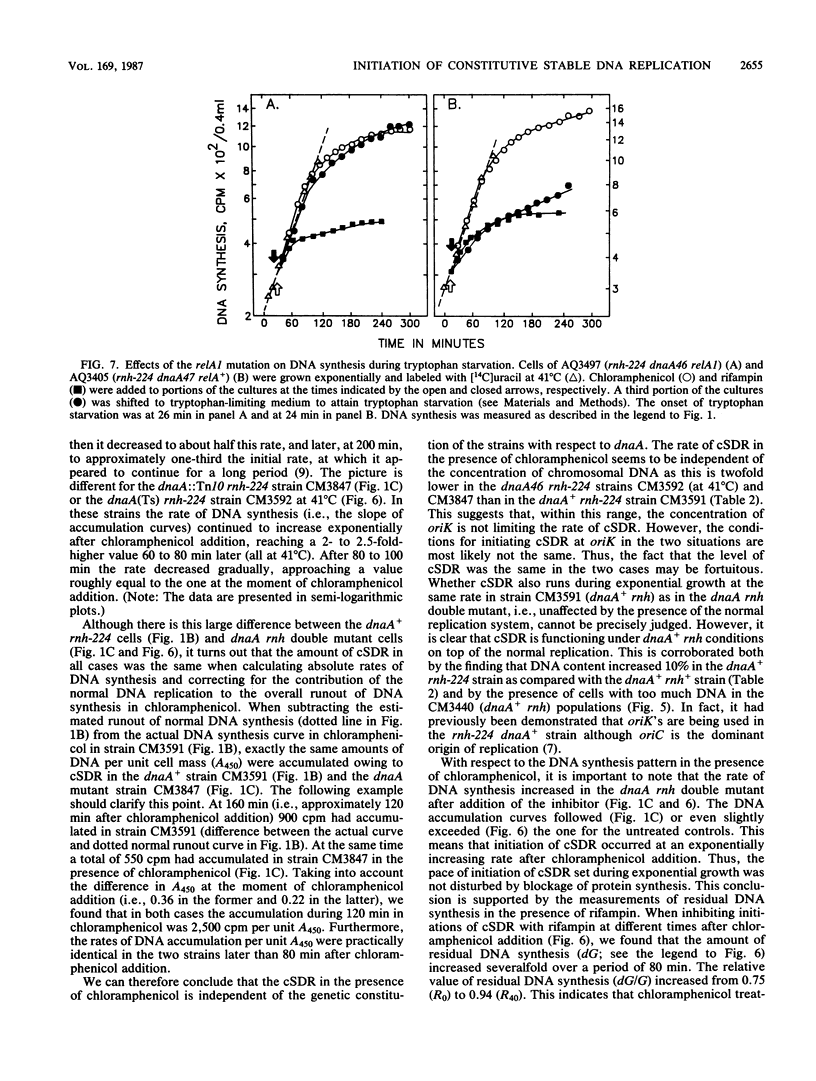
The alternative pathway of DNA replication in rnh mutants of Escherichia coli can be continuously initiated in the presence of chloramphenicol, giving rise to constitutive stable DNA replication (cSDR). We conducted a physiological analysis of cSDR in rnh-224 mutants in the presence or absence of the normal DNA replication system. The following results were obtained. cSDR allowed the cells to grow in the absence of the normal replication system at a 30 to 40% reduced growth rate and with an approximately twofold-decreased DNA content. cSDR initiation was random with respect to time in the cell cycle as well as choice of origins. cSDR initiation continued to increase exponentially for more than one doubling time when protein synthesis was inhibited by chloramphenicol. cSDR initiation was inhibited during amino acid starvation in stringent (relA+) but not in relaxed (relA1) strains, indicating its sensitivity to ppGpp. cSDR initiation was rifampin sensitive, demonstrating that RNA polymerase was involved. cSDR functioned in dnaA+ rnh-224 strains parallel to the normal oriC+ dnaA+-dependent chromosome replication system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Bialy H., Kogoma T. RNase H is not involved in the induction of stable DNA replication in Escherichia coli. J Bacteriol. 1986 Jan;165(1):321–323. doi: 10.1128/jb.165.1.321-323.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye E., Steen H. B., Skarstad K. Flow cytometry of bacteria: a promising tool in experimental and clinical microbiology. J Gen Microbiol. 1983 Apr;129(4):973–980. doi: 10.1099/00221287-129-4-973. [DOI] [PubMed] [Google Scholar]
- Donachie W. D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968 Sep 7;219(5158):1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- Kogoma T. A novel Escherichia coli mutant capable of DNA replication in the absence of protein synthesis. J Mol Biol. 1978 May 5;121(1):55–69. doi: 10.1016/0022-2836(78)90262-0. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Lark K. G. Characterization of the replication of Escherichia coli DNA in the absence of protein synthesis: stable DNA replication. J Mol Biol. 1975 May 15;94(2):243–256. doi: 10.1016/0022-2836(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Kogoma T. RNase H-defective mutants of Escherichia coli. J Bacteriol. 1986 May;166(2):361–363. doi: 10.1128/jb.166.2.361-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Skarstad K., Boye E., von Meyenburg K., Steen H. B. RecA protein acts at the initiation of stable DNA replication in rnh mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):439–444. doi: 10.1128/jb.163.2.439-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Subia N. L., von Meyenburg K. Function of ribonuclease H in initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet. 1985;200(1):103–109. doi: 10.1007/BF00383320. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Torrey T. A., Connaughton M. J. Induction of UV-resistant DNA replication in Escherichia coli: induced stable DNA replication as an SOS function. Mol Gen Genet. 1979 Oct 2;176(1):1–9. doi: 10.1007/BF00334288. [DOI] [PubMed] [Google Scholar]
- Kogoma T., von Meyenburg K. The origin of replication, oriC, and the dnaA protein are dispensable in stable DNA replication (sdrA) mutants of Escherichia coli K-12. EMBO J. 1983;2(3):463–468. doi: 10.1002/j.1460-2075.1983.tb01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L. J., von Meyenburg K. Nonrandom minichromosome replication in Escherichia coli K-12. J Bacteriol. 1987 Jan;169(1):430–433. doi: 10.1128/jb.169.1.430-433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppes L., Nordström K. Insertion of an R1 plasmid into the origin of replication of the E. coli chromosome: random timing of replication of the hybrid chromosome. Cell. 1986 Jan 17;44(1):117–124. doi: 10.1016/0092-8674(86)90490-3. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. A. recA-dependent DNA replication in the absence of protein synthesis: characteristics of a dominant lethal replication mutation, dnaT, and requirement for recA+ function. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):537–549. doi: 10.1101/sqb.1979.043.01.059. [DOI] [PubMed] [Google Scholar]
- Leonard A. C., Helmstetter C. E. Cell cycle-specific replication of Escherichia coli minichromosomes. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5101–5105. doi: 10.1073/pnas.83.14.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett G. W., Orr E., Pritchard R. H. Chloramphenicol releases a block in initiation of chromosome replication in a dnaA strain of Escherichia coli K12. Mol Gen Genet. 1980;178(2):329–336. doi: 10.1007/BF00270480. [DOI] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Boye E., Steen H. B. Timing of initiation of chromosome replication in individual Escherichia coli cells. EMBO J. 1986 Jul;5(7):1711–1717. doi: 10.1002/j.1460-2075.1986.tb04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Cell cycle parameters of slowly growing Escherichia coli B/r studied by flow cytometry. J Bacteriol. 1983 May;154(2):656–662. doi: 10.1128/jb.154.2.656-662.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Steen H. B., Boye E. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J Bacteriol. 1985 Aug;163(2):661–668. doi: 10.1128/jb.163.2.661-668.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen H. B., Lindmo T. Flow cytometry: a high-resolution instrument for everyone. Science. 1979 Apr 27;204(4391):403–404. doi: 10.1126/science.441727. [DOI] [PubMed] [Google Scholar]
- Torrey T. A., Kogoma T. Suppressor mutations (rin) that specifically suppress the recA+ dependence of stable DNA replication in Escherichia coliK-12. Mol Gen Genet. 1982;187(2):225–230. doi: 10.1007/BF00331121. [DOI] [PubMed] [Google Scholar]
- de Massy B., Fayet O., Kogoma T. Multiple origin usage for DNA replication in sdrA(rnh) mutants of Escherichia coli K-12. Initiation in the absence of oriC. J Mol Biol. 1984 Sep 15;178(2):227–236. doi: 10.1016/0022-2836(84)90141-4. [DOI] [PubMed] [Google Scholar]
- van der Ende A., Baker T. A., Ogawa T., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3954–3958. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg Kaspar Transport-limited growth rates in a mutant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):878–888. doi: 10.1128/jb.107.3.878-888.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg K., Hansen F. G., Riise E., Bergmans H. E., Meijer M., Messer W. Origin of replication, oriC, of the Escherichia coli K12 chromosome: genetic mapping and minichromosome replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):121–128. doi: 10.1101/sqb.1979.043.01.018. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., van Deurs B. Physiological and morphological effects of overproduction of membrane-bound ATP synthase in Escherichia coli K-12. EMBO J. 1984 Aug;3(8):1791–1797. doi: 10.1002/j.1460-2075.1984.tb02047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




